Microwave Absorption of α-Fe2O3@diatomite Composites
Abstract
:1. Introduction
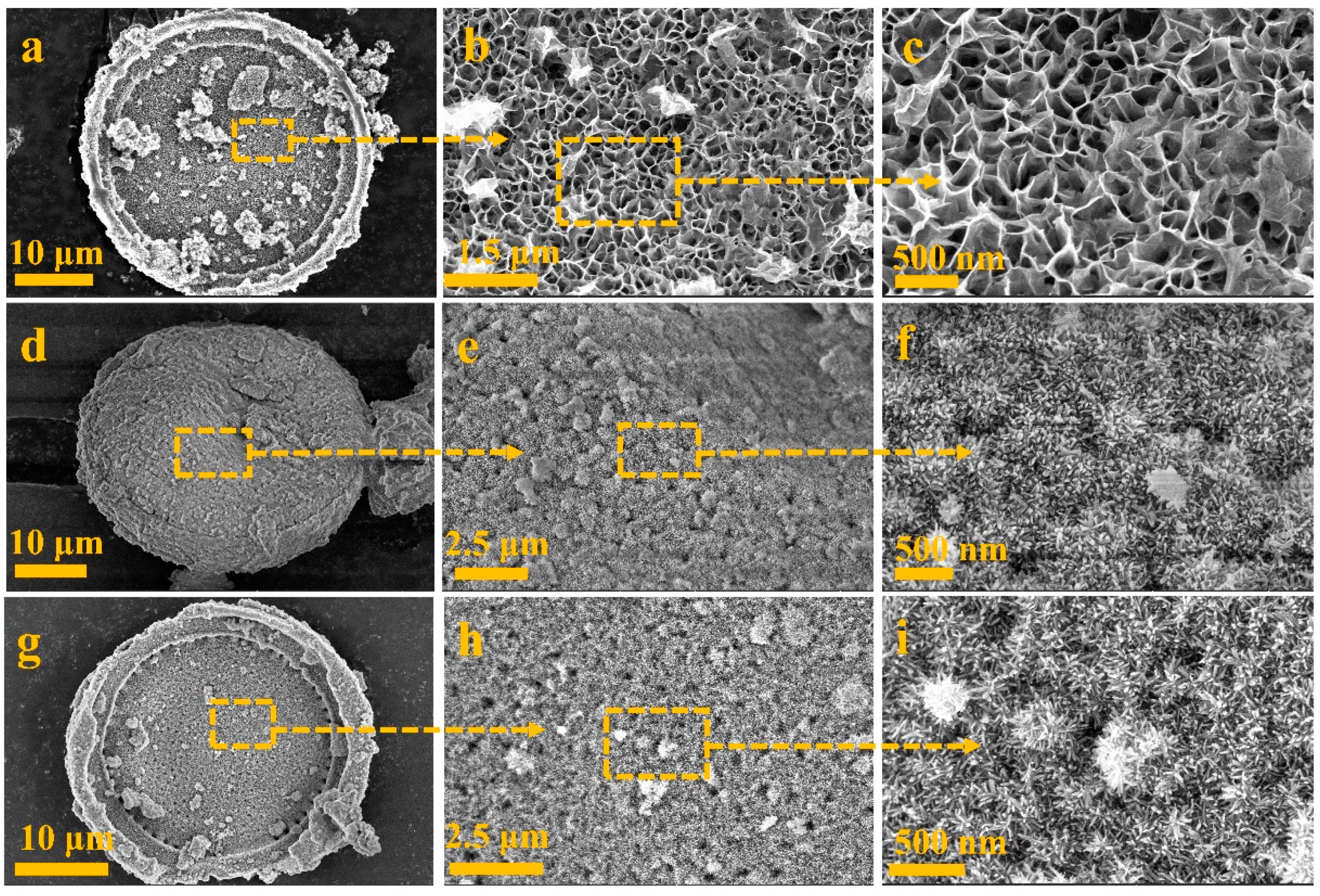
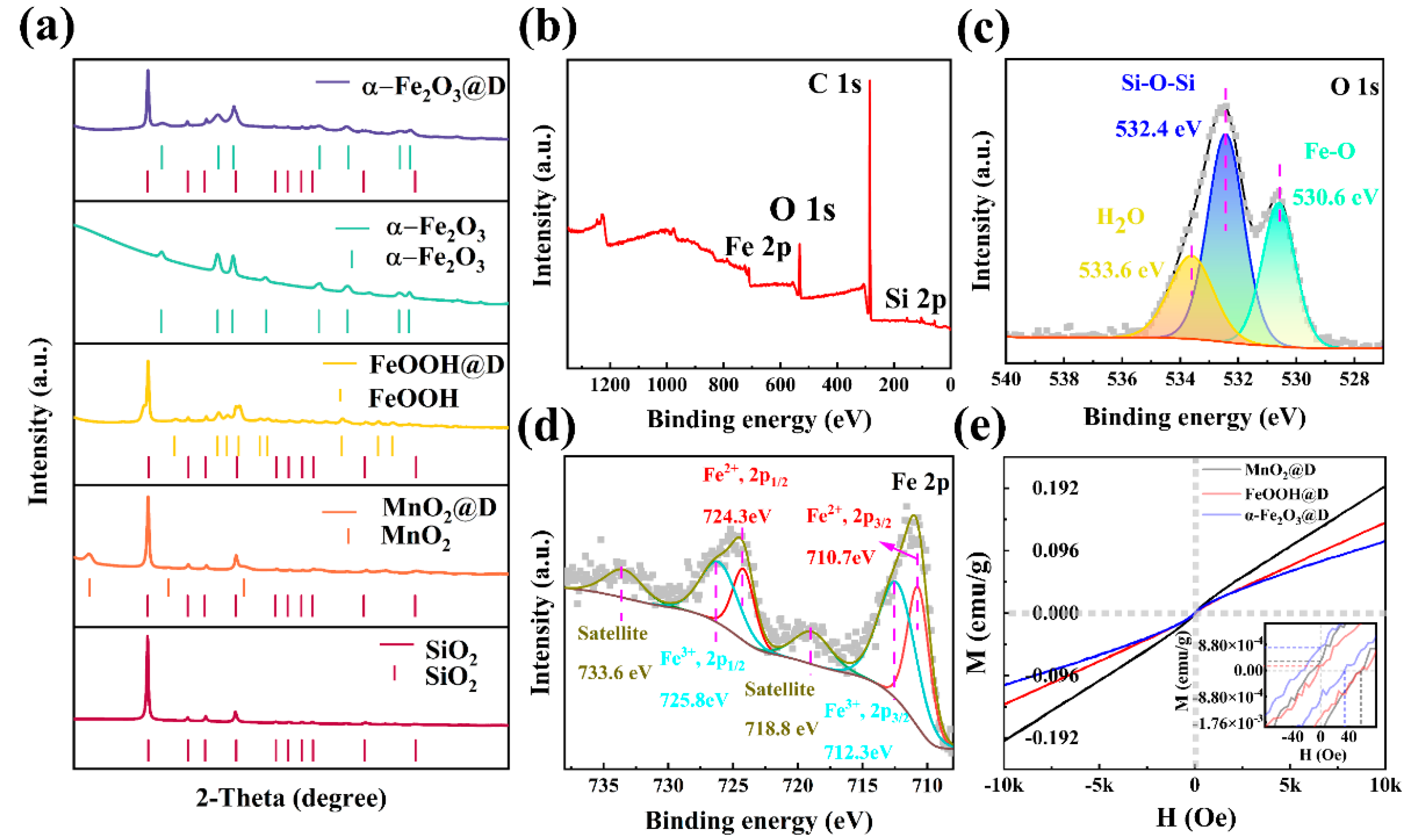
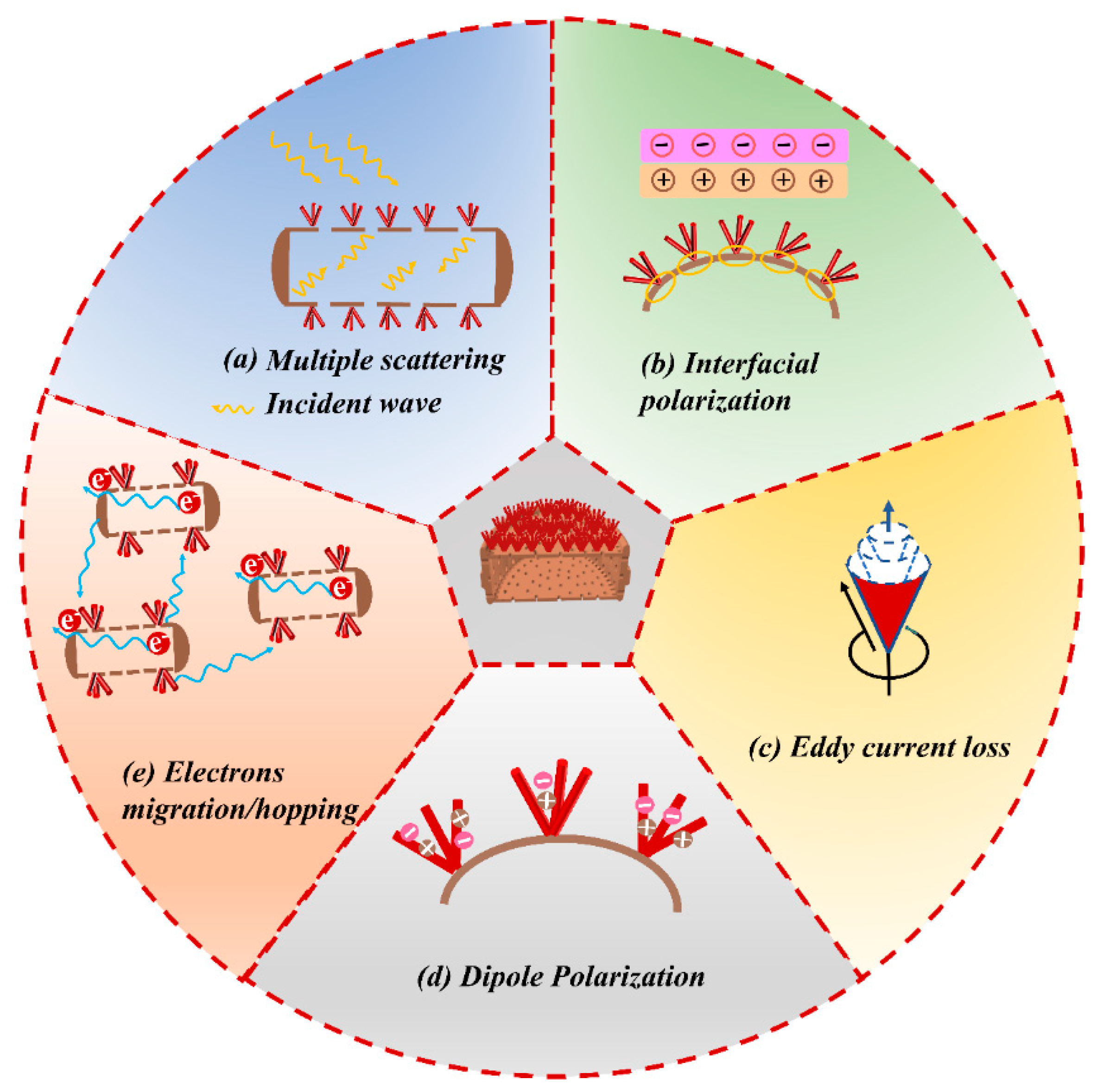
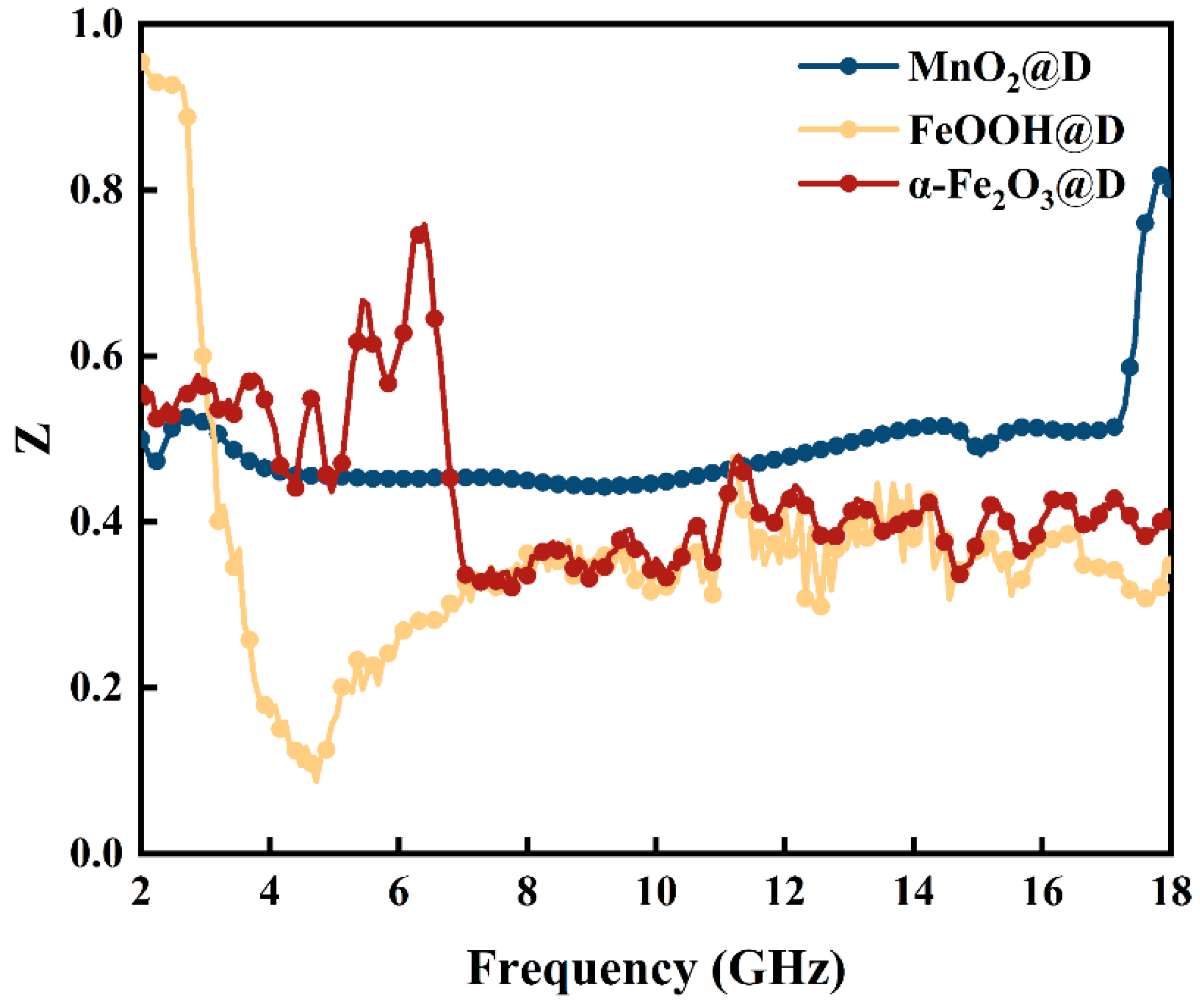
2. Results and Discussion
3. Methods and Materials
3.1. Synthesis of MnO2 Nanosheets on De
3.2. Preparation of Sea-Urchin-Like FeOOH on De
3.3. Preparation of α-Fe2O3@D
3.4. Characterization of Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girgert, R.; Grundker, C.; Emons, G.; Hanf, V. Electromagnetic fields alter the expression of estrogen receptor cofactors in breast cancer cells. Bioelectromagnetics 2008, 29, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.G.; Jiang, J.J.; Geng, D.Y.; Li, B.Q.; Han, Z.; Liu, W.; Zhang, Z.D. Dual nonlinear dielectric resonance and strong natural resonance in Ni/ZnO nanocapsules. Appl. Phys. Lett. 2009, 94, 053119. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Zhao, L.; Hu, Z.; Gu, Y. Research progress on nanostructured radar absorbing materials. Energy Sci. Eng. 2011, 3, 580–584. [Google Scholar] [CrossRef]
- Qin, F.X.; Peng, H.X. Ferromagnetic microwires enabled multifunctional composite materials. Prog. Mater. Sci. 2013, 58, 183–259. [Google Scholar] [CrossRef]
- Yang, P.-A.; Huang, Y.; Li, R.; Huang, X.; Ruan, H.; Shou, M.; Li, W.; Zhang, Y.; Li, N.; Dong, L. Optimization of Fe@Ag core–shell nanowires with improved impedance matching and microwave absorption properties. Chem. Eng. J. 2022, 430, 132878. [Google Scholar] [CrossRef]
- Wang, D.-S.; Mukhtar, A.; Wu, K.-M.; Gu, L.; Cao, X. Multi-segmented nanowires: A high tech bright future. Materials 2019, 12, 3908. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Guner, S.; Slimani, Y.; Hassan, M.; Baykal, A.; Gondal, M.A.; Baig, U.; Trukhanov, S.V.; Trukhanov, A.V. Structural and Magnetic Properties of Co0.5Ni0.5Ga0.01Gd0.01Fe1.98O4/ZnFe2O4 Spinel Ferrite Nanocomposites: Comparative Study between Sol-Gel and Pulsed Laser Ablation in Liquid Approaches. Nanomaterials 2021, 11, 2461. [Google Scholar] [CrossRef]
- Yakovenko, O.S.; Matzui, L.Y.; Vovchenko, L.L.; Oliynyk, V.V.; Zagorodnii, V.V.; Trukhanov, S.V.; Trukhanov, A.V. Electromagnetic Properties of Carbon Nanotube/BaFe12-xGaxO19/Epoxy Composites with Random and Oriented Filler Distributions. Nanomaterials 2021, 11, 2873. [Google Scholar] [CrossRef] [PubMed]
- Trukhanov, A.; Turchenko, V.; Bobrikov, I.; Trukhanov, S.; Kazakevich, I.; Balagurov, A. Crystal structure and magnetic properties of the BaFe12−xAlxO19 (x = 0.1–1.2) solid solutions. J. Magn. Magn. Mater. 2015, 393, 253–259. [Google Scholar] [CrossRef]
- Karpinsky, D.V.; Silibin, M.V.; Trukhanov, S.V.; Trukhanov, A.V.; Zhaludkevich, A.L.; Latushka, S.I.; Zhaludkevich, D.V.; Khomchenko, V.A.; Alikin, D.O.; Abramov, A.S. Peculiarities of the crystal structure evolution of BiFeO3–BaTiO3 ceramics across structural phase transitions. Electromagn. Prop. 2020, 10, 801. [Google Scholar] [CrossRef]
- Vinnik, D.; Zhivulin, V.; Sherstyuk, D.; Starikov, A.Y.; Zezyulina, P.; Gudkova, S.; Zherebtsov, D.; Rozanov, K.; Trukhanov, S.; Astapovich, K. Electromagnetic properties of zinc–nickel ferrites in the frequency range of 0.05–10 GHz. Mater. Today Chem. 2021, 20, 100460. [Google Scholar] [CrossRef]
- Lee, E.-T.; Jang, G.-E.; Kim, C.K.; Yoon, D.-H. Fabrication and gas sensing properties of α-Fe2O3 thin film prepared by plasma enhanced chemical vapor deposition (PECVD). Sens. Actuators B Chem. 2001, 77, 221–227. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.H.; Lo, I.M.C. Selective removal of heavy metals from industrial wastewater using maghemite nanoparticle: Performance and mechanisms. J. Environ. Eng. 2006, 132, 709–715. [Google Scholar] [CrossRef]
- Liao, L.; Zheng, Z.; Yan, B.; Zhang, J.; Gong, H.; Li, J.; Liu, C.; Shen, Z.; Yu, T. Morphology controllable synthesis of α-Fe2O3 1D nanostructures: Growth mechanism and nanodevice based on single nanowire. J. Phys. Chem. C 2008, 112, 10784–10788. [Google Scholar] [CrossRef]
- Javid, M.; Zhou, Y.L.; Wang, D.X.; Li, D.; Shi, G.M.; Kim, U.; Zhou, L.; Dong, X.L.; Zhang, Z.D. Magnetic Behavior, Electromagnetic Multiresonances, and Microwave Absorption of the Interfacial Engineered Fe@FeSi/SiO2 Nanocomposite. ACS Appl. Nano Mater. 2018, 1, 1309–1320. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Wang, Y.; Huang, Y.; Wang, Y.; Luo, J. Core–shell CoNi@graphitic carbon decorated on B, N-codoped hollow carbon polyhedrons toward lightweight and high-efficiency microwave attenuation. ACS Appl. Mater. Interfaces 2019, 11, 25624–25635. [Google Scholar] [CrossRef]
- Grossman, J.H.; McNeil, S.E. Nanotechnology in cancer medicine. Phys. Today 2012, 65, 38. [Google Scholar] [CrossRef]
- Ansari, S.A.M.K.; Ficiarà, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic iron oxide nanoparticles: Synthesis, characterization and functionalization for biomedical applications in the central nervous system. Materials 2019, 12, 465. [Google Scholar] [CrossRef]
- Osipov, V.; Platonov, V.; Uimin, M.; Podkin, A. Laser synthesis of magnetic iron oxide nanopowders. Tech. Phys. 2012, 57, 543–549. [Google Scholar] [CrossRef]
- Safronov, A.; Beketov, I.; Komogortsev, S.; Kurlyandskaya, G.; Medvedev, A.; Leiman, D.; Larrañaga, A.; Bhagat, S. Spherical magnetic nanoparticles fabricated by laser target evaporation. AIP Adv. 2013, 3, 052135. [Google Scholar] [CrossRef]
- Che, R.C.; Peng, L.M.; Duan, X.F.; Chen, Q.; Liang, X.L. Microwave absorption enhancement and complex permittivity and permeability of Fe encapsulated within carbon nanotubes. Adv. Mater. 2004, 16, 401–405. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xiao, G.; Wang, T.S.; Ouyang, Q.Y.; Qi, L.H.; Ma, Y.; Gao, P.; Zhu, C.L.; Cao, M.S.; Jin, H.B. Porous Fe3O4/Carbon Core/Shell Nanorods: Synthesis and Electromagnetic Properties. J. Phys. Chem. C 2011, 115, 13603–13608. [Google Scholar] [CrossRef]
- Cao, J.; Fu, W.; Yang, H.; Yu, Q.; Zhang, Y.; Liu, S.; Sun, P.; Zhou, X.; Leng, Y.; Wang, S.; et al. Large-scale synthesis and microwave absorption enhancement of actinomorphic tubular ZnO/CoFe2O4 nanocomposites. J. Phys. Chem. B 2009, 113, 4642–4647. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Pan, S.; Zhang, J. Facile Preparation and Electromagnetic Properties of Core− Shell Composite Spheres Composed of Aloe-like Nickel Flowers Assembled on Hollow Glass Spheres. J. Phys. Chem. C 2009, 113, 2715–2721. [Google Scholar] [CrossRef]
- Ohlan, A.; Singh, K.; Chandra, A.; Dhawan, S.K. Microwave absorption behavior of core-shell structured poly (3,4-ethylenedioxy thiophene)-barium ferrite nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 927–933. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhang, F.; Zhao, G.G.; Fang, X.Y.; Jin, H.B.; Gao, P.; Zhu, C.L.; Cao, M.S.; Xiao, G. Synthesis, Multi-Nonlinear Dielectric Resonance, and Excellent Electromagnetic Absorption Characteristics of Fe3O4/ZnO Core/Shell Nanorods. J. Phys. Chem. C 2010, 114, 9239–9244. [Google Scholar] [CrossRef]
- Wang, D.; Mukhtar, A.; Humayun, M.; Wu, K.; Du, Z.; Wang, S.; Zhang, Y. A Critical Review on Nanowire-Motors: Design, Mechanism and Applications. Chem. Rec. 2022, 22, e202200016. [Google Scholar] [CrossRef]
- Yun, X.J.; Wu, Q.F.; Feng, L.; Shen, J.C.; Chen, J.; Chu, P.K.; Liu, L.Z.; Wu, X.L. Microwave absorption enhancement of e-Fe3O4@C microspheres by core surface modification. J. Alloys Compd. 2020, 835, 155307. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, X.; Wang, X.; Wu, X.; Zhang, Z.; Shen, J. Hydrothermal self-assembled Fe3O4/CA core-shell composites for broadband microwave absorption. J. Magn. Magn. Mater. 2022, 541, 168511. [Google Scholar] [CrossRef]
- Liu, S.D.; Meng, X.W.; Wang, Z.Z.; Li, Z.H.; Yang, K. Enhancing microwave absorption by constructing core/shell TiN@TiO2 heterostructures through post-oxidation annealing. Mater. Lett. 2019, 257, 126677. [Google Scholar] [CrossRef]
- Fu, W.Y.; Liu, S.K.; Fan, W.H.; Yang, H.B.; Pang, X.F.; Xu, J.; Zou, G.T. Hollow glass microspheres coated with CoFe2O4 and its microwave absorption property. J. Magn. Magn. Mater. 2007, 316, 54–58. [Google Scholar] [CrossRef]
- Mu, G.H.; Pan, X.F.; Shen, H.G.; Gu, M.Y. Preparation and magnetic properties of composite powders of hollow microspheres coated with barium ferrite. Mater. Sci. Eng. A 2007, 445, 563–566. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Zheng, T.; Jiang, D.; Zhou, Z.; Liu, C.; Zhang, X.; Zhang, Y.; Losic, D. Tuning MnO2 to FeOOH replicas with bio-template 3D morphology as electrodes for high performance asymmetric supercapacitors. Chem. Eng. J. 2019, 370, 136–147. [Google Scholar] [CrossRef]
- Li, K.; Feng, S.; Jing, C.; Chen, Y.; Liu, X.; Zhang, Y.; Zhou, L. Assembling a double shell on a diatomite skeleton ternary complex with conductive polypyrrole for the enhancement of supercapacitors. ChemComm 2019, 55, 13773–13776. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, K.; Le, Q.; Zhu, S.; Guo, X.; Jiang, D.; Zhang, Y. Tuning parallel manganese dioxide to hollow parallel hydroxyl oxidize iron replicas for high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 2021, 594, 812–823. [Google Scholar] [CrossRef]
- Li, K.; Hu, Z.; Zhao, R.; Zhou, J.; Jing, C.; Sun, Q.; Rao, J.; Yao, K.; Dong, B.; Liu, X. A multidimensional rational design of nickel–iron sulfide and carbon nanotubes on diatomite via synergistic modulation strategy for supercapacitors. J. Colloid Interface Sci. 2021, 603, 799–809. [Google Scholar] [CrossRef]
- Li, K.; Teng, H.; Dai, X.; Wang, Y.; Wang, D.; Zhang, X.; Yao, Y.; Liu, X.; Feng, L.; Rao, J. Atomic scale modulation strategies and crystal phase transition of flower-like CoAl layered double hydroxides for supercapacitors. CrystEngComm 2022, 24, 2081–2088. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, R.; Wang, D.; Li, K.; Sun, Q.; Xiao, Y.; Teng, H.; Huang, X.; Sun, T.; Liu, Z.; et al. Lightweight, Low-Cost Co2SiO4@diatomite Core-Shell Composite Material for High-Efficiency Microwave Absorption. Molecules 2022, 27, 1055. [Google Scholar] [CrossRef]
- Lv, H.; Liang, X.; Cheng, Y.; Zhang, H.; Tang, D.; Zhang, B.; Ji, G.; Du, Y. Coin-like α-Fe2O3@ CoFe2O4 core–shell composites with excellent electromagnetic absorption performance. ACS Appl. Mater. Interfaces 2015, 7, 4744–4750. [Google Scholar] [CrossRef]
- Guo, W.; Wang, S.; Ren, Q.; Jin, Z.; Ding, Y.; Xiong, C.; Li, J.; Chen, J.; Zhu, Y.; Oh, W.-C. Microwave absorption and photocatalytic activity of MgxZn1−x ferrite/diatomite composites. J. Korean Ceram. Soc. 2022, 59, 252–262. [Google Scholar] [CrossRef]
- Wu, Z.C.; Pei, K.; Xing, L.S.; Yu, X.F.; You, W.B.; Che, R.C. Enhanced Microwave Absorption Performance from Magnetic Coupling of Magnetic Nanoparticles Suspended within Hierarchically Tubular Composite. Adv. Funct. Mater. 2019, 29, 1901448. [Google Scholar] [CrossRef]
- Kuang, P.Y.; Zhang, L.Y.; Cheng, B.; Yu, J.G. Enhanced charge transfer kinetics of Fe2O3/CdS composite nanorod arrays using cobalt-phosphate as cocatalyst. Appl. Catal. B 2017, 218, 570–580. [Google Scholar] [CrossRef]
- Rui, Q.; Wang, L.; Zhang, Y.; Feng, C.; Zhang, B.; Fu, S.; Guo, H.; Hu, H.; Bi, Y. Synergistic effects of P-doping and a MnO2 cocatalyst on Fe2O3 nanorod photoanodes for efficient solar water splitting. J. Mater. Chem. A 2018, 6, 7021–7026. [Google Scholar] [CrossRef]
- Bu, X.B.; Gao, Y.X.; Zhang, S.H.; Tian, Y. Amorphous cerium phosphate on P-doped Fe2O3 nanosheets for efficient photoelectrochemical water oxidation. Chem. Eng. J. 2019, 355, 910–919. [Google Scholar] [CrossRef]
- Zheng, P.L.; Zhang, Y.; Dai, Z.F.; Zheng, Y.; Dinh, K.N.; Yang, J.; Dangol, R.; Liu, X.B.; Yan, Q.Y. Constructing Multifunctional Heterostructure of Fe2O3@Ni3Se4 Nanotubes. Small 2018, 14, 1704065. [Google Scholar] [CrossRef]
- Bayazit, M.K.; Cao, E.; Gavriilidis, A.; Tang, J. A microwave promoted continuous flow approach to self-assembled hierarchical hematite superstructures. Green Chem. 2016, 18, 3057–3065. [Google Scholar] [CrossRef]
- Zhou, W.M.; Zhang, J.; Liu, Y.B.; Li, X.L.; Niu, X.M.; Song, Z.T.; Min, G.Q.; Wan, Y.Z.; Shi, L.Y.; Feng, S.L. Characterization of anti-adhesive self-assembled monolayer for nanoimprint lithography. Appl. Surf. Sci. 2008, 255, 2885–2889. [Google Scholar] [CrossRef]
- Huang, B.; Yue, J.L.; Wei, Y.S.; Huang, X.Z.; Tang, X.Z.; Du, Z.J. Enhanced microwave absorption properties of carbon nanofibers functionalized by FeCo coatings. Appl. Surf. Sci. 2019, 483, 98–105. [Google Scholar] [CrossRef]
- Cao, M.S.; Wang, X.X.; Cao, W.Q.; Fang, X.Y.; Wen, B.; Yuan, J. Thermally Driven Transport and Relaxation Switching Self-Powered Electromagnetic Energy Conversion. Small 2018, 14, 1800987. [Google Scholar] [CrossRef]
- Ahmad, S.H.; Abdullah, M.H.; Hui, D.; Yusoff, A.N.; Puryanti, D. Magnetic and microwave absorbing properties of magnetite–thermoplastic natural rubber nanocomposites. J. Magn. Magn. Mater. 2010, 322, 3401–3409. [Google Scholar]
- Kurlyandskaya, G.; Safronov, A.; Bhagat, S.; Lofland, S.; Beketov, I.; Prieto, L.M. Tailoring functional properties of Ni nanoparticles-acrylic copolymer composites with different concentrations of magnetic filler. J. Appl. Phys. 2015, 117, 123917. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bai, X.; Wang, M. Facile preparation, characterization and highly effective microwave absorption performance of porous α-Fe2O3 nanorod–graphene composites. J. Mater. Sci. Mater. Electron. 2018, 29, 3381–3390. [Google Scholar] [CrossRef]
- Mensah, E.E.; Abbas, Z. Experimental and Computational Study of the Microwave Absorption Properties of Recycled α-Fe2O3/OPEFB Fiber/PCL Multi-Layered Composites. J. Mater. Sci. Chem. Eng. 2022, 10, 30–41. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Y.; Wang, M. Synthesis of graphene/thorns-like polyaniline/alpha-Fe2O3@SiO2 nanocomposites for lightweight and highly efficient electromagnetic wave absorber. J. Colloid Interface Sci. 2018, 530, 212–222. [Google Scholar] [CrossRef]


| Sample Name | RL(min) | Effective Absorption Bandwidth (RL < −20 dB) |
|---|---|---|
| MnO2@D | −7.9 dB | 0 |
| FeOOH@D | −17.8 dB | 0 |
| α-Fe2O3@D | −54.2 dB | 8.24 GHz |
| Sample Name | Percentage (wt.%) | RLmin (dB) | Absorberthickness (mm) | EAB(RL < −10 dB) (GHz) | Reference |
|---|---|---|---|---|---|
| Fe2O4/α-Fe2O3 | 20 | −52.69 | 3 | 5.36 | [52] |
| α-Fe2O3-graphene | 15 | −30.6 | 4 | 5.5 | [53] |
| α-Fe2O3/OPEFB fiber/PCL | 25 | −38 | 6 | 3 | [54] |
| RGO/PANI/α-Fe2O3@SiO2 with 1:4 | 16.7 | −31.06 | 5 | 2 | [55] |
| RGO/PANI/α-Fe2O3@SiO2 with 1:6 | 16.7 | −25.88 | 4 | 3 | [55] |
| α-Fe2O3@D | 20 | −54.2 | 3 | 8.24 | this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, D.; Dong, L.; Li, K.; Zhang, Y.; Yang, P.; Yi, S.; Dai, X.; Yin, C.; Du, Z.; et al. Microwave Absorption of α-Fe2O3@diatomite Composites. Int. J. Mol. Sci. 2022, 23, 9362. https://doi.org/10.3390/ijms23169362
Zhang C, Wang D, Dong L, Li K, Zhang Y, Yang P, Yi S, Dai X, Yin C, Du Z, et al. Microwave Absorption of α-Fe2O3@diatomite Composites. International Journal of Molecular Sciences. 2022; 23(16):9362. https://doi.org/10.3390/ijms23169362
Chicago/Turabian StyleZhang, Chenzhi, Dashuang Wang, Lichao Dong, Kailin Li, Yifan Zhang, Pingan Yang, Shuang Yi, Xingjian Dai, Changqing Yin, Zhilan Du, and et al. 2022. "Microwave Absorption of α-Fe2O3@diatomite Composites" International Journal of Molecular Sciences 23, no. 16: 9362. https://doi.org/10.3390/ijms23169362
APA StyleZhang, C., Wang, D., Dong, L., Li, K., Zhang, Y., Yang, P., Yi, S., Dai, X., Yin, C., Du, Z., Zhang, X., Zhou, Q., Yi, Z., Rao, J., & Zhang, Y. (2022). Microwave Absorption of α-Fe2O3@diatomite Composites. International Journal of Molecular Sciences, 23(16), 9362. https://doi.org/10.3390/ijms23169362







