The Chaperone System in Salivary Glands: Hsp90 Prospects for Differential Diagnosis and Treatment of Malignant Tumors
Abstract
1. Introduction
2. Results
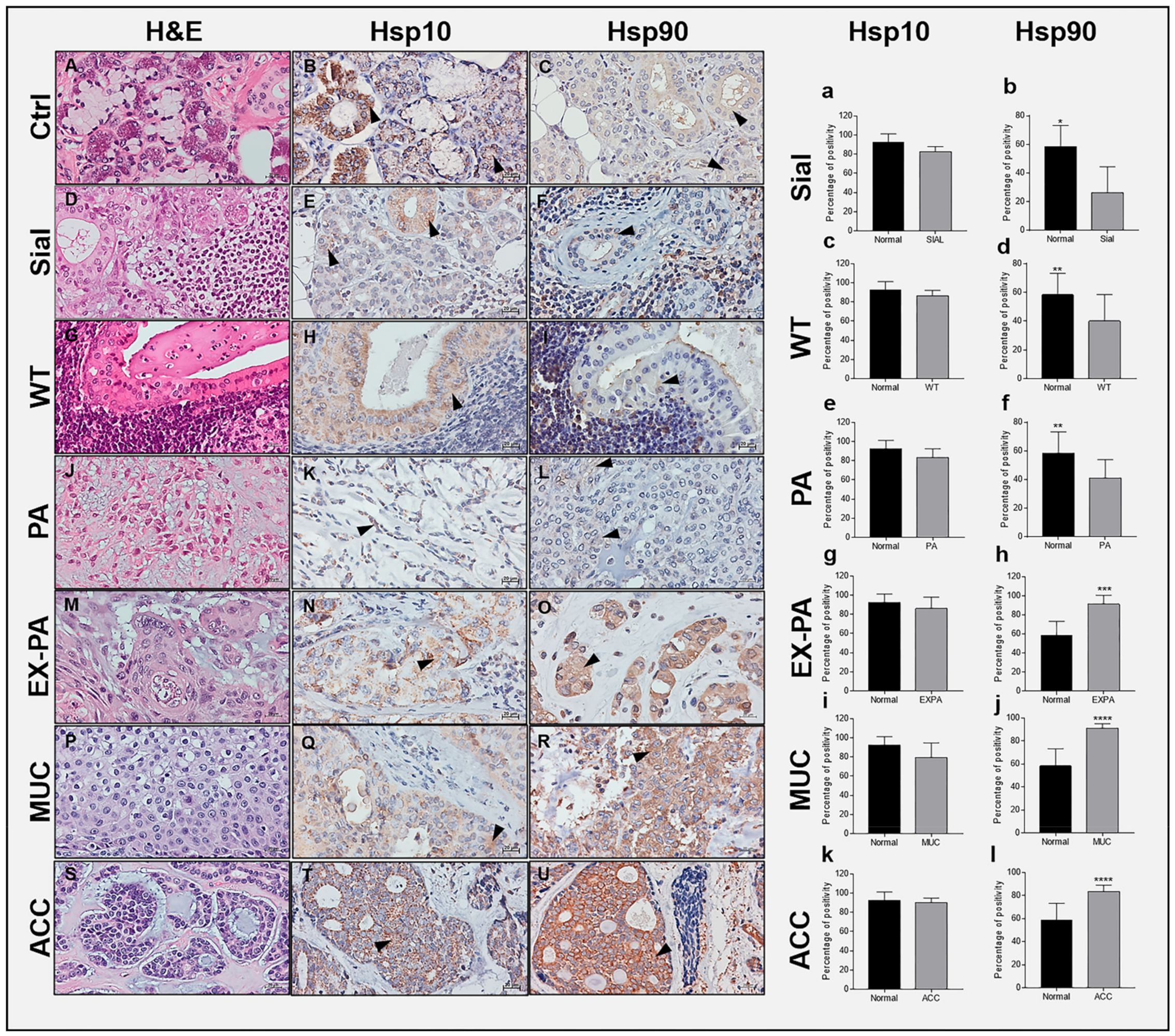
2.1. Immunohistochemical Assessment of Hsp10 and Hsp90
2.2. Hsp10 Levels and Topography in the Tissue of Normal and Pathological Salivary Glands
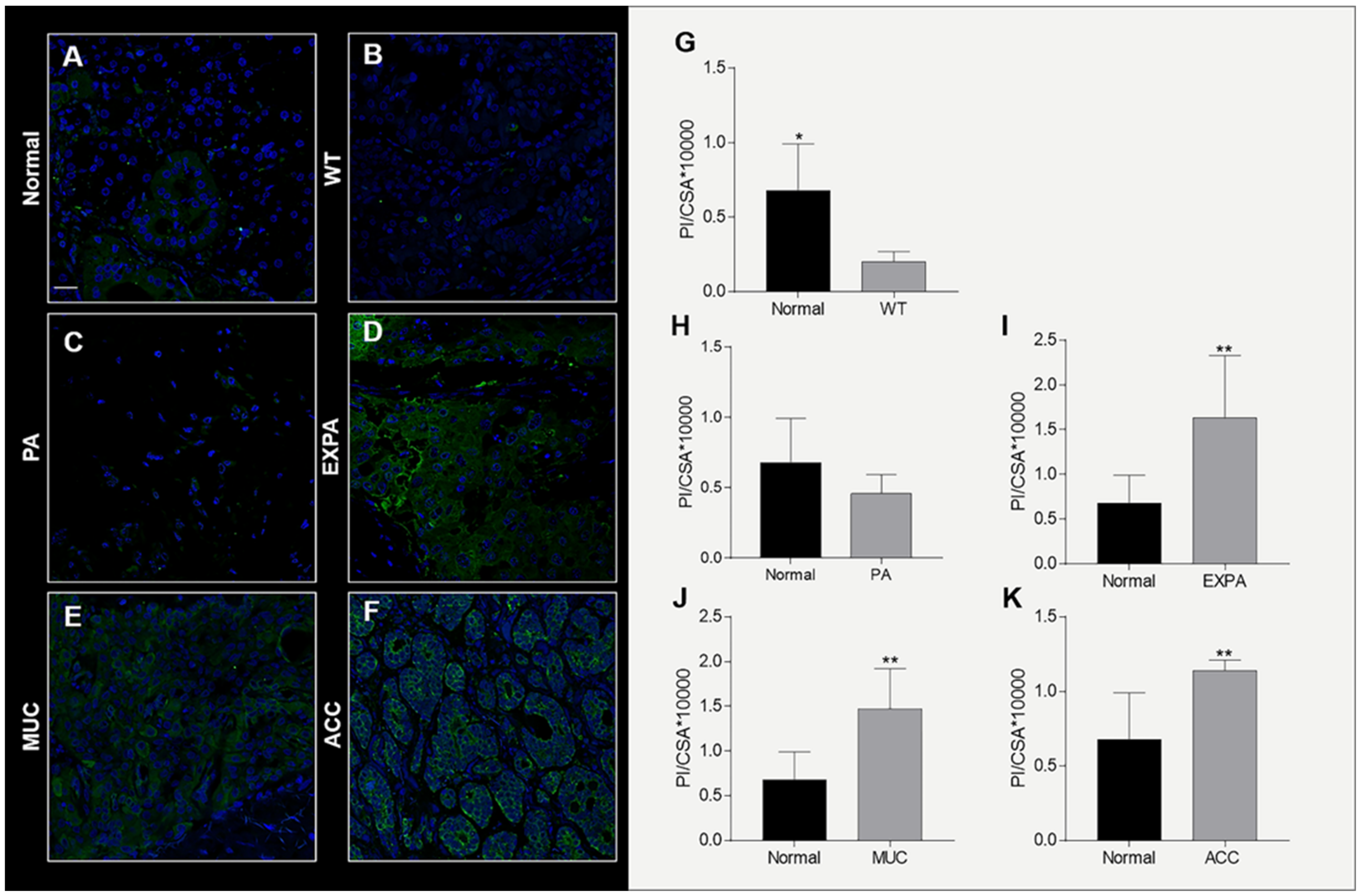
2.3. Hsp90 Tissue Levels Decrease in Sialadenitis
2.4. Hsp90 Tissue Levels Decrease in Benign Tumors
2.5. Hsp90 Tissue Levels Increase in Malignant Tumors
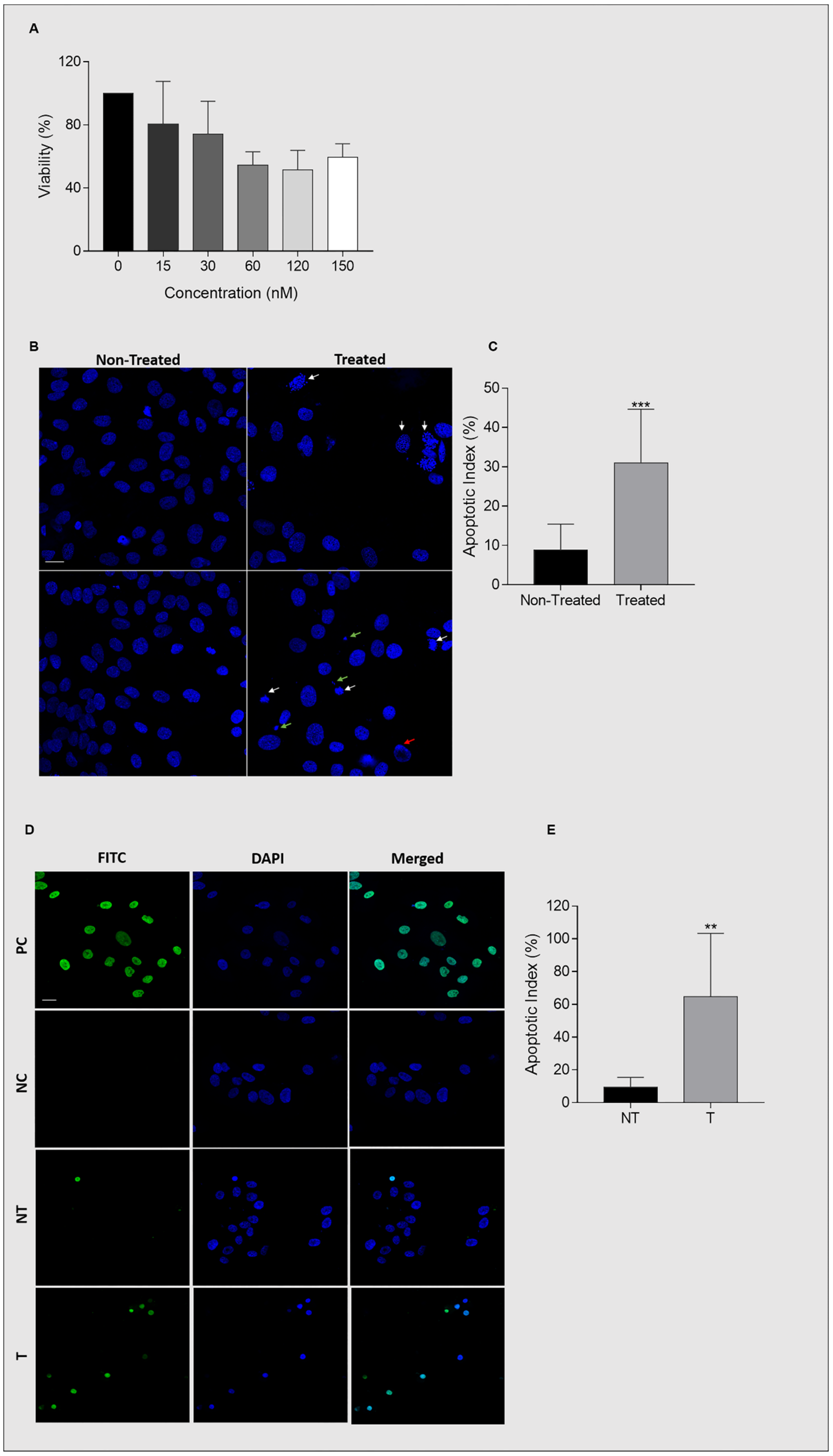
2.6. Ganetespib Reduces UM-HACC-2A Cell Viability by Inducing Apoptosis
2.7. Ganetespib Reduces Cell Proliferation and Migration
2.8. Expression of Hsp90 in Ganetespib-Treated UM-HACC-2A Cells
2.9. Expression of Akt in Ganetespib-Treated UM-HACC-2A Cells
2.10. Expression of NF-κB in Ganetespib-Treated UM-HACC-2A Cells
2.11. Expression of Caspase 3 in Ganetespib-Treated UM-HACC-2A Cells
2.12. Expression of VEGF in Ganetespib-Treated UM-HACC-2A Cells
3. Discussion
4. Material and Methods
4.1. Ethics Statement
4.2. Specimens
4.3. Histopathology
4.4. Immunohistochemistry
4.5. Immunofluorescence and Confocal Microscopy
4.6. UM-HACC-2A Cells
4.7. Cell Viability
4.8. Wound Healing Assay
4.9. Cell Proliferation
4.10. DAPI Staining
4.11. TUNEL Assay
4.12. Western Blot
4.13. Statistical Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | Chaperone system |
| Hsp | Heat shock protein |
| UPS | Ubiquitin-proteasome system |
| IS | Immune system |
| WT | Warthin’s tumor |
| PA | Pleomorphic adenoma |
| SMG | Submandibular gland |
| RT | Radiotherapy |
| CT | Chemotherapy |
| AE | Adverse effects |
| PG | Parotid gland |
| EX-PA | Carcinoma ex-pleomorphic adenoma |
| ACC | Adenoid cystic carcinoma |
| MUC | Mucoepidermoid carcinoma |
| H&E | Hematoxylin and Eosin |
| IHC | Immunohistochemistry |
| HPF | High-power field |
| IF | Immunofluorescence |
| PI | Pixel intensity |
| cross-sectional area | CSA |
| OSGM | Optimal salivary gland medium |
| DMEM | Dulbecco’s modified eagle medium |
| FBS | Fetal bovine serum |
| rhEGF | Human epidermal growth factor |
| BBE | Bovine brain extract |
| rhInsulin | Recombinant human insulin |
| DMSO | Dimethylsulfoxide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| SDS | Sodium dodecyl sulfate |
| VEGF | Vascular endothelial growth factor |
| t-akt | Total Akt |
| p-akt | Phospho-Akt |
| GIST | Gastrointestinal stromal tumors |
| OSCC | Oral squamous cell carcinoma |
| GA | Geldanamycin |
| RC | Radicicol |
References
- Seethala, R.R.; Stenman, G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Salivary Gland. Head Neck Pathol. 2017, 11, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Spitz, M.R.; Sider, J.G.; Newell, G.R. Salivary gland cancer and risk of subsequent skin cancer. Head Neck 1990, 12, 254–256. [Google Scholar] [CrossRef]
- Del Signore, A.G.; Megwalu, U.C. The Rising Incidence of Major Salivary Gland Cancer in the United States. Ear Nose Throat J. 2017, 96, E13–E16. [Google Scholar] [CrossRef]
- Young, A.; Okuyemi, O.T. Malignant Salivary Gland Tumors; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Guzzo, M.; Locati, L.D.; Prott, F.J.; Gatta, G.; McGurk, M.; Licitra, L. Major and minor salivary gland tumors. Crit. Rev. Oncol. Hematol. 2010, 74, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA. Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.J.L.; Conway de Macario, E. Chaperone proteins and chaperonopathies. In Stress: Physiology, Biochemistry, and Pathology Handbook of Stress Series; Academic Press: Cambridge, MA, USA, 2019; Volume 3, pp. 135–152. ISBN 9780128131466. [Google Scholar]
- Macario, A.J.L.; Conway de Macario, E.; Cappello, F. The Chaperonopathies; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–33. [Google Scholar] [CrossRef]
- Fouani, M.; Basset, C.A.; Mangano, G.D.; Leone, L.G.; Lawand, N.B.; Leone, A.; Barone, R. Heat Shock Proteins Alterations in Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 2806. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Macario, A.J.L.; Conway de Macario, E. Chaperonins in cancer: Expression, function, and migration in extracellular vesicles. Semin. Cancer Biol. 2021; in press. [Google Scholar] [CrossRef]
- Basset, C.A.; Rappa, F.; Lentini, V.L.; Barone, R.; Pitruzzella, A.; Unti, E.; Cappello, F.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. Hsp27 and Hsp60 in human submandibular salivary gland: Quantitative patterns in healthy and cancerous tissues with potential implications for differential diagnosis and carcinogenesis. Acta Histochem. 2021, 123, 151771. [Google Scholar] [CrossRef]
- Basset, C.A.; Cappello, F.; Rappa, F.; Jurjus, A.R.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. Chaperonin Hsp60 and Cancer Therapies; Springer: Dordrecht, The Netherlands, 2020; pp. 1–22. [Google Scholar]
- Macario, A.J.L.; Conway de Macario, E. Sick chaperones, cellular stress, and disease. N. Engl. J. Med. 2005, 353, 1489–1501. [Google Scholar] [CrossRef]
- Campanella, C.; Rappa, F.; Sciumè, C.; Marino Gammazza, A.; Barone, R.; Bucchieri, F.; David, S.; Curcurù, G.; Caruso Bavisotto, C.; Pitruzzella, A.; et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 2015, 121, 3230–3239. [Google Scholar] [CrossRef] [PubMed]
- Basset, C.A.; Cappello, F.; Rappa, F.; Lentini, V.L.; Jurjus, A.R.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. Molecular chaperones in tumors of salivary glands. J. Mol. Histol. 2020, 51, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Hoter, A.; Rizk, S.; Naim, H.Y. The multiple roles and therapeutic potential of molecular chaperones in prostate cancer. Cancers 2019, 11, 1194. [Google Scholar] [CrossRef]
- Wang, G.; Gu, X.; Chen, L.; Wang, Y.; Cao, B.; Qun, E. Comparison of the expression of 5 heat shock proteins in benign and malignant salivary gland tumor tissues. Oncol. Lett. 2013, 5, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.J.; Guerrero-Giménez, M.E.; Prince, T.L.; Ackerman, A.; Bonorino, C.; Calderwood, S.K. Heat shock proteins are essential components in transformation and tumor progression: Cancer cell intrinsic pathways and beyond. Int. J. Mol. Sci. 2019, 20, 4507. [Google Scholar] [CrossRef]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of hsp90 in cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef]
- Edkins, A.L.; Boshoff, A. General Structural and Functional Features of Molecular Chaperones. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1340, pp. 11–73. [Google Scholar]
- Cappello, F.; David, S.; Ardizzone, N.; Rappa, F.; Marasà, L.; Bucchieri, F.; Zummo, G. Expression of Heat Shock Proteins HSP10, HSP27, HSP60, HSP70, and HSP90 in Urothelial Carcinoma of Urinary Bladder. J. Cancer Mol. 2006, 2, 73–77. [Google Scholar]
- Cappello, F.; Rappa, F.; David, S.; Anzalone, R.; Zummo, G. Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res. 2003, 23, 1325–1331. [Google Scholar]
- Rappa, F.; Sciume, C.; Lo Bello, M.; Bavisotto, C.C.; Gammazza, A.M.; Barone, R.; Campanella, C.; David, S.; Carini, F.; Zarcone, F.; et al. Comparative analysis of Hsp10 and Hsp90 expression in healthy mucosa and adenocarcinoma of the large bowel. Anticancer Res. 2014, 34, 4153–4160. [Google Scholar]
- Rappa, F.; Unti, E.; Baiamonte, P.; Cappello, F.; Scibetta, N. Different immunohistochemical levels of Hsp60 and Hsp70 in a subset of brain tumors and putative role of Hsp60 in neuroepithelial tumorigenesis. Eur. J. Histochem. 2013, 57, e20. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Arrigo, A.P.; Calderwood, S.K. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch. Toxicol. 2013, 87, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 family: Structure, regulation, function, and implications in health and disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Li, L.; Jiang, J.; Zheng, Z.; Shang, J.; Wang, C.; Chen, W.; Bao, Q.; Xu, X.; et al. Small-molecule inhibitor targeting the Hsp90-Cdc37 protein-protein interaction in colorectal cancer. Sci. Adv. 2019, 5, 2277. [Google Scholar] [CrossRef]
- Bakkar, N.; Guttridge, D.C. NF-κB signaling: A tale of two pathways in skeletal myogenesis. Physiol. Rev. 2010, 90, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. Onco. Targets. Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Brown, K.D.; Claudio, E.; Siebenlist, U. The roles of the classical and alternative nuclear factor-κB pathways: Potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, 212. [Google Scholar] [CrossRef]
- Yeramian, A.; García, V.; Bergadà, L.; Domingo, M.; Santacana, M.; Valls, J.; Martinez-Alonso, M.; Carceller, J.A.; Cussac, A.L.; Dolcet, X.; et al. Bioluminescence Imaging to Monitor the Effects of the Hsp90 Inhibitor NVP-AUY922 on NF-κB Pathway in Endometrial Cancer. Mol. Imaging Biol. 2016, 18, 545–556. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Li, M.; Yan, H.; Sun, M.; Fan, T. Management of salivary gland carcinomas—A review. Oncotarget 2017, 8, 3946–3956. [Google Scholar] [CrossRef]
- Geiger, J.L.; Ismaila, N.; Beadle, B.; Caudell, J.J.; Chau, N.; Deschler, D.; Glastonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of salivary gland malignancy: ASCO guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Zou, Q.; Liu, Q.; Lin, Y.; Chen, S. HSP90 inhibitor ganetespib (STA-9090) inhibits tumor growth in c-Myc-dependent esophageal squamous cell carcinoma. Onco. Targets. Ther. 2020, 13, 2997–3011. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Lu, Y.; Li, Z.; Li, L.; Niu, D.; Xu, W.; Liu, J.; Fu, L.; Zhou, Z.; Gu, Y.; et al. Ganetespib overcomes resistance to PARP inhibitors in breast cancer by targeting core proteins in the DNA repair machinery. Investig. New Drugs 2017, 35, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Deycmar, S.; Mara, E.; Kerschbaum-Gruber, S.; Waller, V.; Georg, D.; Pruschy, M. Ganetespib selectively sensitizes cancer cells for proximal and distal spread-out Bragg peak proton irradiation. Radiat. Oncol. 2022, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.S.; Warner, E.A.; Giaccone, G. Ganetespib for small cell lung cancer. Expert Opin. Investig. Drugs 2017, 26, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, E.; Shien, K.; Torigoe, H.; Takeda, T.; Takahashi, Y.; Ogoshi, Y.; Yoshioka, T.; Namba, K.; Sato, H.; Suzawa, K.; et al. Ganetespib in epidermal growth factor receptor-tyrosine kinase inhibitor-resistant non-small cell lung cancer. Anticancer Res. 2019, 39, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Saini, N.; Parris, A.B.; Zhao, M.; Yang, X. Ganetespib induces G2/M cell cycle arrest and apoptosis in gastric cancer cells through targeting of receptor tyrosine kinase signaling. Int. J. Oncol. 2017, 51, 967–974. [Google Scholar] [CrossRef]
- Lin, S.F.; Der Lin, J.; Hsueh, C.; Chou, T.C.; Yeh, C.N.; Chen, M.H.; Wong, R.J. Efficacy of an HSP90 inhibitor, ganetespib, in preclinical thyroid cancer models. Oncotarget 2017, 8, 41294–41304. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Saini, N.; Howard, E.W.; Parris, A.B.; Ma, Z.; Zhao, Q.; Zhao, M.; Liu, B.; Edgerton, S.M.; Thor, A.D.; et al. Ganetespib targets multiple levels of the receptor tyrosine kinase signaling cascade and preferentially inhibits ErbB2-overexpressing breast cancer cells. Sci. Rep. 2018, 8, 6829. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Braicu, I.; Berger, R.; Mahner, S.; Sehouli, J.; Pujade-Lauraine, E.; Cassier, P.A.; Moll, U.M.; Ulmer, H.; Leunen, K.; et al. Part I of GANNET53: A European Multicenter Phase I/II Trial of the Hsp90 Inhibitor Ganetespib Combined with Weekly Paclitaxel in Women with High-Grade, Platinum-Resistant Epithelial Ovarian Cancer—A Study of the GANNET53 Consortium. Front. Oncol. 2019, 9, 832. [Google Scholar] [CrossRef]
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S.J. Old and New Approaches to Target the Hsp90 Chaperone. Curr. Cancer Drug Targets 2019, 20, 253–270. [Google Scholar] [CrossRef]
- Eyermann, C.E.; Haley, J.D.; Alexandrova, E.M. The HSP-RTK-Akt axis mediates acquired resistance to Ganetespib in HER2-positive breast cancer. Cell Death Dis. 2021, 12, 126. [Google Scholar] [CrossRef]
- Acquaviva, J.; Smith, D.L.; Jimenez, J.P.; Zhang, C.; Sequeira, M.; He, S.; Sang, J.; Bates, R.C.; Proia, D.A. Overcoming acquired BRAF inhibitor resistance in melanoma via targeted inhibition of hsp90 with ganetespib. Mol. Cancer Ther. 2014, 13, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Huang, E.H.B.; Christie, I.; Kurland, B.F.; Burns, T.F. Acquired resistance to the Hsp90 inhibitor, ganetespib, in KRAS-Mutant NSCLC is mediated via reactivation of the ERK-p90RSK-mTOR signaling network. Mol. Cancer Ther. 2017, 16, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Long, T.E.; Park, W.; Landry, J.C.; Taliaferro-Smith, L.; Farris, A.B.; Diaz, R.; El-Rayes, B.F. Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol. Carcinog. 2015, 54, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.H.; Kun, W.; Fu, X.L.; Zhou, P.J.; Liu, Z.; Xu, D.D.; Wang, Y.F.; Yang, D.P.; Xie, Q.L.; Liu, Q.Y. Hsp90 inhibitor induces KG-1a cell differentiation and apoptosis via Akt/NF-κB signaling. Oncol. Rep. 2017, 38, 1517–1524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. Synergistic cytotoxicity of BIIB021 with triptolide through suppression of PI3K/Akt/mTOR and NF-κB signal pathways in thyroid carcinoma cells. Biomed. Pharmacother. 2016, 83, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Cao, P.; Goeddel, D.V. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol. Cell 2002, 9, 401–410. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, L.; Li, L.; Li, Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020, 6, 112. [Google Scholar] [CrossRef]
- Lin, T.Y.; Bear, M.; Du, Z.; Foley, K.P.; Ying, W.; Barsoum, J.; London, C. The novel HSP90 inhibitor STA-9090 exhibits activity against Kit-dependent and -independent malignant mast cell tumors. Exp. Hematol. 2008, 36, 1266–1277. [Google Scholar] [CrossRef]
- Zhai, H.Y.; Zhou, Q.F.; Dou, J.P.; Liu, F.Y.; Zhu, X.Y.; Yu, J.; Liang, P. Hepatic Microwave Ablation–Induced Tumor Destruction and Animal End Point Survival Can Be Improved by Suppression of Heat Shock Protein 90. J. Ultrasound Med. 2020, 39, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Park, K.S.; Lee, D.H.; Alberobello, A.T.; Raffeld, M.; Pierobon, M.; Pin, E.; Petricoin, E.F.; Wang, Y.; Giaccone, G. HSP-90 inhibitor ganetespib is synergistic with doxorubicin in small cell lung cancer. Oncogene 2014, 33, 4867–4876. [Google Scholar] [CrossRef]
- McCleese, J.K.; Bear, M.D.; Fossey, S.L.; Mihalek, R.M.; Foley, K.P.; Ying, W.; Barsoum, J.; London, C.A. The novel HSP90 inhibitor STA-1474 exhibits biologic activity against osteosarcoma cell lines. Int. J. Cancer 2009, 125, 2792–2801. [Google Scholar] [CrossRef]
- Mac Gabhann, F.; Popel, A.S. Dimerization of VEGF receptors and implications for signal transduction: A computational study. Biophys. Chem. 2007, 128, 125–139. [Google Scholar] [CrossRef]
- Yamada-Kanazawa, S.; Kajihara, I.; Fukushima, S.; Jinnin, M.; Masuzawa, M.; Masuzawa, M.; Amoh, Y.; Hoshina, D.; Abe, R.; Ihn, H. Inhibition of heat shock protein 90 exerts an antitumour effect in angiosarcoma: Involvement of the vascular endothelial growth factor signalling pathway. Br. J. Dermatol. 2017, 177, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Ganji, P.N.; Park, W.; Wen, J.; Mahaseth, H.; Landry, J.; Farris, A.B.; Willingham, F.; Sullivan, P.S.; Proia, D.A.; El-Hariry, I.; et al. Antiangiogenic effects of ganetespib in colorectal cancer mediated through inhibition of HIF-1α and STAT-3. Angiogenesis 2013, 16, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Gilkes, D.M.; Chaturvedi, P.; Luo, W.; Hu, H.; Takano, N.; Liang, H.; Semenza, G.L. Ganetespib blocks HIF-1 activity and inhibits tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in orthotopic mouse models of triple-negative breast cancer. J. Mol. Med. 2014, 92, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Nonni, A.; Sergentanis, T.N.; Papadimitriou, C.A.; Michalopoulos, N.V.; Lazaris, A.C.; Patsouris, E.; Zografos, G.C. Heat shock protein90 in lobular neoplasia of the breast. BMC Cancer 2008, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Diehl, M.C.; Idowu, M.O.; Kimmelshue, K.; York, T.P.; Elmore, L.W.; Holt, S.E. Elevated expression of nuclear Hsp90 in invasive breast tumors. Cancer Biol. Ther. 2009, 8, 1952–1961. [Google Scholar] [CrossRef]
- Xu, Q.; Tu, J.; Dou, C.; Zhang, J.; Yang, L.; Liu, X.; Lei, K.; Liu, Z.; Wang, Y.; Li, L.; et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol. Cancer 2017, 16, 178. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; He, S.L.; Li, L.J.; Wang, C. Hsp90 up-regulates PD-L1 to promote HPV-positive cervical cancer via HER2/PI3K/AKT pathway. Mol. Med. 2021, 27, 130. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Guo, M.; Xu, S.; Song, X.; Bai, B.; Li, Z.; Chen, J.; An, Y.; Nie, Y.; Wu, K.; et al. HSP90-dependent PUS7 overexpression facilitates the metastasis of colorectal cancer cells by regulating LASP1 abundance. J. Exp. Clin. Cancer Res. 2021, 40, 170. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.H.; Lee, E.J.; Jang, K.T.; Kim, K.M.; Park, C.K.; Lee, C.S.; Kang, D.Y.; Lee, S.H.; Sohn, T.S.; Kim, S. Expression of HSP90 in gastrointestinal stromal tumours and mesenchymal tumours. Histopathology 2010, 56, 694–701. [Google Scholar] [CrossRef]
- Shiraishi, N.; Onda, T.; Hayashi, K.; Onidani, K.; Watanabe, K.; Sekikawa, S.; Shibahara, T. Heat shock protein 90 as a molecular target for therapy in oral squamous cell carcinoma: Inhibitory effects of 17⇑dmag and ganetespib on tumor cells. Oncol. Rep. 2021, 45, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Tsai, P.T.; Lin, C.K.; Shieh, Y.S.; Chen, Y.W. Expression pattern of heat shock protein 90 in patients with oral squamous cell carcinoma in northern Taiwan. Br. J. Oral Maxillofac. Surg. 2017, 55, 281–286. [Google Scholar] [CrossRef]
- Ono, K.; Eguchi, T.; Sogawa, C.; Calderwood, S.K.; Futagawa, J.; Kasai, T.; Seno, M.; Okamoto, K.; Sasaki, A.; Kozaki, K. ichi HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell. Biochem. 2018, 119, 7350–7362. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xie, G.; Zhan, Y.; Lu, J.; Xu, L.; Fan, S.; Wang, W. Elevated HSP90 associates with expression of HIF-1α and p-AKT and is predictive of poor prognosis in nasopharyngeal carcinoma. Histopathology 2019, 75, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Bar, J.K.; Cierpikowski, P.; Lis-Nawara, A.; Duc, P.; Hałoń, A.; Radwan-Oczko, M. Comparison of p53, HSP90, E-cadherin and HPV in oral lichen planus and oral squamous cell carcinoma. Acta Otorhinolaryngol. Ital. 2021, 41, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Dimas, D.T.H.; Perlepe, C.D.; Sergentanis, T.N.; Misitzis, I.; Kontzoglou, K.; Patsouris, E.; Kouraklis, G.; Psaltopoulou, T.; Nonni, A. The prognostic significance of hsp70/hsp90 expression in breast cancer: A systematic review and meta-analysis. Anticancer Res. 2018, 38, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Multhoff, G.; Farkas, B.; Wild, P.J.; Landthaler, M.; Stolz, W.; Vogt, T. Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp. Dermatol. 2004, 13, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zoroquiain, P.; Faingold, D.; Algahmdi, S.; Vila, N.; Logan, P.; Sanft, D.M.; Dias, A.B.T.; Aldrees, S.; Bravo-Filho, V.; Burnier, J.; et al. Analysis of HSP90 expression is valuable in the differential diagnosis of ocular surface squamous lesions. In Proceedings of the American Journal of Clinical Pathology; Oxford Academic: Oxford, UK, 2016; Volume 145, pp. 385–392. [Google Scholar]
- Zagouri, F.; Sergentanis, T.; Nonni, A.; Papadimitriou, C.; Pazaiti, A.; Michalopoulos, N.V.; Safioleas, P.; Lazaris, A.; Theodoropoulos, G.; Patsouris, E.; et al. Decreased Hsp90 expression in infiltrative lobular carcinoma: An immunohistochemical study. BMC Cancer 2010, 10, 409. [Google Scholar] [CrossRef]
- Biamonte, M.A.; Van De Water, R.; Arndt, J.W.; Scannevin, R.H.; Perret, D.; Lee, W.C. Heat shock protein 90: Inhibitors in clinical trials. J. Med. Chem. 2010, 53, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Huryn, D.M.; Wipf, P. Natural Product Chemistry and Cancer Drug Discovery. In Cancer Drug Design and Discovery: Second Edition; Academic Press: Cambridge, MA, USA, 2013; pp. 91–120. ISBN 9780123965219. [Google Scholar]
- Warner, K.A.; Oklejas, A.E.; Pearson, A.T.; Zhang, Z.; Wu, W.; Divi, V.; Rodriguez-Ramirez, C.; Castilho, R.M.; Polverini, P.J.; Nör, J.E. UM-HACC-2A: MYB-NFIB fusion-positive human adenoid cystic carcinoma cell line. Oral Oncol. 2018, 87, 21–28. [Google Scholar] [CrossRef]
- Prahallad, A.; Sun, C.; Huang, S.; Di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012, 483, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Fransén, K.; Klintenäs, M.; Österström, A.; Dimberg, J.; Monstein, H.J.; Söderkvist, P. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis 2004, 25, 527–533. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Guo, S.T.; Wang, J.Y.; Yan, X.G.; Farrelly, M.; Zhang, Y.Y.; Liu, F.; Yari, H.; La, T.; Lei, F.X.; et al. Reactivation of ERK and Akt confers resistance of mutant BRAF colon cancer cells to the HSP90 inhibitor AUY922. Oncotarget 2016, 7, 49597–49610. [Google Scholar] [CrossRef] [PubMed]
- Peyssonnaux, C.; Eychène, A. The Raf/MEK/ERK pathway: New concepts of activation. Biol. Cell 2001, 93, 53–62. [Google Scholar] [CrossRef]
- Goldman, J.W.; Raju, R.N.; Gordon, G.A.; El-Hariry, I.; Teofilivici, F.; Vukovic, V.M.; Bradley, R.; Karol, M.D.; Chen, Y.; Guo, W.; et al. A first in human, safety, pharmacokinetics, and clinical activity phase I study of once weekly administration of the Hsp90 inhibitor ganetespib (STA-9090) in patients with solid malignancies. BMC Cancer 2013, 13, 152. [Google Scholar] [CrossRef]
- Ghadban, T.; Dibbern, J.L.; Reeh, M.; Miro, J.T.; Tsui, T.Y.; Wellner, U.; Izbicki, J.R.; Güngör, C.; Vashist, Y.K. HSP90 is a promising target in gemcitabine and 5-fluorouracil resistant pancreatic cancer. Apoptosis 2017, 22, 369–380. [Google Scholar] [CrossRef]
- Chettiar, S.T.; Malek, R.; Annadanam, A.; Nugent, K.M.; Kato, Y.; Wang, H.; Cades, J.A.; Taparra, K.; Belcaid, Z.; Ballew, M.; et al. Ganetespib radiosensitization for liver cancer therapy. Cancer Biol. Ther. 2016, 17, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wu, W.; Zhou, Y.; Xie, Q.; Liu, T.; Jin, J.; Liu, K. HSP27 expression levels are associated with the sensitivity of hepatocellular carcinoma cells to 17-allylamino-17-demethoxygeldanamycin. Futur. Oncol. 2013, 9, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. The effect of 17-allylamino-17-demethoxygeldanamycin alone or in combination with paclitaxel on anaplastic thyroid carcinoma cells. Endocrine 2015, 48, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.V.; Valenti, M.; Miranda, S.; Maloney, A.; Eccles, S.A.; Thomas, G.; Clarke, P.A.; Workman, P. Mode of cell death induced by the HSP90 inhibitor 17-AAG (tanespimycin) is dependent on the expression of pro-apoptotic bax. Oncotarget 2013, 4, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Broemer, M.; Krappmann, D.; Scheidereit, C. Requirement of Hsp90 activity for IκB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-κB activation. Oncogene 2004, 23, 5378–5386. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, W.; Li, Y.; Yang, D.; Li, X.; Shen, C.; Liu, Y.; Ke, X.; Guo, S.; Guo, Z. HSP90AA1-mediated autophagy promotes drug resistance in osteosarcoma. J. Exp. Clin. Cancer Res. 2018, 37, 201. [Google Scholar] [CrossRef]
- Da Rocha Dias, S.; Friedlos, F.; Light, Y.; Springer, C.; Workman, P.; Marais, R. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2005, 65, 10686–10691. [Google Scholar] [CrossRef]
- Grbovic, O.M.; Basso, A.D.; Sawai, A.; Ye, Q.; Friedlander, P.; Solit, D.; Rosen, N. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc. Natl. Acad. Sci. USA 2006, 103, 57–62. [Google Scholar] [CrossRef]
- Siligardi, G.; Panaretou, B.; Meyerc, P.; Singh, S.; Woolfson, D.N.; Piper, P.W.; Pearl, L.H.; Prodromou, C. Regulation of Hsp90 ATPase activity by the Co-chaperone Cdc37p/p50 cdc37. J. Biol. Chem. 2002, 277, 20151–20159. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.K.; Gohlke, U.; Sobott, F.; Good, V.M.; Ali, M.M.U.; Prodromou, C.; Robinson, C.V.; Saibil, H.R.; Pearl, L.H. Structure of an Hsp90-Cdc37-Cdk4 Complex. Mol. Cell 2006, 23, 697–707. [Google Scholar] [CrossRef]
- Liu, K.; Jin, H.; Guo, Y.; Liu, Y.; Wan, Y.; Zhao, P.; Zhou, Z.; Wang, J.; Wang, M.; Zou, C.; et al. CFTR interacts with Hsp90 and regulates the phosphorylation of AKT and ERK1/2 in colorectal cancer cells. FEBS Open Bio. 2019, 9, 1119–1127. [Google Scholar] [CrossRef]
- Redlak, M.J.; Miller, T.A. Targeting PI3K/Akt/HSP90 signaling sensitizes gastric cancer cells to deoxycholate-induced apoptosis. Dig. Dis. Sci. 2011, 56, 323–329. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Zhou, X.; Tang, M.; Tan, W.; Sun, T.; Wang, Y.; Deng, Y. miR-485-5p/HSP90 axis blocks Akt1 phosphorylation to suppress osteosarcoma cell proliferation and migration via PI3K/AKT pathway. J. Physiol. Biochem. 2020, 76, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Ou, W.; Meng, F.; Zhou, H.; Wang, A. Targeting HSP90 in ovarian cancers with multiple receptor tyrosine kinase coactivation. Mol. Cancer 2011, 10, 125. [Google Scholar] [CrossRef]
- Farhana, L.; Dawson, M.I.; Fontana, J.A. Apoptosis induction by a novel retinoid-related molecule requires nuclear factor-κB activation. Cancer Res. 2005, 65, 4909–4917. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, Y.; Li, T.; Shi, K.; Li, Z.; Ma, Y.; Li, F.; Luo, H.; Yang, Y.; Xu, C. Heat shock protein 90-mediated inactivation of nuclear factor-kB switches autophagy to apoptosis through becn1 transcriptional inhibition in selenite-induced NB4 cells. Mol. Biol. Cell 2011, 22, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shao, F.; Liu, Z.; Zhang, J.; Wang, S.; Liu, J.; Liu, H.; Chen, H.; Liu, K.; Xia, M.; et al. The Hsp90 inhibitor SNX-2112, induces apoptosis in multidrug resistant K562/ADR cells through suppression of Akt/NF-κB and disruption of mitochondria-dependent pathways. Chem. Biol. Interact. 2013, 205, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Na, B.H.; Hoang, T.X.; Kim, J.Y. Hsp90 inhibition reduces TLR5 surface expression and NF-B activation in human myeloid leukemia THP-1 cells. Biomed Res. Int. 2018, 2018, 4319369. [Google Scholar] [CrossRef]
- Hertlein, E.; Wagner, A.J.; Jones, J.; Lin, T.S.; Maddocks, K.J.; Towns, W.H.; Goettl, V.M.; Zhang, X.; Jarjoura, D.; Raymond, C.A.; et al. 17-DMAG targets the nuclear factor-κB family of proteins to induce apoptosis in chronic lymphocytic leukemia: Clinical implications of HSP90 inhibition. Blood 2010, 116, 45–53. [Google Scholar] [CrossRef]
- Walsby, E.; Pearce, L.; Burnett, A.K.; Fegan, C.; Pepper, C. The Hsp90 inhibitor NVP-AUY922-AG inhibits NF-κB signaling, overcomes microenvironmental cytoprotection and is highly synergistic with fludarabine in primary CLL cells. Oncotarget 2012, 3, 525–534. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Matta, H.; Chaudhary, P.M. A purine scaffold HSP90 inhibitor BIIB021 has selective activity against KSHV-associated primary effusion lymphoma and blocks vFLIP k13-induced NF-κB. Clin. Cancer Res. 2013, 19, 5016–5026. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Rogut, M.; Mielczarek-Lewandowska, A.; Wozniak, M.; Czyz, M. 17-aminogeldanamycin inhibits constitutive nuclear factor-kappa b (Nf-κb) activity in patient-derived melanoma cell lines. Int. J. Mol. Sci. 2020, 21, 3749. [Google Scholar] [CrossRef]
- Bai, L.; Xu, S.; Chen, W.; Li, Z.; Wang, X.; Tang, H.; Lin, Y. Blocking NF-κB and Akt by Hsp90 inhibition sensitizes Smac mimetic compound 3-induced extrinsic apoptosis pathway and results in synergistic cancer cell death. Apoptosis 2011, 16, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Parascandolo, A.; Rappa, F.; Cappello, F.; Kim, J.; Cantu, D.A.; Chen, H.; Mazzoccoli, G.; Hematti, P.; Castellone, M.D.; Salvatore, M.; et al. Extracellular Superoxide Dismutase Expression in Papillary Thyroid Cancer Mesenchymal Stem/Stromal Cells Modulates Cancer Cell Growth and Migration. Sci. Rep. 2017, 7, 41416. [Google Scholar] [CrossRef]
- Barone, R.; Macaluso, F.; Sangiorgi, C.; Campanella, C.; Gammazza, A.M.; Moresi, V.; Coletti, D.; Conway de Macario, E.; Macario, A.J.L.; Cappello, F.; et al. Skeletal muscle Heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 α1 expression. Sci. Rep. 2016, 6, 19781. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Rappa, F.; Macaluso, F.; Caruso Bavisotto, C.; Sangiorgi, C.; Di Paola, G.; Tomasello, G.; Di Felice, V.; Marcianò, V.; Farina, F.; et al. Alcoholic liver disease: A mouse model reveals protection by Lactobacillus fermentum. Clin. Transl. Gastroenterol. 2016, 7, e138. [Google Scholar] [CrossRef] [PubMed]
- Rappa, F.; Pitruzzella, A.; Marino Gammazza, A.; Barone, R.; Mocciaro, E.; Tomasello, G.; Carini, F.; Farina, F.; Zummo, G.; Conway de Macario, E.; et al. Quantitative patterns of Hsps in tubular adenoma compared with normal and tumor tissues reveal the value of Hsp10 and Hsp60 in early diagnosis of large bowel cancer. Cell Stress Chaperones 2016, 21, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Sangiorgi, C.; Marino Gammazza, A.; D’Amico, D.; Salerno, M.; Cappello, F.; Pomara, C.; Zummo, G.; Farina, F.; Di Felice, V.; et al. Effects of Conjugated Linoleic Acid Associated with Endurance Exercise on Muscle Fibres and Peroxisome Proliferator-Activated Receptor γ Coactivator 1 α Isoforms. J. Cell. Physiol. 2017, 232, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, B. NF-κB promotes iNOS and VEGF expression in salivary gland adenoid cystic carcinoma cells and enhances endothelial cell motility in vitro. Cell Prolif. 2009, 42, 150–161. [Google Scholar] [CrossRef]


| Specimen | Hsp10 | Hsp90 1 | |||
|---|---|---|---|---|---|
| Percentage of Positive Cells | Intensity | Percentage of Positive Cells | Intensity | ||
| Normal submandibular (SMG) and parotid (PG) gland | Ducts | 96.6 | +++ | 75.5 | +/+++ |
| Acini | 92.5 | ++/+++ | 36.7 | +/++ | |
| Sialadenitis | Ducts | 90 | ++/+++ | 26.4 | +/+++ |
| Acini | 75 | ++ | 26.4 | +/+++ | |
| Tumor 2 | Warthin’s tumor (WT) | 86.2 | ++ | 40 | +/+++ |
| Pleomorphic adenoma (PA) | 83.3 | +++ | 41 | +/++ | |
| Carcinoma ex-pleomorphic adenoma (EX-PA) | 86 | ++/+++ | 91.2 | ++/+++ | |
| Adenoid cystic carcinoma (ACC) | 90 | ++/+++ | 83.3 | ++/+++ | |
| Mucoepidermoid carcinoma (MUC) | 79.6 | ++/+++ | 91 | +++ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basset, C.A.; Rappa, F.; Barone, R.; Florena, A.M.; Porcasi, R.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. The Chaperone System in Salivary Glands: Hsp90 Prospects for Differential Diagnosis and Treatment of Malignant Tumors. Int. J. Mol. Sci. 2022, 23, 9317. https://doi.org/10.3390/ijms23169317
Basset CA, Rappa F, Barone R, Florena AM, Porcasi R, Conway de Macario E, Macario AJL, Leone A. The Chaperone System in Salivary Glands: Hsp90 Prospects for Differential Diagnosis and Treatment of Malignant Tumors. International Journal of Molecular Sciences. 2022; 23(16):9317. https://doi.org/10.3390/ijms23169317
Chicago/Turabian StyleBasset, Charbel A., Francesca Rappa, Rosario Barone, Ada Maria Florena, Rossana Porcasi, Everly Conway de Macario, Alberto J. L. Macario, and Angelo Leone. 2022. "The Chaperone System in Salivary Glands: Hsp90 Prospects for Differential Diagnosis and Treatment of Malignant Tumors" International Journal of Molecular Sciences 23, no. 16: 9317. https://doi.org/10.3390/ijms23169317
APA StyleBasset, C. A., Rappa, F., Barone, R., Florena, A. M., Porcasi, R., Conway de Macario, E., Macario, A. J. L., & Leone, A. (2022). The Chaperone System in Salivary Glands: Hsp90 Prospects for Differential Diagnosis and Treatment of Malignant Tumors. International Journal of Molecular Sciences, 23(16), 9317. https://doi.org/10.3390/ijms23169317









