Drug-Loaded Polymeric Micelles Based on Smart Biocompatible Graft Copolymers with Potential Applications for the Treatment of Glaucoma
Abstract
1. Introduction
2. Results
2.1. Micellar Sizes and Stability
2.2. FTIR Spectroscopy
2.3. Assessment of the Haemolysis Degree
2.4. In Vitro Cytotoxicity Analysis
2.5. Drug Release Kinetics
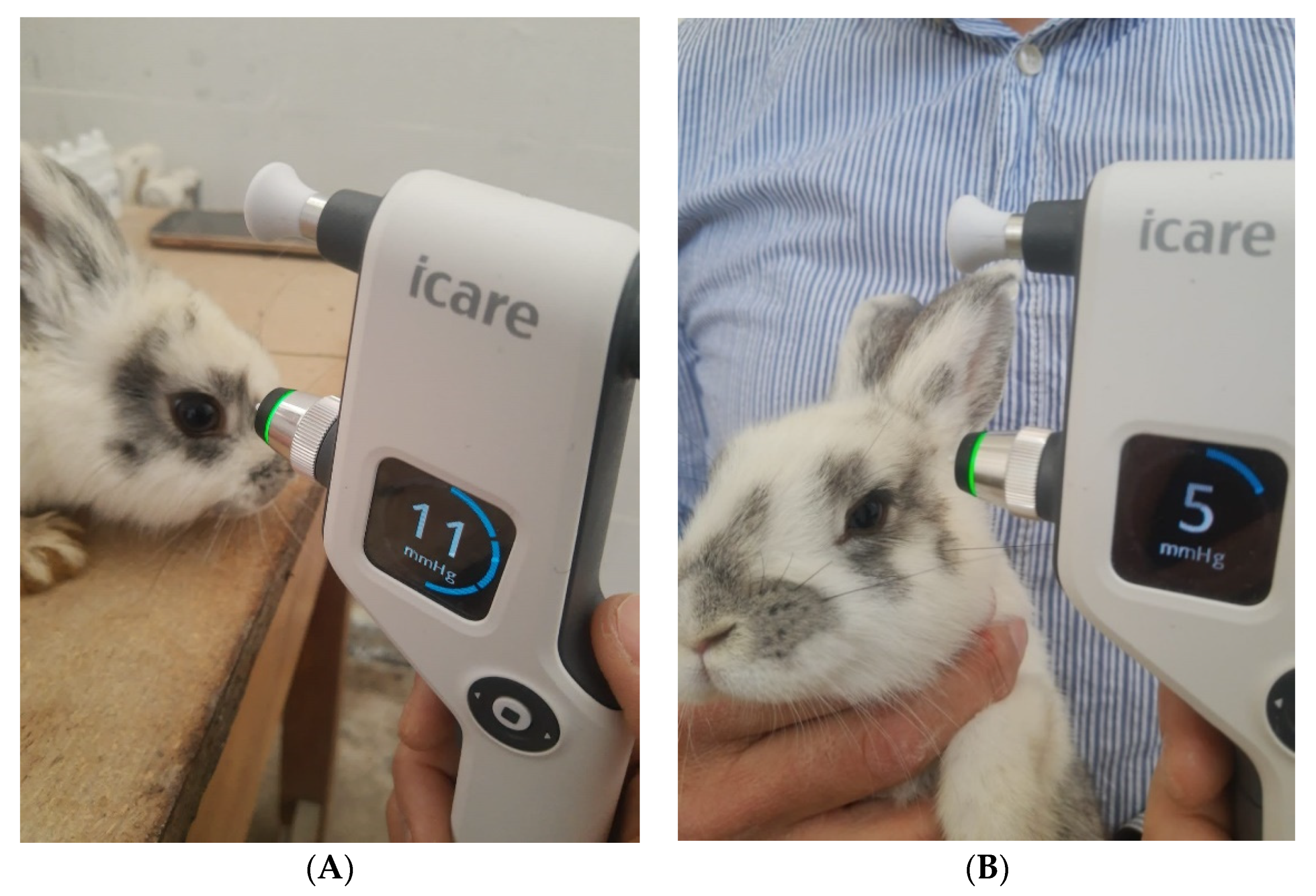
2.6. In Vivo Evaluation of the IOP
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Micelle Preparation Procedure
3.2.2. Physicochemical Characterisation Methods
3.2.3. In Vitro Biological Characterisation Methods
3.2.4. In Vivo Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mélik Parsadaniantz, S.; Réaux-le Goazigo, A.; Sapienza, A.; Habas, C.; Baudouin, C. Glaucoma: A Degenerative Optic Neuropathy Related to Neuroinflammation? Cells 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.; Pandit, J.; Mondal, R.S.; Mishra, A.K.; Chuttani, K.; Aqil, M.; Ali, A.; Sultana, Y. In situ gelling dorzolamide loaded chitosan nanoparticles for the treatment of glaucoma. Carbohydr. Polym. 2014, 102, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Deshmukh, R.; Said, D.G.; Dua, H.S. The impact of COVID-19 pandemic on ophthalmology services: Are we ready for the aftermath? Ther. Adv. Ophthalmol. 2020, 12, 2515841420964099. [Google Scholar] [CrossRef] [PubMed]
- Occhiutto, M.L.; Maranhão, R.C.; Costa, V.P.; Konstas, A.G. Nanotechnology for Medical and Surgical Glaucoma Therapy—A Review. Adv. Ther. 2020, 37, 155–199. [Google Scholar] [CrossRef]
- Kadam, R.S.; Jadhav, G.; Ogidigben, M.; Kompella, U.B. Ocular Pharmacokinetics of Dorzolamide and Brinzolamide After Single and Multiple Topical Dosing: Implications for Effects on Ocular Blood Flow. Drug Metab. Dispos. 2011, 39, 1529–1537. [Google Scholar] [CrossRef]
- Hotchkiss, M.L.; Robin, A.L.; Pollack, I.P.; Quigley, H.A. Nonsteroidal Anti-inflammatory Agents after Argon Laser Trabeculoplasty: A Trial with Flurbiprofen and Indomethacin. Ophthalmology 1984, 91, 969–976. [Google Scholar] [CrossRef]
- Lichter, M.; Feldman, F.; Clark, L.; Cohen, M.M. Effect of Indomethacin on the Ocular Hypotensive Action of Timolol Maleate. Am. J. Ophthalmol. 1984, 98, 79–81. [Google Scholar] [CrossRef]
- Tuulonen, A. The effect of topical indomethacin on acute pressure elevation of laser trabeculoplasty in capsular glaucoma. Acta Ophthalmol. 1985, 63, 245–249. [Google Scholar] [CrossRef]
- Goethals, M.; Missotten, L.; The Belgian Study Group on Glaucoma. Efficacy and safety of Indomethacin 0.1% versus Flurbiprofen 0.03% eyedrops in inflammation after argon laser trabeculoplasty. Doc. Ophthalmol. 1994, 85, 287–293. [Google Scholar] [CrossRef]
- Ammar, H.O.; Salama, H.; Ghorab, M.; Mahmoud, A.A. Nanoemulsion as a Potential Ophthalmic Delivery System for Dorzolamide Hydrochloride. AAPS PharmSciTech 2009, 10, 808–819. [Google Scholar] [CrossRef]
- Afify, E.A.M.R.; Elsayed, I.; Gad, M.K.; Mohamed, M.I.; Afify, A.E.-M.M.R. Enhancement of pharmacokinetic and pharmacological behavior of ocular dorzolamide after factorial optimization of self-assembled nanostructures. PLoS ONE 2018, 13, e0191415. [Google Scholar] [CrossRef] [PubMed]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.A.; Cronemberger, S.; Nogueira, J.C.; et al. Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 570, 118662. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, N.K.; Zafar, A.; Imam, S.S.; Alharbi, K.S.; Alotaibi, N.H.; Alshehri, S.; Alhakamy, N.A.; Alzarea, A.I.; Afzal, M.; Elmowafy, M. Stimulus Responsive Ocular Gentamycin-Ferrying Chitosan Nanoparticles Hydrogel: Formulation Optimization, Ocular Safety and Antibacterial Assessment. Int. J. Nanomed. 2020, 15, 4717–4737. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Pérez, S.; Andrés-Guerrero, V.; López-Cano, J.J.; Molina-Martínez, I.; Herrero-Vanrell, R.; Bravo-Osuna, I. Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents. Pharmaceutics 2020, 12, 306. [Google Scholar] [CrossRef]
- Fouda, N.H.; Abdelrehim, R.T.; Hegazy, D.A.; Habib, B.A. Sustained ocular delivery of Dorzolamide-HCl via proniosomal gel formulation: In-vitro characterization, statistical optimization, and in-vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2018, 25, 1340–1349. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Sosnik, A.; Chiappetta, D.A.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Single and mixed poloxamine micelles as nanocarriers for solubilization and sustained release of ethoxzolamide for topical glaucoma therapy. J. R. Soc. Interface 2012, 9, 2059–2069. [Google Scholar] [CrossRef]
- Durgun, M.E.; Güngör, S.; Özsoy, Y. Micelles: Promising Ocular Drug Carriers for Anterior and Posterior Segment Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 323–341. [Google Scholar] [CrossRef]
- La, S.B.; Okano, T.; Kataoka, K. Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly(β-benzyl L-aspartate) block copolymer micelles. J. Pharm. Sci. 1996, 85, 85–90. [Google Scholar] [CrossRef]
- Shin, I.G.; Kim, S.Y.; Lee, Y.M.; Cho, C.S.; Sung, Y.K. Methoxy poly(ethylene glycol)/ε-caprolactone amphiphilic block copolymeric micelle containing indomethacin.: I. Preparation and characterization. J. Control. Release 1998, 51, 1–11. [Google Scholar] [CrossRef]
- Ouahab, A.; Shen, Y.; Tu, J.-S. Novel oral delivery system of indomethacin by solidified mPEG-PDLLA micelles: In vivo study. Drug Deliv. 2012, 19, 232–237. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Tan, X.; Li, S.; Zhou, Y.; Geng, P.; Hua, A.; Deng, A.; Yu, Z. Indomethacin-based stimuli-responsive micelles combined with paclitaxel to overcome multidrug resistance. Oncotarget 2017, 8, 111281–111294. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.R.; Firouzian, F.; Haddadi, R.; Nourian, A. Indomethacin loaded dextran stearate polymeric micelles improve adjuvant-induced arthritis in rats: Design and in vivo evaluation. Inflammopharmacology 2021, 29, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Sipos, B.; Csóka, I.; Ambrus, R.; Schelz, Z.; Zupkó, I.; Balogh, G.T.; Katona, G. Spray-dried indomethacin-loaded polymeric micelles for the improvement of intestinal drug release and permeability. Eur. J. Pharm. Sci. 2022, 174, 106200. [Google Scholar] [CrossRef]
- Winninger, J.; Iurea, D.M.; Atanase, L.I.; Salhi, S.; Delaite, C.; Riess, G. Micellization of novel biocompatible thermo-sensitive graft copolymers based on poly(ε-caprolactone), poly(N-vinylcaprolactam) and poly(N-vinylpyrrolidone). Eur. Polym. J. 2019, 119, 74–82. [Google Scholar] [CrossRef]
- Atanase, L.; Desbrieres, J.; Riess, G. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization. Polymers 2020, 12, 1018. [Google Scholar] [CrossRef]
- Dupeyrón, D.; Kawakami, M.; Ferreira, A.M.; Cáceres-Vélez, P.R.; Rieumont, J.; Azevedo, R.B.; Carvalho, J.C. Design of indomethacin-loaded nanoparticles: Effect of polymer matrix and surfactant. Int. J. Nanomed. 2013, 8, 3467–3477. [Google Scholar] [CrossRef]
- Badri, W.; El Asbahani, A.; Miladi, K.; Baraket, A.; Agusti, G.; Nazari, Q.A.; Errachid, A.; Fessi, H.; Elaissari, A. Poly (ε-caprolactone) nanoparticles loaded with indomethacin and Nigella sativa L. essential oil for the topical treatment of inflammation. J. Drug Deliv. Sci. Technol. 2018, 46, 234–242. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Adam, M.; LaRue, I.; Sheiko, S.S.; Rubinstein, M. Diblock Copolymer Micelles in a Dilute Solution. Macromolecules 2005, 38, 5330–5351. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; González-Gómez, Á.; Lizardi-Mendoza, J.; Vázquez-Lasa, B.; Goycoolea, F.M.; San Román, J.; Argüelles-Monal, W.M. Effect of the molecular architecture on the thermosensitive properties of chitosan-g-poly(N-vinylcaprolactam). Carbohydr. Polym. 2015, 134, 92–101. [Google Scholar] [CrossRef]
- Massoumi, B.; Ramezani, M.; Jaymand, M.; Ahmadinejad, M. Multi-walled carbon nanotubes-g-[poly(ethylene glycol)-b-poly(ε-caprolactone)]: Synthesis, characterization, and properties. J. Polym. Res. 2015, 22, 214. [Google Scholar] [CrossRef]
- Cadinoiu, A.; Peptu, C.A.; Fache, B.; Chailan, J.F.; Popa, M. Microparticulated systems based on chitosan and poly(vinyl alcohol) with potential ophthalmic applications. J. Microencapsul. 2015, 32, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Burlui, V.; Popa, M.; Cadinoiu, A.N.; Stadoleanu, C.; Mihalache, G.; Zamaru, V.; Dârţu, L.; Folescu, E.; Raţă, D.M. Physico-chemical characterization and in vitro hemolysis evaluation of titanium dioxide nanoparticles. Int. J. Med. Dent. 2015, 19, 124–130. [Google Scholar]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I. Biocompatible injectable polysaccharide materials for drug delivery. In Polysaccharide Carriers for Drug Delivery; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 127–154. [Google Scholar]
- Alupei, L.; Lisa, G.; Butnariu, A.; Desbrieres, J.; Cadinoiu, A.N.; Peptu, C.A.; Calin, G.; Popa, M. New folic acid-chitosan derivative based nanoparticles–potential applications in cancer therapy. Cellul. Chem. Technol. 2017, 51, 631–648. [Google Scholar]
- Prabhu, P.; Kumar, R.N.; Koland, M.; Harish, N.M.; Vijayanarayan, K.; Dhondge, G.; Charyulu, R.N. Preparation and Evaluation of Nano-vesicles of Brimonidine Tartrate as an Ocular Drug Delivery System. J. Young Pharm. 2010, 2, 356–361. [Google Scholar] [CrossRef]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.-M.; Gherghel, D.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef]
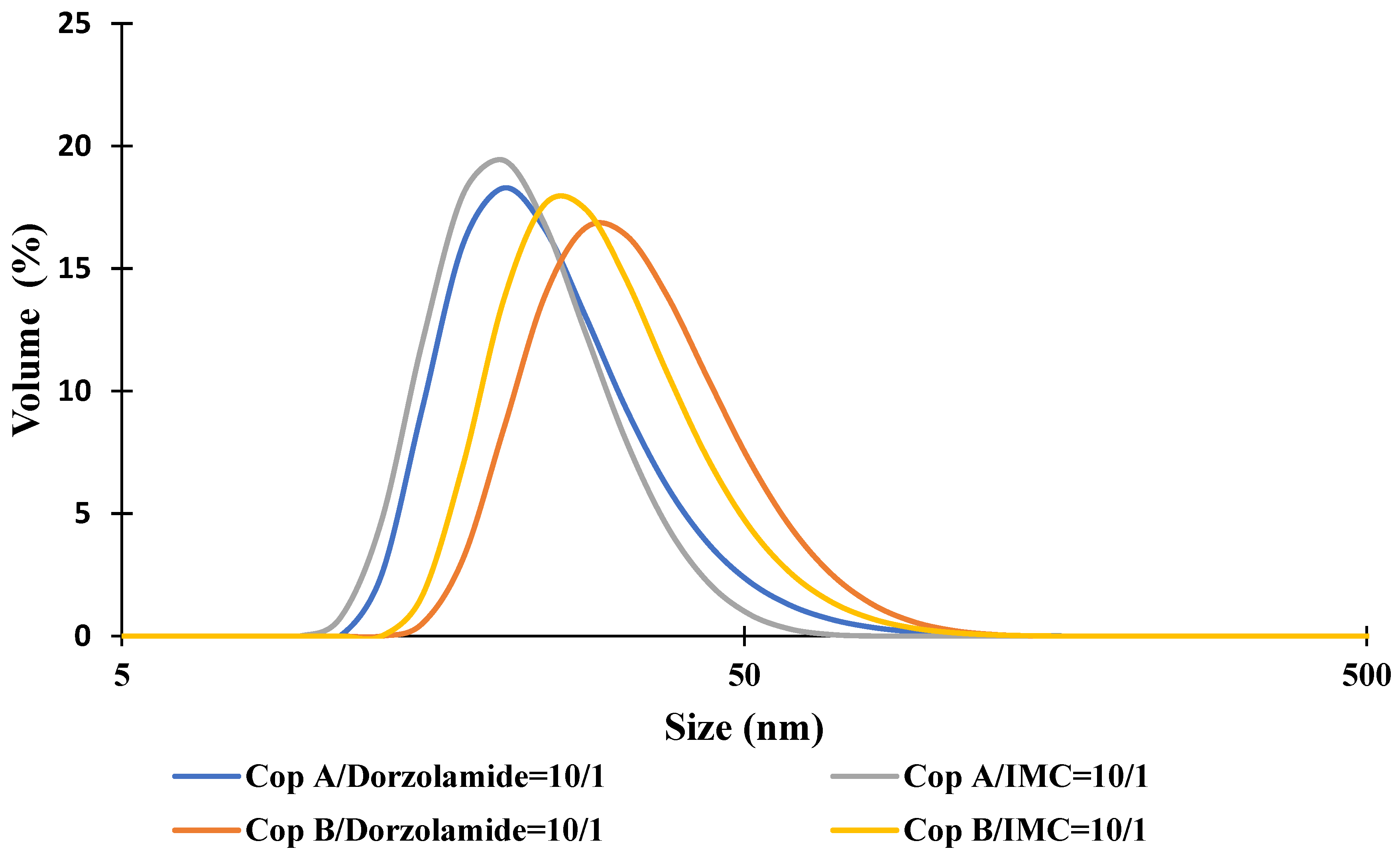


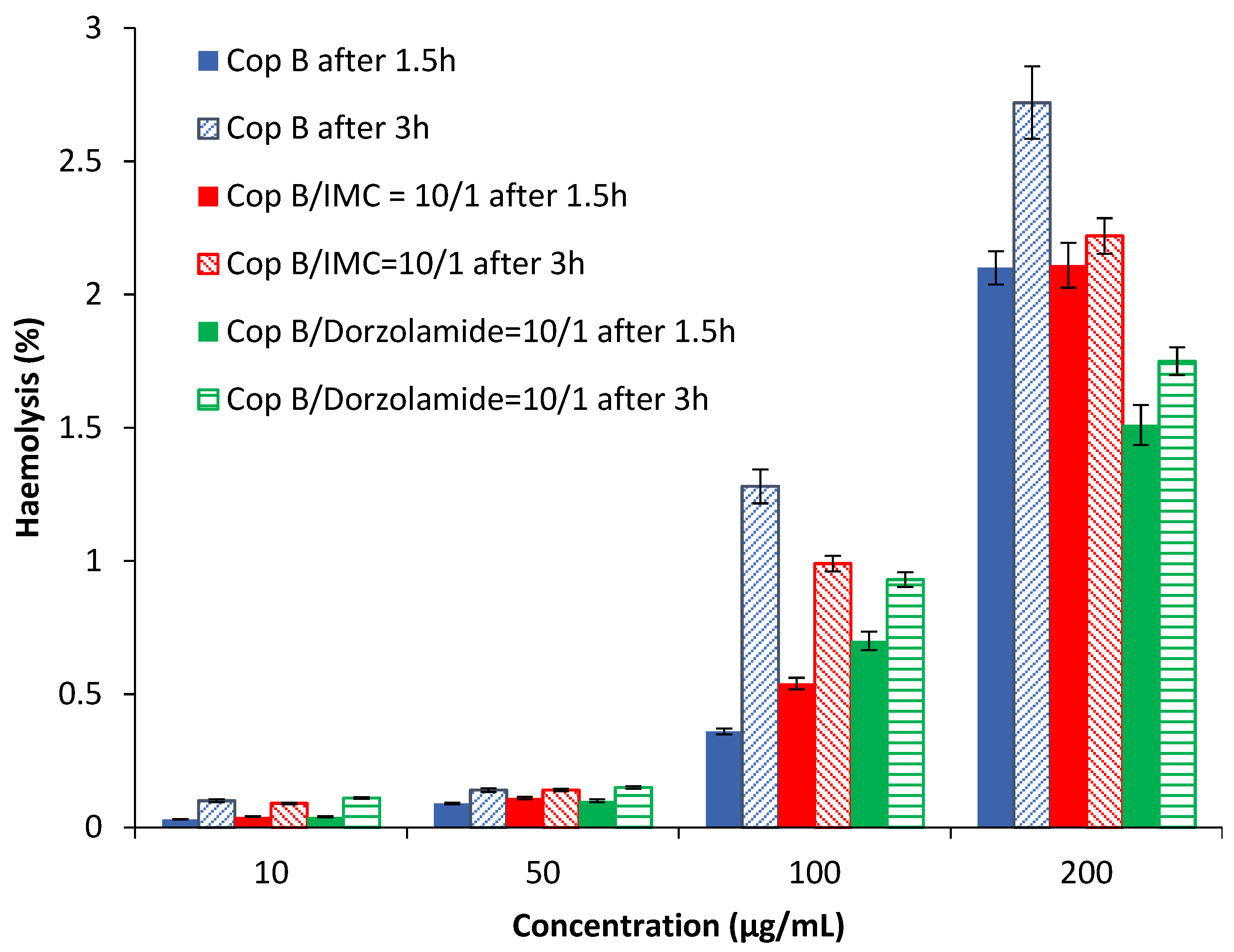
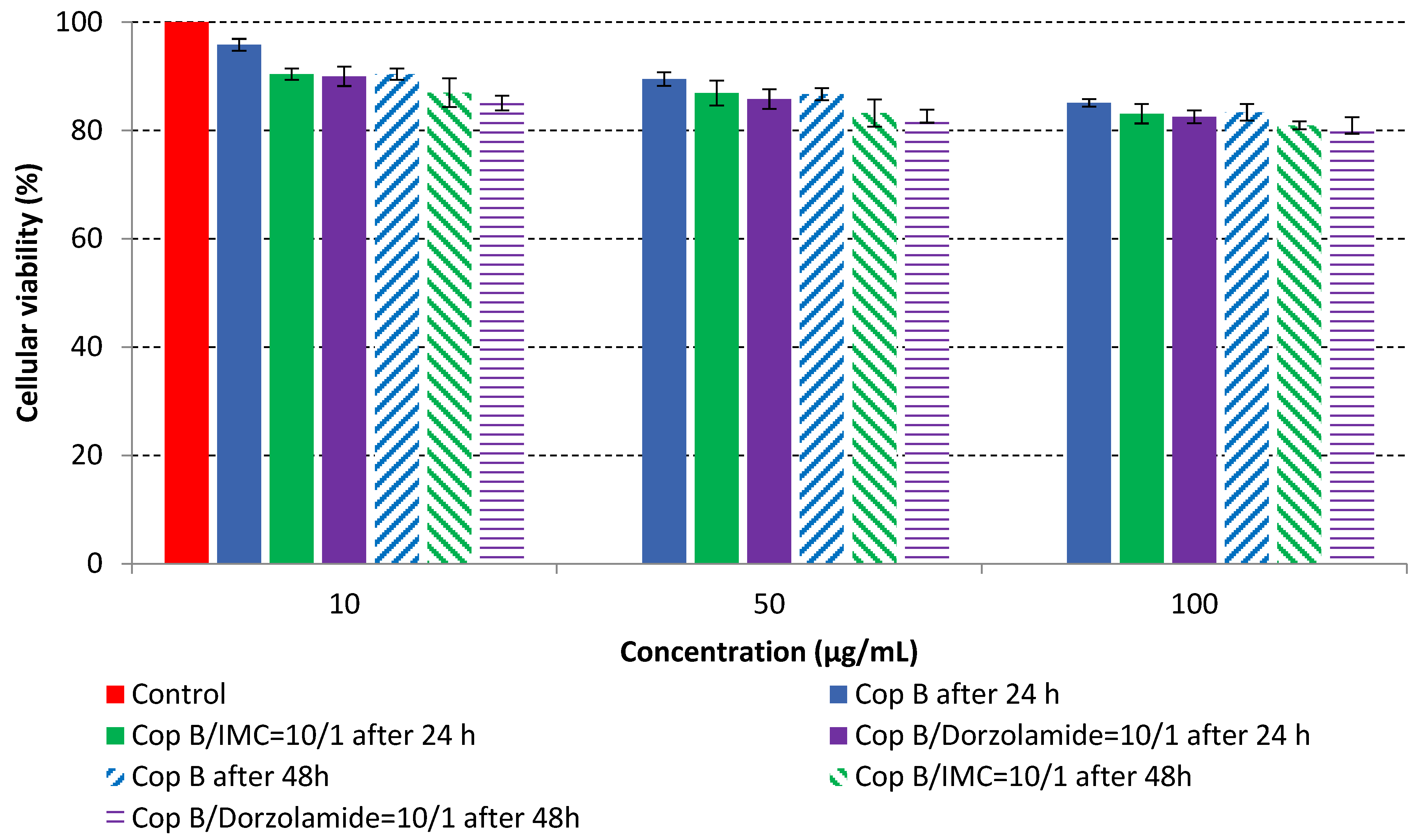
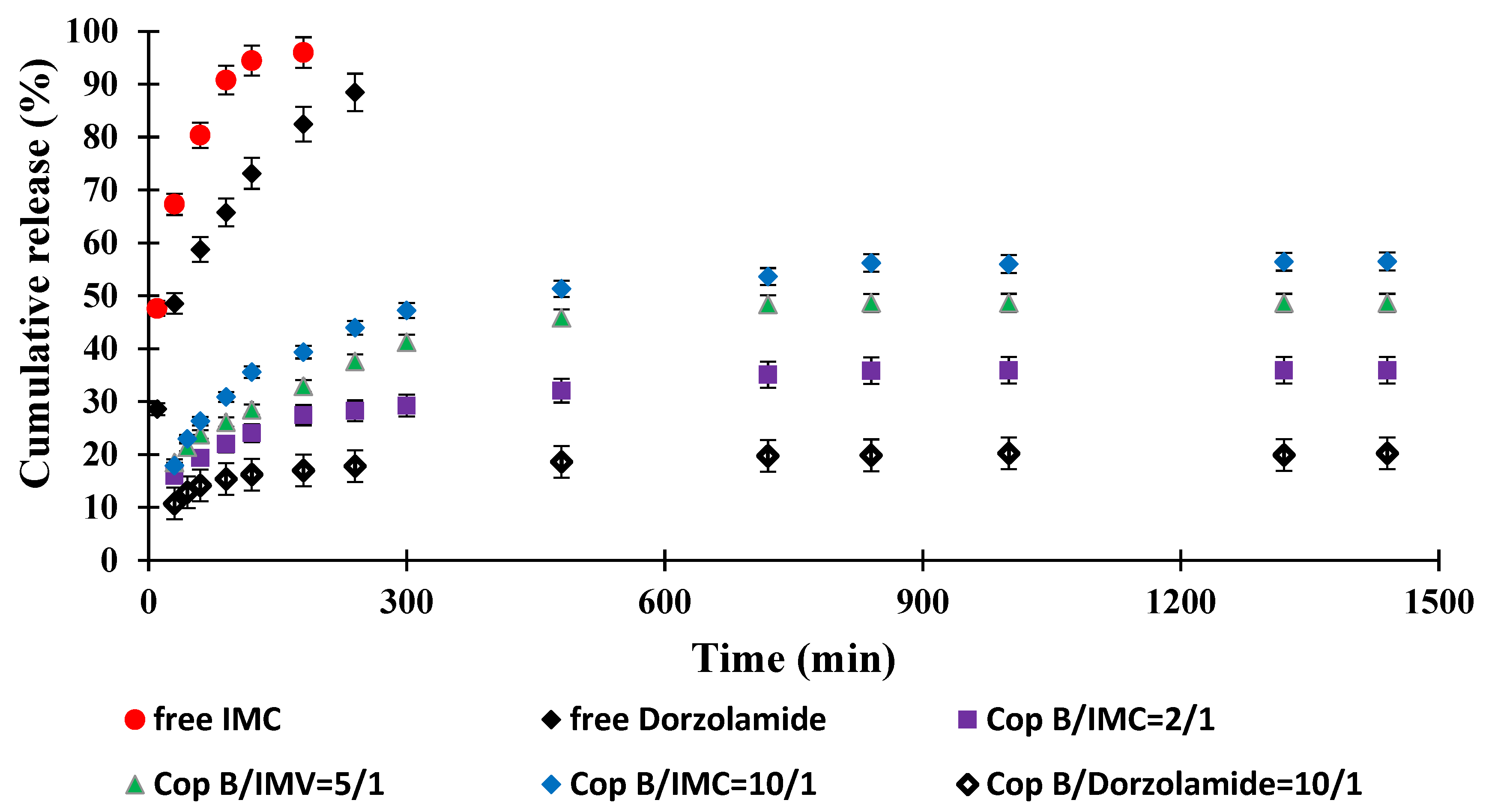
| Sample | Z-Average (nm) | Dv (nm) | PDI | ZP (mV) | DEE (%) | DLE (%) |
|---|---|---|---|---|---|---|
| Cop A | 39.4 ± 0.1 | 24.2 ± 0.2 | 0.197 | −3.5 | - | - |
| Cop A/Dorzolamide = 10/1 (wt/wt) | 41.3 ± 0.4 | 24.5 ± 0.5 | 0.258 | −3.3 | 20.2 | 4.4 |
| Cop A/IMC = 10/1 (wt/wt) | 29.6 ± 0.3 | 23.0 ± 0.1 | 0.154 | −7.5 | 68.5 | 6.3 |
| Cop B | 47.2 ± 0.2 | 34.8 ± 0.4 | 0.200 | −3.3 | - | - |
| Cop B/Dorzolamide = 10/1 (wt/wt) | 54.8 ± 0.3 | 37.5 ± 0.2 | 0.354 | −3.3 | 34.0 | 6.7 |
| Cop B/IMC = 10/1 (wt/wt) | 41.8 ± 0.4 | 31.0 ± 0.1 | 0.161 | −5.5 | 75.1 | 8.2 |
| Cop B/Dorzolamide = 10/1+Cop B/IMC = 10/1 (50/50 wt/wt) | 45.7 ± 0.5 | 33.9 ± 0.2 | 0.252 | −4.1 | - | - |
| Sample | 24 h | 48 h | |
|---|---|---|---|
| Control | 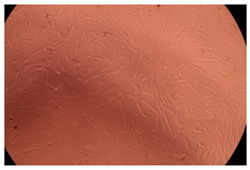 | 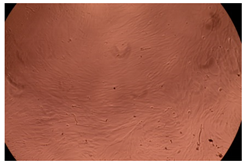 | |
| Cop B | 10 µg/mL |  | 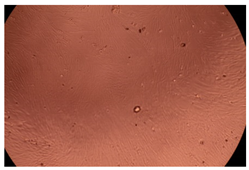 |
| 50 µg/mL | 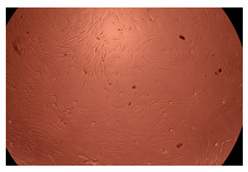 |  | |
| 100 µg/mL |  | 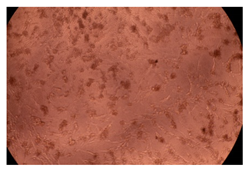 | |
| Cop B/IMC = 10/1 (wt/wt) | 10 µg/mL | 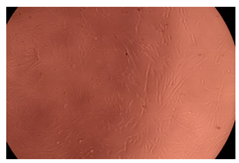 |  |
| 50 µg/mL |  | 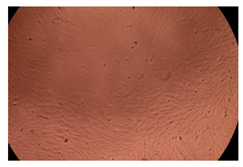 | |
| 100 µg/mL | 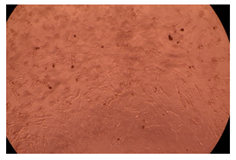 | 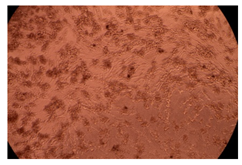 | |
| Cop B/Dorzolamide = 10/1 (wt/wt) | 10 µg/mL |  | 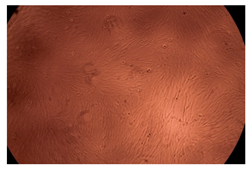 |
| 50 µg/mL | 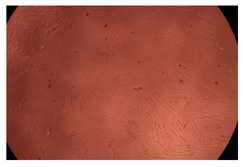 | 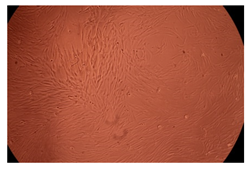 | |
| 100 µg/mL |  |  | |
| Sample | IOP (mmHg) | |||||
|---|---|---|---|---|---|---|
| After 15 Days of DEX Administration | After 15 Days of Treatment | |||||
| Morning | Noon | Evening | Morning | Noon | Evening | |
| LC | 5 | 6 | 7 | 5 | 6 | 7 |
| LE1 | 9 | 10 | 11 | 9 | 10 | 11 |
| LE2 | 9 | 10 | 11 | 9 | 10 | 11 |
| LE3 | 9 | 10 | 11 | 7 | 8 | 9 |
| LE4 | 9 | 10 | 11 | 4 | 5 | 5 |
| LE5 | 9 | 11 | 12 | 10 | 11 | 13 |
| Copolymer | Sample Name | PNVCL (mol %) | PNVP (mol%) | DPn, Average per Graft |
|---|---|---|---|---|
| PCL120-g-P(NVCL507-co-NVP128) | Cop A | 64 | 20 | 42 |
| PCL120-g-P(NVCL1253-co-NVP139) | Cop B | 82 | 10 | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozturk, M.-R.; Popa, M.; Rata, D.M.; Cadinoiu, A.N.; Parfait, F.; Delaite, C.; Atanase, L.I.; Solcan, C.; Daraba, O.M. Drug-Loaded Polymeric Micelles Based on Smart Biocompatible Graft Copolymers with Potential Applications for the Treatment of Glaucoma. Int. J. Mol. Sci. 2022, 23, 9382. https://doi.org/10.3390/ijms23169382
Ozturk M-R, Popa M, Rata DM, Cadinoiu AN, Parfait F, Delaite C, Atanase LI, Solcan C, Daraba OM. Drug-Loaded Polymeric Micelles Based on Smart Biocompatible Graft Copolymers with Potential Applications for the Treatment of Glaucoma. International Journal of Molecular Sciences. 2022; 23(16):9382. https://doi.org/10.3390/ijms23169382
Chicago/Turabian StyleOzturk, Manuela-Ramona (Blanaru), Marcel Popa, Delia Mihaela Rata, Anca Niculina Cadinoiu, Frederique Parfait, Christelle Delaite, Leonard Ionut Atanase, Carmen Solcan, and Oana Maria Daraba. 2022. "Drug-Loaded Polymeric Micelles Based on Smart Biocompatible Graft Copolymers with Potential Applications for the Treatment of Glaucoma" International Journal of Molecular Sciences 23, no. 16: 9382. https://doi.org/10.3390/ijms23169382
APA StyleOzturk, M.-R., Popa, M., Rata, D. M., Cadinoiu, A. N., Parfait, F., Delaite, C., Atanase, L. I., Solcan, C., & Daraba, O. M. (2022). Drug-Loaded Polymeric Micelles Based on Smart Biocompatible Graft Copolymers with Potential Applications for the Treatment of Glaucoma. International Journal of Molecular Sciences, 23(16), 9382. https://doi.org/10.3390/ijms23169382










