Isolation of Reporter Cells That Respond to Vitamin A and/or D Using a piggyBac Transposon Promoter-Trapping Vector System
Abstract
:1. Introduction
2. Results
2.1. Cloning of Calcitriol- or Bexarotene-Responsive Cells Using a Transposon-Mediated Promoter-Trapping Vector System
2.2. Identification of Responsive Genes by 5′ Rapid Amplification of cDNA Ends (5′ RACE)
2.3. Low Induction Levels of the Newly Identified Responsive Genes Revealed by Real-Time PCR Analysis
2.4. Luciferase Assay of Reporter Cells for Responsiveness to Various Vitamin A and D Analogs
3. Discussion
4. Materials and Methods
4.1. Establishment of Reporter Cells Responsive to Bexarotene or Calcitriol
4.2. Identification of Responsive Genes by 5′ Rapid Amplification of cDNA Ends
4.3. Real-Time PCR Analysis
4.4. Luciferase Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishikawa, K.; Kobayashi, Y.; Wakabayashi, Y.; Watanabe, S.; Semba, K. A highly sensitive trap vector system for isolating reporter cells and identification of responsive genes. Biol. Methods Protoc. 2018, 3, bpy003. [Google Scholar] [CrossRef] [PubMed]
- Ivics, Z.; Li, M.A.; Mátés, L.; Boeke, J.D.; Nagy, A.; Bradley, A.; Izsvák, Z. Transposon-mediated genome manipulation in vertebrates. Nat. Methods 2009, 6, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, K.; Suster, M.L.; Mizusawa, K.; Nagayoshi, S.; Kotani, T.; Urasaki, A.; Kishimoto, Y.; Hibi, M.; Kawakami, K. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA 2008, 105, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Tamamura, S.; Semba, K.; Watanabe, S. Establishment of reporter cells that respond to glucocorticoids by a transposon-mediated promoter-trapping system. Eur. J. Pharm. Sci. 2021, 162, 105819. [Google Scholar] [CrossRef]
- Yusa, K.; Zhou, L.; Li, M.A.; Bradley, A.; Craig, N.L. A hyperactive piggyBac transposase for mammalian applications. Proc. Natl. Acad. Sci. USA 2011, 108, 1531–1536. [Google Scholar] [CrossRef]
- Grabundzija, I.; Irgang, M.; Mátés, L.; Belay, E.; Matrai, J.; Gogol-Döring, A.; Kawakami, K.; Chen, W.; Ruiz, P.; Chuah, M.K.; et al. Comparative analysis of transposable element vector systems in human cells. Mol. Ther. 2010, 18, 1200–1209. [Google Scholar] [CrossRef]
- Gogol-Döring, A.; Ammar, I.; Gupta, S.; Bunse, M.; Miskey, C.; Chen, W.; Uckert, W.; Schulz, T.F.; Izsvák, Z.; Ivics, Z. Genome-wide Profiling Reveals Remarkable Parallels Between Insertion Site Selection Properties of the MLV Retrovirus and the piggyBac Transposon in Primary Human CD4+ T Cells. Mol. Ther. 2016, 24, 592–606. [Google Scholar] [CrossRef]
- Tashiro, K.; Abe, T.; Oue, N.; Yasui, W.; Ryoji, M. Characterization of vitamin D-mediated induction of the CYP 24 transcription. Mol. Cell. Endocrinol. 2004, 226, 27–32. [Google Scholar] [CrossRef]
- Sakaki, T.; Kagawa, N.; Yamamoto, K.; Inouye, K. Metabolism of vitamin D3 by cytochromes P450. Front. Biosci. 2005, 10, 119–134. [Google Scholar] [CrossRef]
- Leeb-Lundberg, L.M.; Marceau, F.; Müller-Esterl, W.; Pettibone, D.J.; Zuraw, B.L. International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 2005, 57, 27–77. [Google Scholar] [CrossRef]
- Couture, R.; Blaes, N.; Girolami, J.P. Kinin receptors in vascular biology and pathology. Curr. Vasc. Pharmacol. 2014, 12, 223–248. [Google Scholar] [CrossRef]
- Graness, A.; Adomeit, A.; Heinze, R.; Wetzker, R.; Liebmann, C. A novel mitogenic signaling pathway of bradykinin in the human colon carcinoma cell line SW-480 involves sequential activation of a Gq/11 protein, phosphatidylinositol 3-kinase beta, and protein kinase Cepsilon. J. Biol. Chem. 1998, 273, 32016–32022. [Google Scholar] [CrossRef]
- Ichikawa, T.; Horie-Inoue, K.; Ikeda, K.; Blumberg, B.; Inoue, S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J. Biol. Chem. 2006, 281, 16927–16934. [Google Scholar] [CrossRef]
- Deng, X.; Li, Y.; Guo, C.; Zhao, Z.; Yuan, G. Novel roles of Tsukushi in signaling pathways and multiple disease processes. Biofactors 2021, 47, 512–521. [Google Scholar] [CrossRef]
- Di Masi, A.; Leboffe, L.; De Marinis, E.; Pagano, F.; Cicconi, L.; Rochette-Egly, C.; Lo-Coco, F.; Ascenzi, P.; Nervi, C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol. Asp. Med. 2015, 41, 1–115. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Li, R.; Chen, G. Transcriptional Factors Mediating Retinoic Acid Signals in the Control of Energy Metabolism. Int. J. Mol. Sci. 2015, 16, 14210–14244. [Google Scholar] [CrossRef]
- Ramagopalan, S.V.; Heger, A.; Berlanga, A.J.; Maugeri, N.J.; Lincoln, M.R.; Burrell, A.; Handunnetthi, L.; Handel, A.E.; Disanto, G.; Orton, S.M.; et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010, 20, 1352–1360. [Google Scholar] [CrossRef]
- Haussler, M.R.; Haussler, C.A.; Jurutka, P.W.; Thompson, P.D.; Hsieh, J.C.; Remus, L.S.; Selznick, S.H.; Whitfield, G.K. The vitamin D hormone and its nuclear receptor: Molecular actions and disease states. J. Endocrinol. 1997, 154, S57–S73. [Google Scholar]
- Carlberg, C.; Seuter, S. A genomic perspective on vitamin D signaling. Anticancer Res. 2009, 29, 3485–3493. [Google Scholar]
- Rochette-Egly, C. Retinoic Acid-Regulated Target Genes During Development: Integrative Genomics Analysis. In The Biochemistry of Retinoid Signaling III; Subcellular Biochemistry; Springer: Cham, Switzerland, 2020; Volume 95, pp. 57–85. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and Its Target Genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Dunlop, T.W. The impact of chromatin organization of vitamin D target genes. Anticancer Res. 2006, 26, 2637–2645. [Google Scholar] [PubMed]
- Siebring-van Olst, E.; Vermeulen, C.; de Menezes, R.X.; Howell, M.; Smit, E.F.; van Beusechem, V.W. Affordable luciferase reporter assay for cell-based high-throughput screening. J. Biomol. Screen. 2013, 18, 453–461. [Google Scholar] [CrossRef] [PubMed]
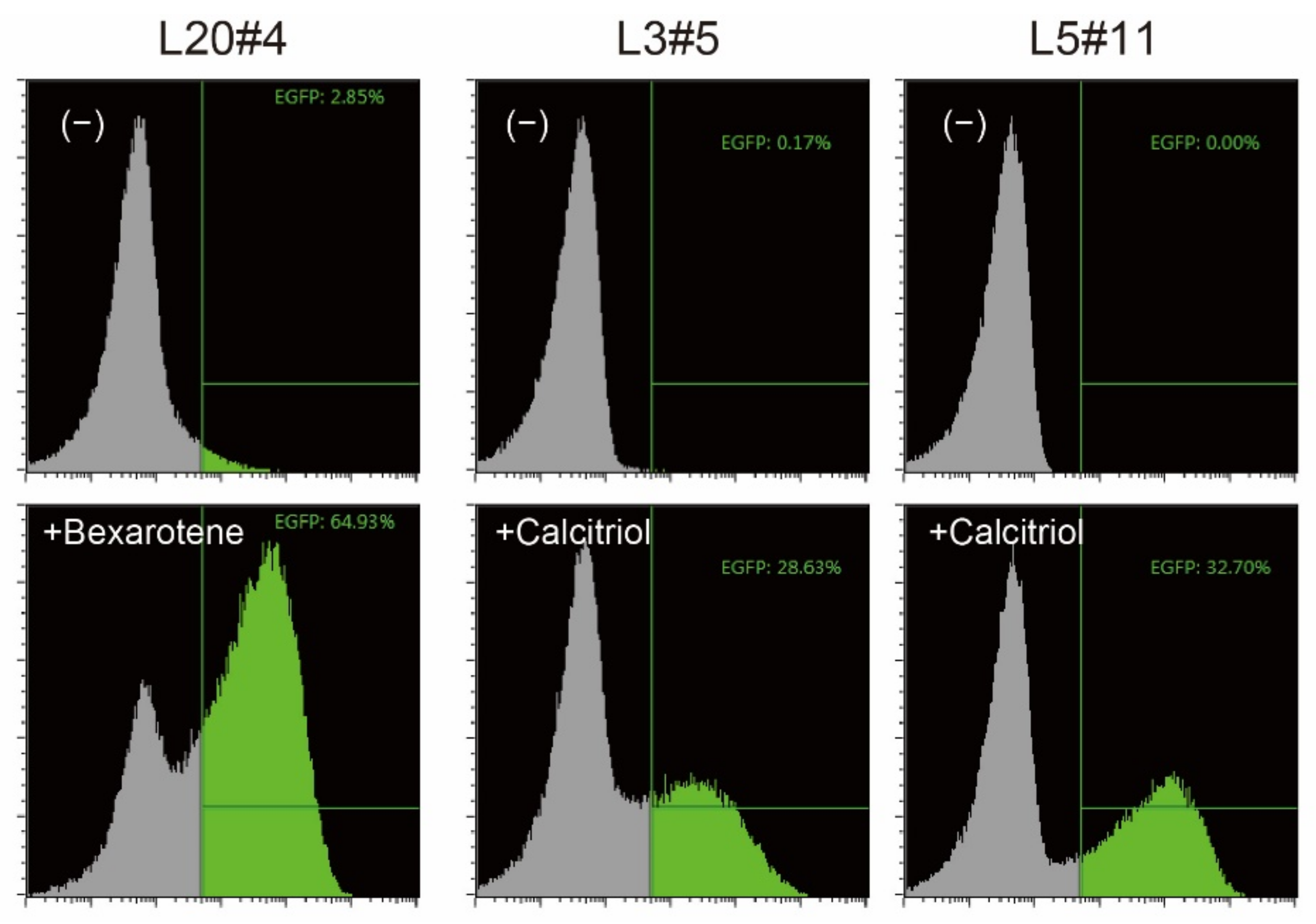
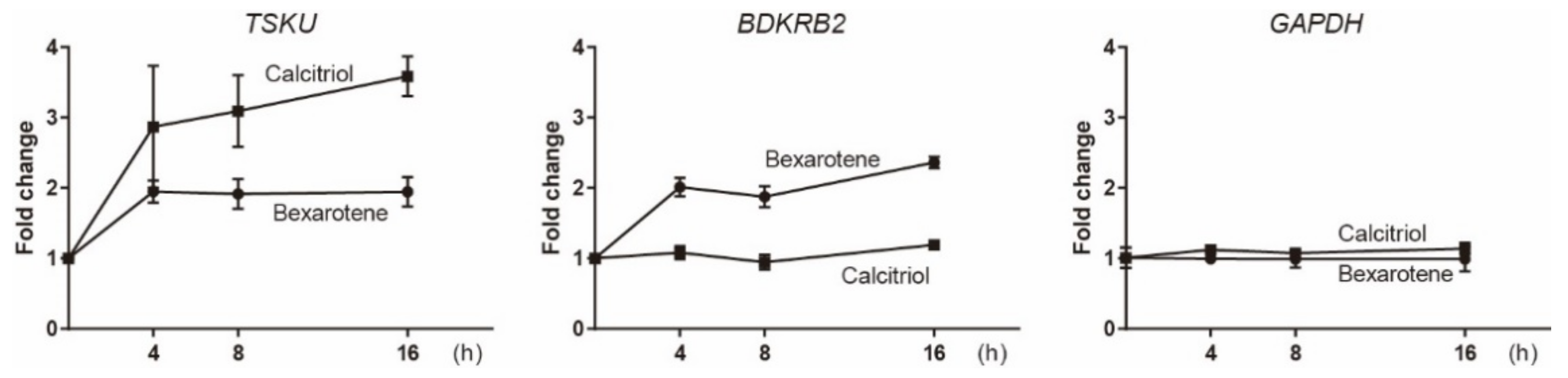
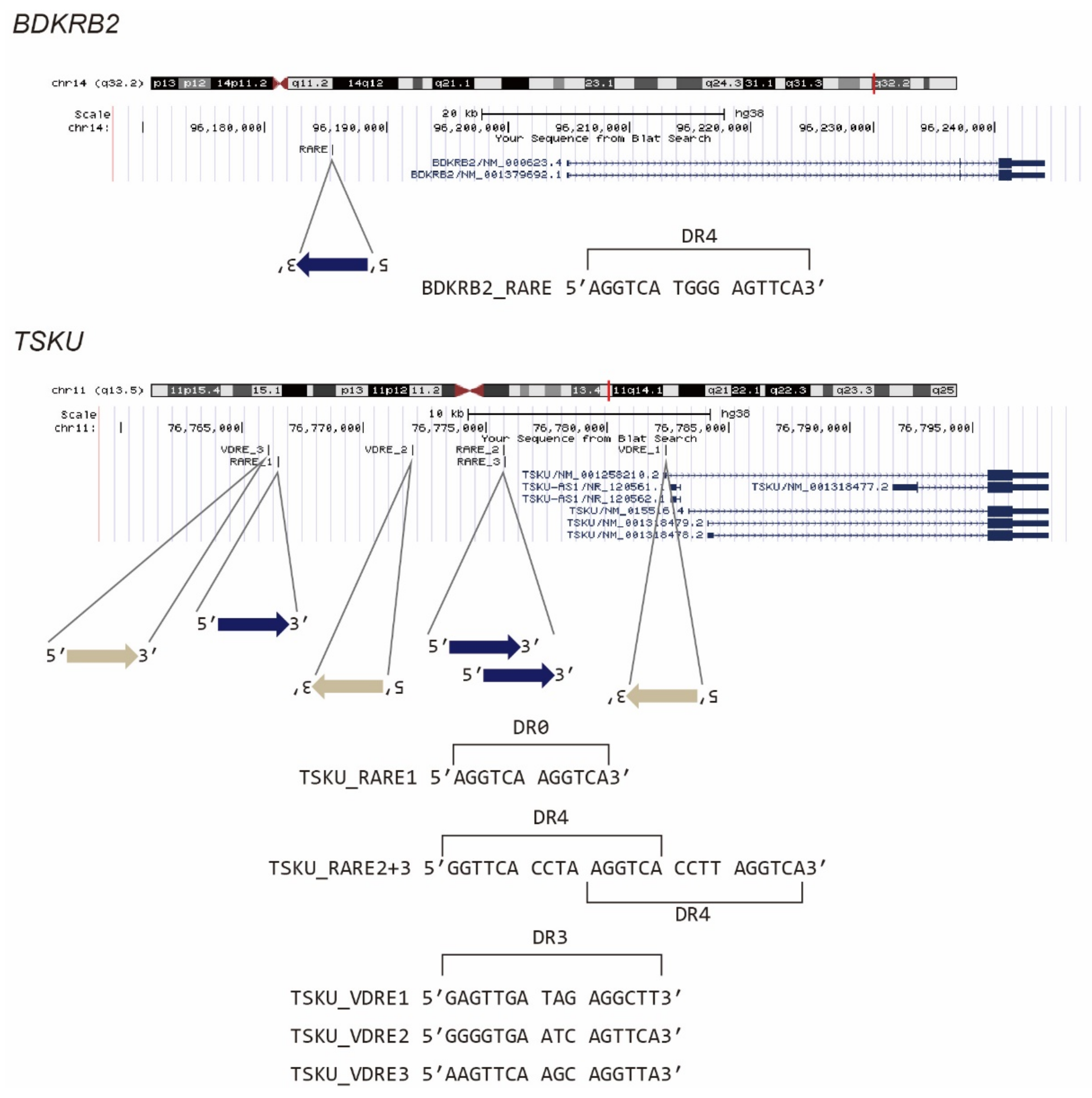
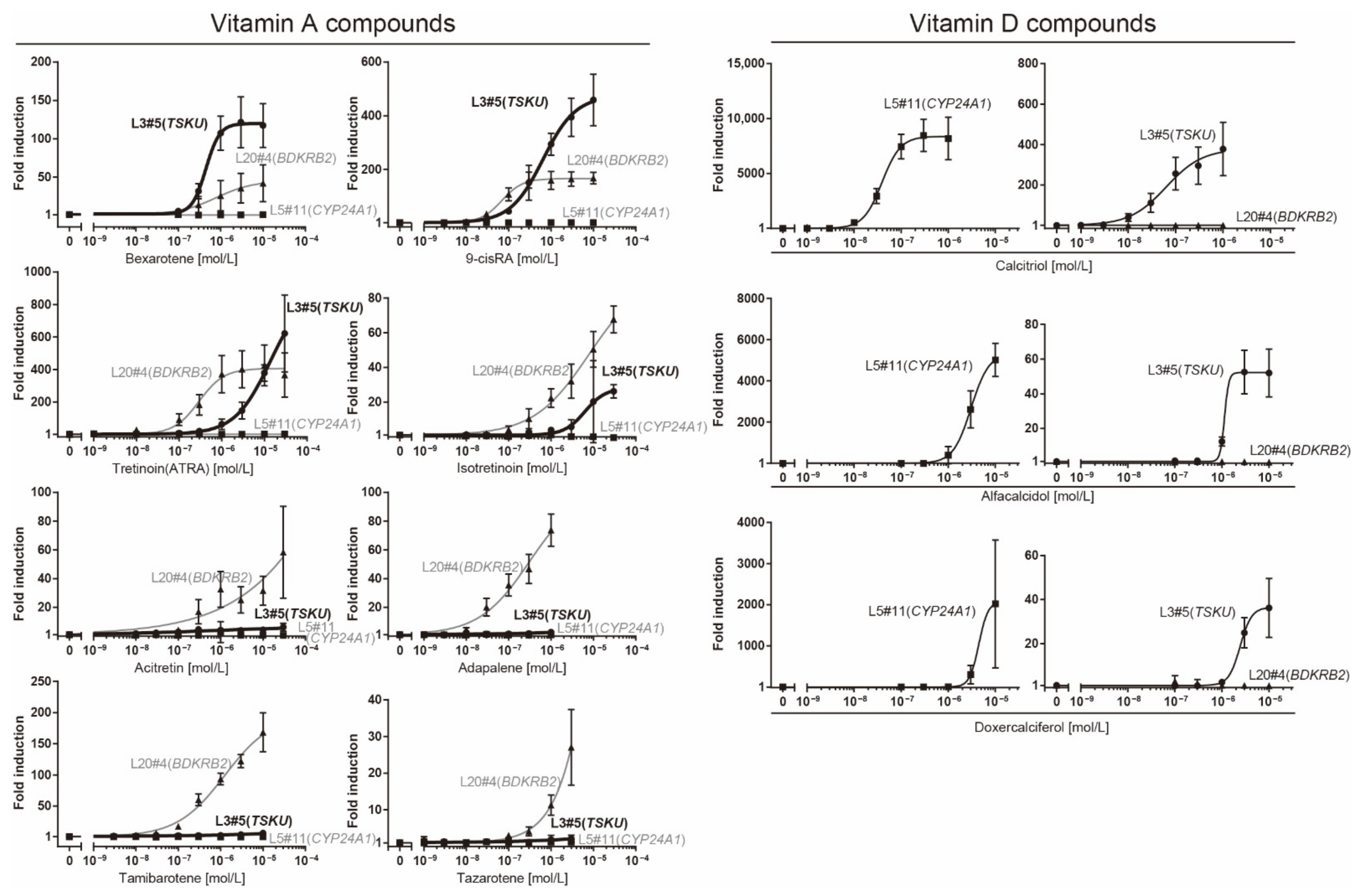
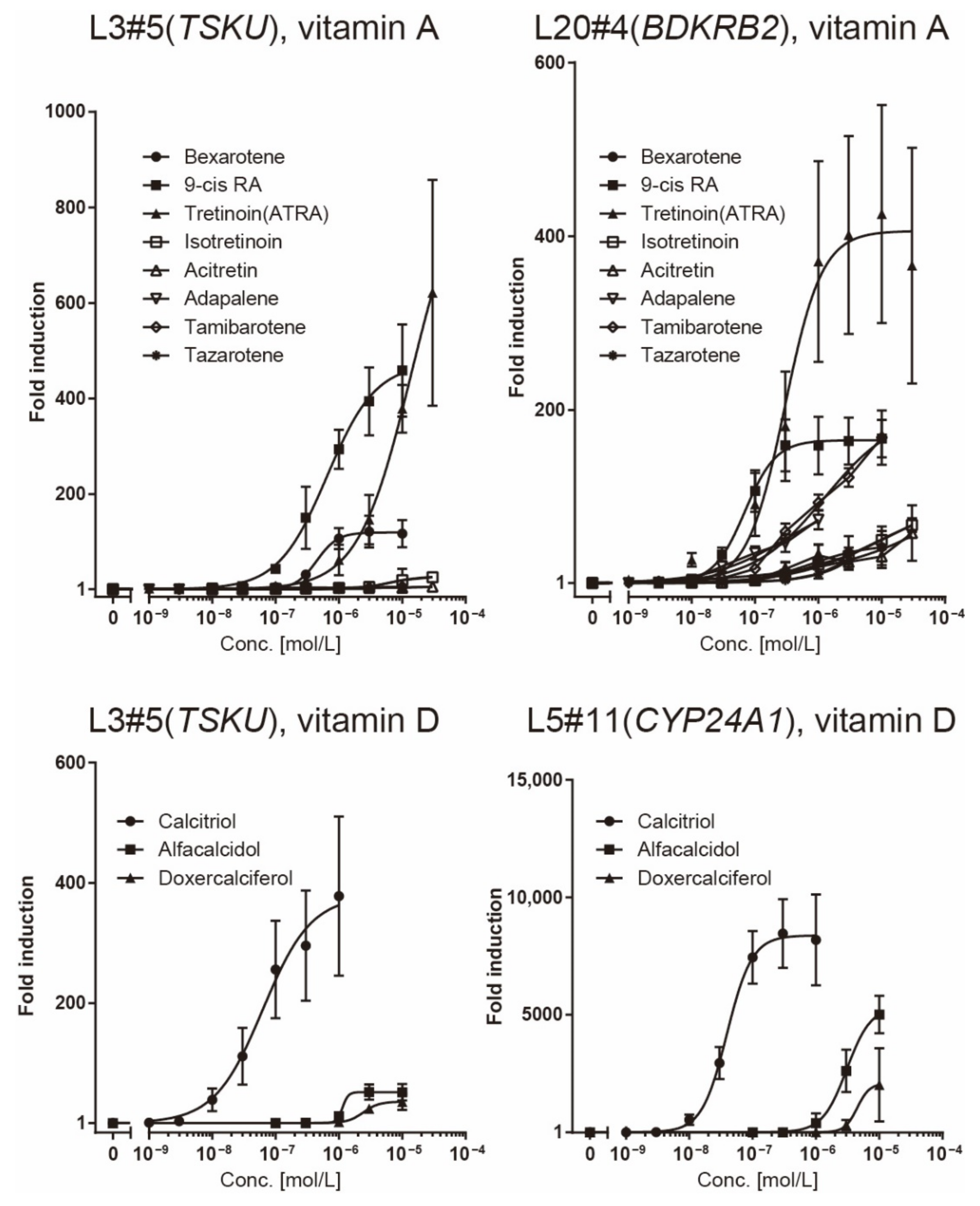
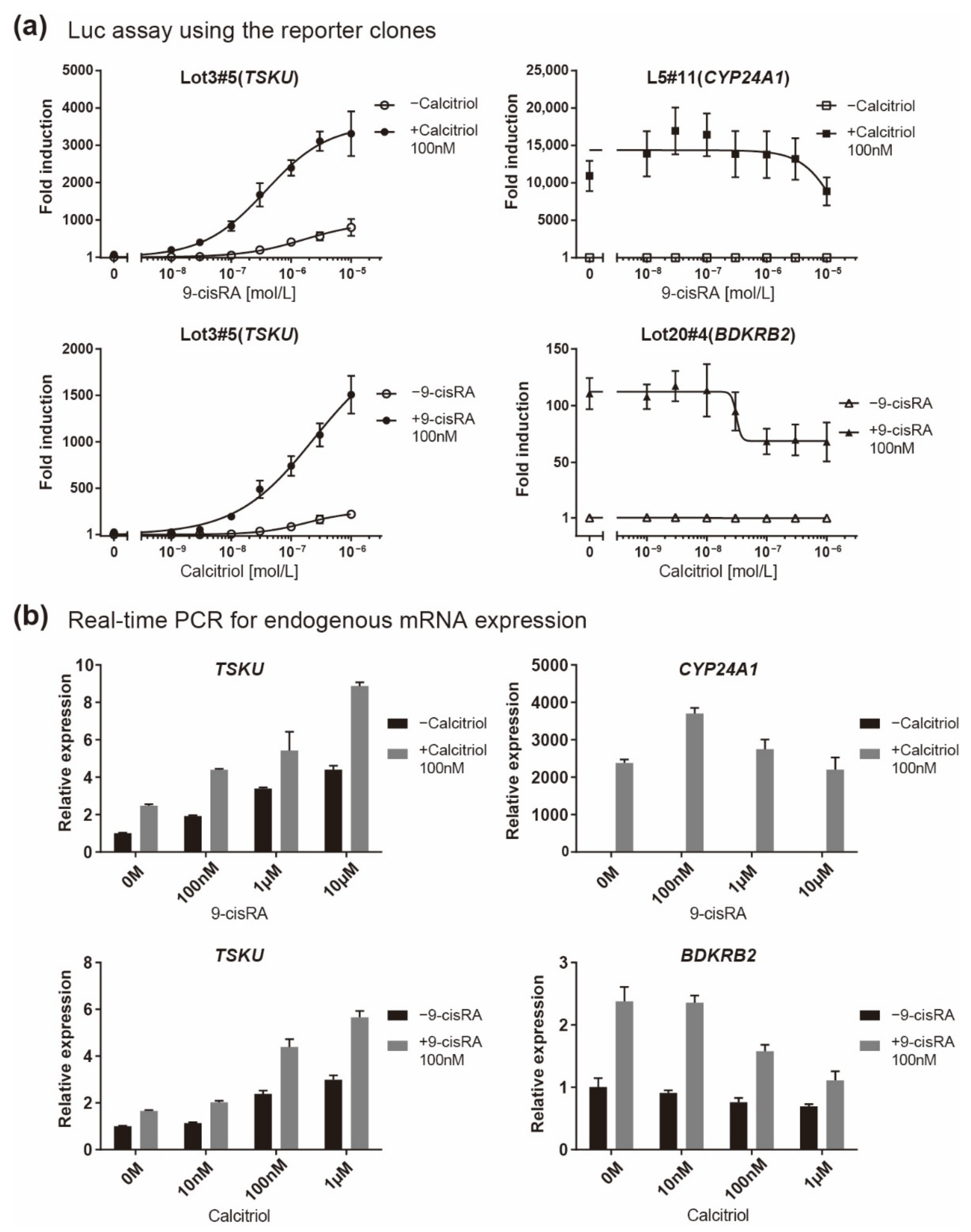
| Clone# | Reagent Used for Screening | Responsive Gene |
|---|---|---|
| L20#4 | Bexarotene (vitamin A) | BDKRB2 |
| L3#5 | Calcitriol (vitamin D) | TSKU |
| L5#11 | Calcitriol (vitamin D) | CYP24A1 |
| Gene | Primer Sequence (5′→3′) |
|---|---|
| TSKU | CTGAGCGACGTGAACCTTAGC |
| CCTGACTGTGCGTCGTGAAG | |
| BDKRB2 | GTACCAGGGAGCGACTGAAG |
| GGGCAAAGGTCCCGTTAAGA | |
| CYP24A1 | CTCATGCTAAATACCCAGGTG |
| TCGCTGGCAAAACGCGATGGG | |
| GAPDH | GAAGGTGAAGGTCGGAGTC |
| GAAGATGGTGATGGGATTTC | |
| HPRT1 | TGACACTGGCAAAACAATGCA |
| GGTCCTTTTCACCAGCAAGCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, K.; Tamamura, S.; Takahashi, N.; Takagi, M.; Semba, K.; Watanabe, S. Isolation of Reporter Cells That Respond to Vitamin A and/or D Using a piggyBac Transposon Promoter-Trapping Vector System. Int. J. Mol. Sci. 2022, 23, 9366. https://doi.org/10.3390/ijms23169366
Ishikawa K, Tamamura S, Takahashi N, Takagi M, Semba K, Watanabe S. Isolation of Reporter Cells That Respond to Vitamin A and/or D Using a piggyBac Transposon Promoter-Trapping Vector System. International Journal of Molecular Sciences. 2022; 23(16):9366. https://doi.org/10.3390/ijms23169366
Chicago/Turabian StyleIshikawa, Kosuke, Sakura Tamamura, Nobuhito Takahashi, Motoki Takagi, Kentaro Semba, and Shinya Watanabe. 2022. "Isolation of Reporter Cells That Respond to Vitamin A and/or D Using a piggyBac Transposon Promoter-Trapping Vector System" International Journal of Molecular Sciences 23, no. 16: 9366. https://doi.org/10.3390/ijms23169366
APA StyleIshikawa, K., Tamamura, S., Takahashi, N., Takagi, M., Semba, K., & Watanabe, S. (2022). Isolation of Reporter Cells That Respond to Vitamin A and/or D Using a piggyBac Transposon Promoter-Trapping Vector System. International Journal of Molecular Sciences, 23(16), 9366. https://doi.org/10.3390/ijms23169366






