Angioimmunoblastic T-Cell Lymphoma with Exuberant CD30-Positive Follicular Dendritic Cell Proliferation in a SARS-CoV-2 Patient: The Role of Mutational Analysis to Exclude an Associated Follicular Dendritic Cell Sarcoma
Abstract
:1. Introduction
2. Results
2.1. Clinical Data
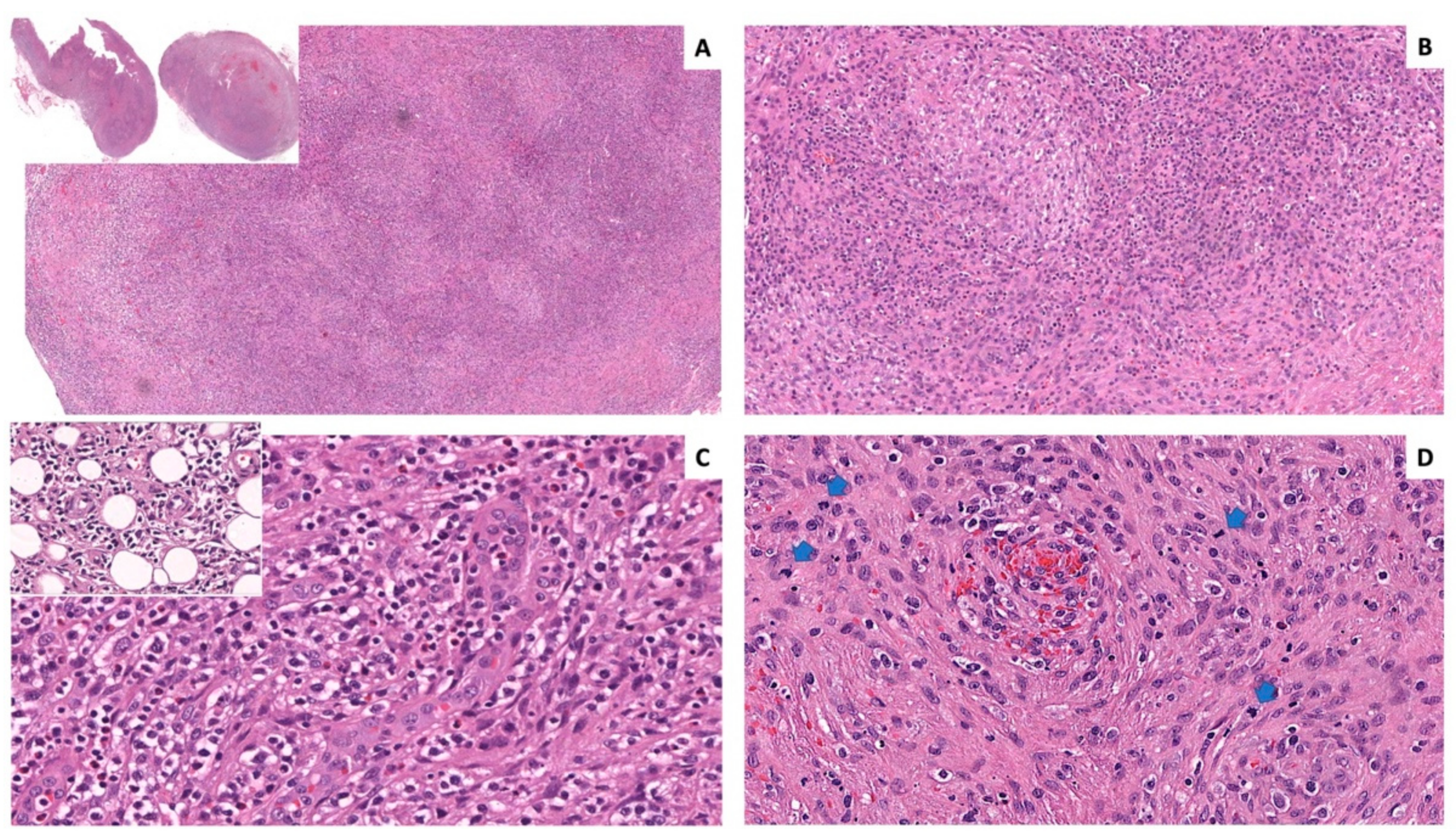
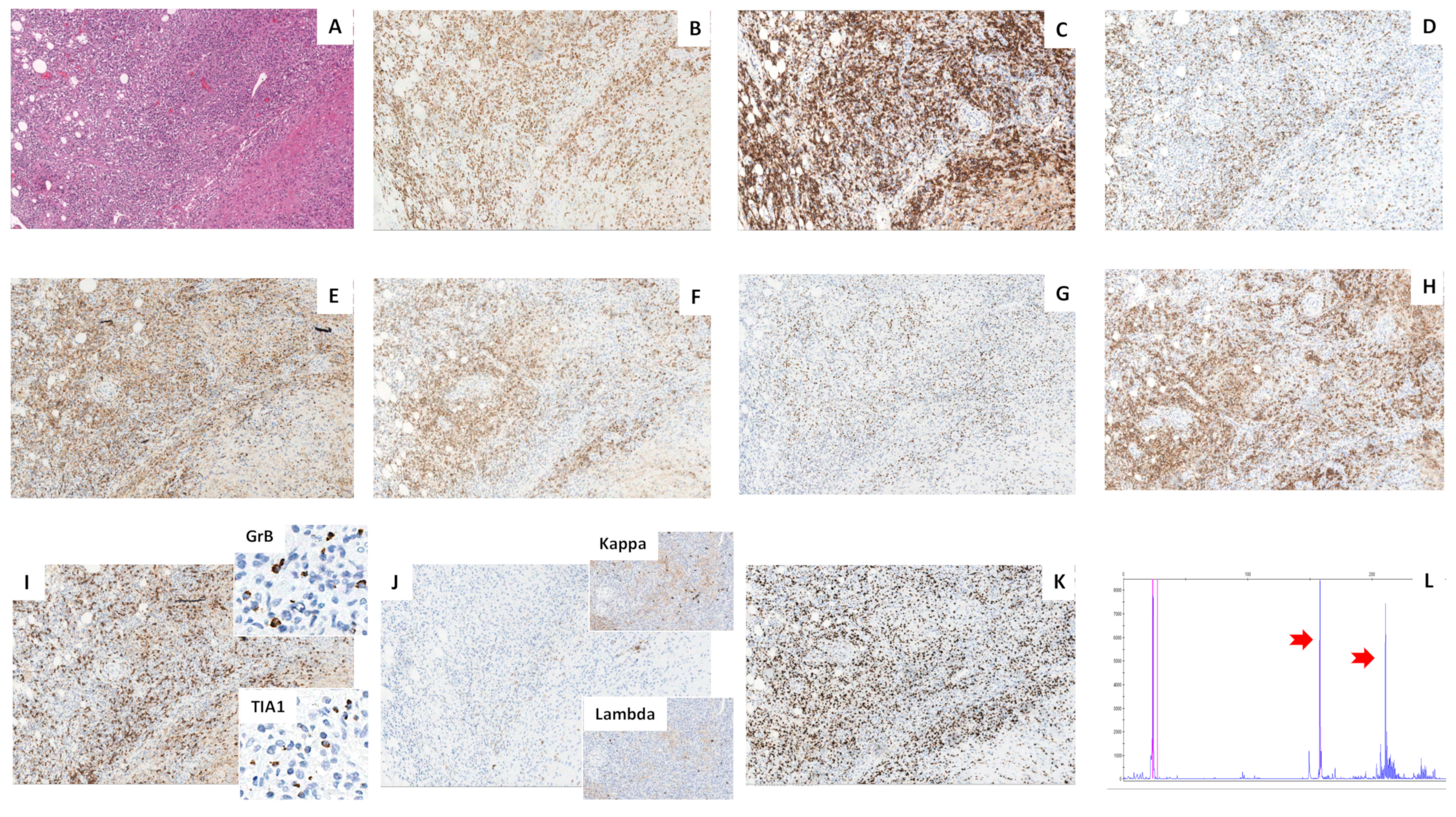
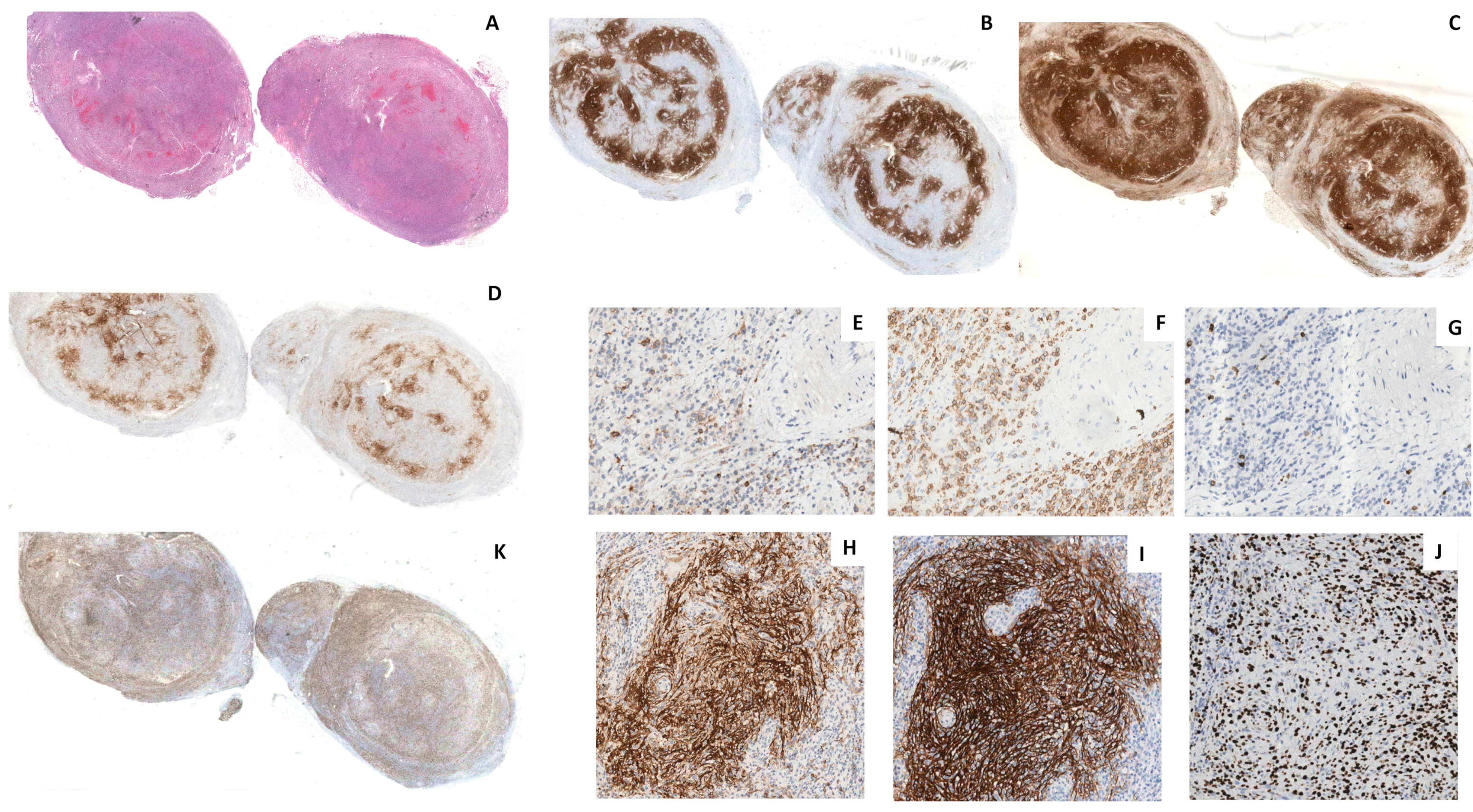
2.2. Histology
2.3. Molecular Analyses
2.4. Treatment and Outcome
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. Angioimmunoblastic T-cell lymphoma. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2017; pp. 408–410. [Google Scholar]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. Follicular dendritic cell sarcoma. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2017; pp. 476–477. [Google Scholar]
- Chan, J.K.C.; Tsang, W.Y.W.; Ng, C.S. Follicular dendritic cell tumor and vascular neoplasm complicating hya-line-vascular Castleman’s disease. Am. J. Surg. Pathol. 1994, 18, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.L.; Arber, D.A.; Pittaluga, S.; Martinez, A.; Burke, J.S.; Raffeld, M.; Camos, M.; Warnke, R.; Jaffe, E.S. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: Evidence for transdifferentiation of the follicular lymphoma clone. Blood 2008, 111, 5433–5439. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Xi, L.; Raffeld, M.; Feldman, A.L.; Ketterling, R.P.; Knudson, R.; Rodriguez-Canales, J.; Hanson, J.; Pittaluga, S.; Jaffe, E.S. Clonally related histiocytic/dendritic cell sarcoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: A study of seven cases. Mod. Pathol. 2011, 24, 1421–1432. [Google Scholar] [CrossRef]
- Starkey, C.R.; Corn, A.I.; Porensky, R.S.; Viswanatha, D.; Wilson, C.S. Peripheral T-cell lymphoma with extensive dendritic cell network mimicking follicular dendritic cell tumor: A case report with pathologic, immunophenotypic, and molecular findings. Am. J. Clin. Pathol. 2006, 126, 230–234. [Google Scholar] [CrossRef]
- Benharroch, D.; Zekzer, M.; Nalbandyan, K. Angioimmunoblastic T-Cell Lymphoma: A Questionable Association with Follicular Dendritic Cell Sarcoma. Case Rep. Hematol. 2017, 2017, 9601094. [Google Scholar] [CrossRef]
- Heavican, T.B.; Bouska, A.; Yu, J.; Lone, W.; Amador, C.; Gong, Q.; Zhang, W.; Li, Y.; Dave, B.J.; Nairismägi, M.L.; et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma. Blood 2019, 133, 1664–1676. [Google Scholar] [CrossRef]
- Griffin, G.K.; Sholl, L.M.; Lindeman, N.I.; Fletcher, C.D.; Hornick, J.L. Targeted genomic sequencing of follicular dendritic cell sarcoma reveals recurrent alterations in NF-κB regulatory genes. Mod. Pathol. 2016, 29, 67–74. [Google Scholar] [CrossRef]
- Van der Weyden, C.A.; Pileri, S.A.; Feldman, A.L.; Whisstock, J.; Prince, H.M. Understanding CD30 biology and therapeutic targeting: A historical perspective providing insight into future directions. Blood Cancer J. 2017, 7, e603. [Google Scholar] [CrossRef]
- Yakushijin, Y.; Shikata, H.; Kito, K.; Ohshima, K.; Kojima, K.; Hato, T.; Hasegawa, H.; Yasukawa, M. Follicular dendritic cell tumor as an unknown primary tumor. Int. J. Clin. Oncol. 2007, 12, 56–58. [Google Scholar] [CrossRef]
- Viola, P.; Vroobel, K.M.; Devaraj, A.; Jordan, S.; Ladas, G.; Dusmet, M.; Montero, A.; Rice, A.; Wotherspoon, A.C.; Nicholson, A.G. Follicular dendritic cell tumour/sarcoma: A commonly misdiagnosed tumour in the thorax. Histopathology 2016, 69, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Wu, F.; Xu, C.P.; Chen, G.L.; Zhang, Y.; Chen, W.; Yan, X.C.; Duan, G.J. Mediastinal follicular dendritic cell sarcoma: A rare, potentially under-recognized, and often misdiagnosed disease. Diagn. Pathol. 2019, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiang, C.; Wu, M.; Hu, S. Follicular Dendritic Cell Sarcoma with Co-Expression of CD4 and CD30 Mimics Anaplastic Large Cell Lymphoma. Front. Oncol. 2020, 10, 876. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Attygalle, A.D.; Kyriakou, C.; Dupuis, J.; Grogg, K.L.; Diss, T.C.; Wotherspoon, A.C.; Chuang, S.S.; Cabeçadas, J.; Isaacson, P.G.; Du, M.Q.; et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: Clinical correlation and insights into natural history and disease progression. Am. J. Surg. Pathol. 2007, 31, 1077–1088. [Google Scholar] [CrossRef]
- Sun, X.; Chang, K.C.; Abruzzo, L.V.; Lai, R.; Younes, A.; Jones, D. Epidermal growth factor receptor expression in follicular dendritic cells: A shared feature of follicular dendritic cell sarcoma and Castleman’s disease. Hum. Pathol. 2003, 34, 835–840. [Google Scholar] [CrossRef]
- Vermi, W.; Giurisato, E.; Lonardi, S.; Balzarini, P.; Rossi, E.; Medicina, D.; Bosisio, D.; Sozzani, S.; Pellegrini, W.; Doglioni, C.; et al. Ligand-dependent activation of EGFR in follicular dendritic cells sarcoma is sustained by local production of cognate ligands. Clin. Cancer Res. 2013, 19, 5027–5038. [Google Scholar] [CrossRef]
- Jain, P.; Prieto, V.G.; Manning, J.T.; Fowler, N.; Medeiros, L.J.; Kanagal-Shamanna, R. Synchronous presentation of intra-nodal follicular dendritic cell sarcoma and Castleman disease. Am. J. Hematol. 2017, 92, 478–479. [Google Scholar] [CrossRef]
- Chan, A.C.; Chan, K.W.; Chan, J.K.; Au, W.Y.; Ho, W.K.; Ng, W.M. Development of follicular dendritic cell sarcoma in hyaline-vascular Castleman’s disease of the nasopharynx: Tracing its evolution by sequential biopsies. Histopathology 2001, 38, 510–518. [Google Scholar] [CrossRef]
- Buscarlet, M.; Provost, S.; Zada, Y.F.; Barhdadi, A.; Bourgoin, V.; Lépine, G.; Mollica, L.; Szuber, N.; Dubé, M.P.; Busque, L. DNMT3A and TET2dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood 2017, 130, 753–762. [Google Scholar] [CrossRef]
- Horspool, A.M.; Kieffer, T.; Russ, B.P.; DeJong, M.A.; Wolf, M.A.; Karakiozis, J.M.; Hickey, B.J.; Fagone, P.; Tacker, D.H.; Bevere, J.R.; et al. Interplay of Antibody and Cytokine Production Reveals CXCL13 as a Potential Novel Biomarker of Lethal SARS-CoV-2 Infection. mSphere 2021, 6, e01324-20. [Google Scholar] [CrossRef] [PubMed]
- Ansel, K.M.; Ngo, V.N.; Hyman, P.L.; Luther, S.A.; Förster, R.; Sedgwick, J.D.; Browning, J.L.; Lipp, M.; Cyster, J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 2000, 406, 309–314. [Google Scholar] [CrossRef] [PubMed]
| Gene | Reference | Chr | cDNA | Consequence | AAChange | COSMIC/dbSNP * | TIER ** | VAF *** |
|---|---|---|---|---|---|---|---|---|
| RHOA | NM_001664.2 | 3 | c.50G > T | Missense | p.G17V | COSV69041529/NA | I | 14% |
| DNMT3A | NM_022552.4 | 2 | c.1015-2A > G | Splice site | p.? | rs920946076 | II | 27.9% |
| TET2 | NM_001127208.2 | 4 | c.5471delG | Frameshift | p.G1824Vfs*9 | NA | II | 30.1% |
| TET2 | NM_001127208.2 | 4 | c.1859dupA | Insertion—Frameshift | p.Y620* | COSV54426384 | II | 24.4% |
| IDH2 | NM_002168.2 | 15 | c.516G > C | Missense | p.R172S | COSV57468772/rs1057519736 | II | 16.3% |
| NSD1 | NM_022455.4 | 5 | c.4210C > T | Missense | p.R1404C | NA | III | 20.3% |
| PIK3C2G | NM_004570.4 | 12 | c.2171C > G | Missense | p.A724G | COSV5683687/rs189828472 | III | 50.6% |
| DICER1 | NM_177438.2 | 14 | c.3631G > A | Missense | p.V1211M | rs764470378 | III | 47.7% |
| CDK12 | NM_016507.2 | 17 | c.4151G > T | Missense | p.G1384V | NA | III | 48.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogges, E.; Pelliccia, S.; Lopez, G.; Barresi, S.; Tafuri, A.; Alaggio, R.; Di Napoli, A. Angioimmunoblastic T-Cell Lymphoma with Exuberant CD30-Positive Follicular Dendritic Cell Proliferation in a SARS-CoV-2 Patient: The Role of Mutational Analysis to Exclude an Associated Follicular Dendritic Cell Sarcoma. Int. J. Mol. Sci. 2022, 23, 9349. https://doi.org/10.3390/ijms23169349
Rogges E, Pelliccia S, Lopez G, Barresi S, Tafuri A, Alaggio R, Di Napoli A. Angioimmunoblastic T-Cell Lymphoma with Exuberant CD30-Positive Follicular Dendritic Cell Proliferation in a SARS-CoV-2 Patient: The Role of Mutational Analysis to Exclude an Associated Follicular Dendritic Cell Sarcoma. International Journal of Molecular Sciences. 2022; 23(16):9349. https://doi.org/10.3390/ijms23169349
Chicago/Turabian StyleRogges, Evelina, Sabrina Pelliccia, Gianluca Lopez, Sabina Barresi, Agostino Tafuri, Rita Alaggio, and Arianna Di Napoli. 2022. "Angioimmunoblastic T-Cell Lymphoma with Exuberant CD30-Positive Follicular Dendritic Cell Proliferation in a SARS-CoV-2 Patient: The Role of Mutational Analysis to Exclude an Associated Follicular Dendritic Cell Sarcoma" International Journal of Molecular Sciences 23, no. 16: 9349. https://doi.org/10.3390/ijms23169349
APA StyleRogges, E., Pelliccia, S., Lopez, G., Barresi, S., Tafuri, A., Alaggio, R., & Di Napoli, A. (2022). Angioimmunoblastic T-Cell Lymphoma with Exuberant CD30-Positive Follicular Dendritic Cell Proliferation in a SARS-CoV-2 Patient: The Role of Mutational Analysis to Exclude an Associated Follicular Dendritic Cell Sarcoma. International Journal of Molecular Sciences, 23(16), 9349. https://doi.org/10.3390/ijms23169349







