Shockwaves Increase In Vitro Resilience of Rhizopus oryzae Biofilm under Amphotericin B Treatment
Abstract
1. Introduction
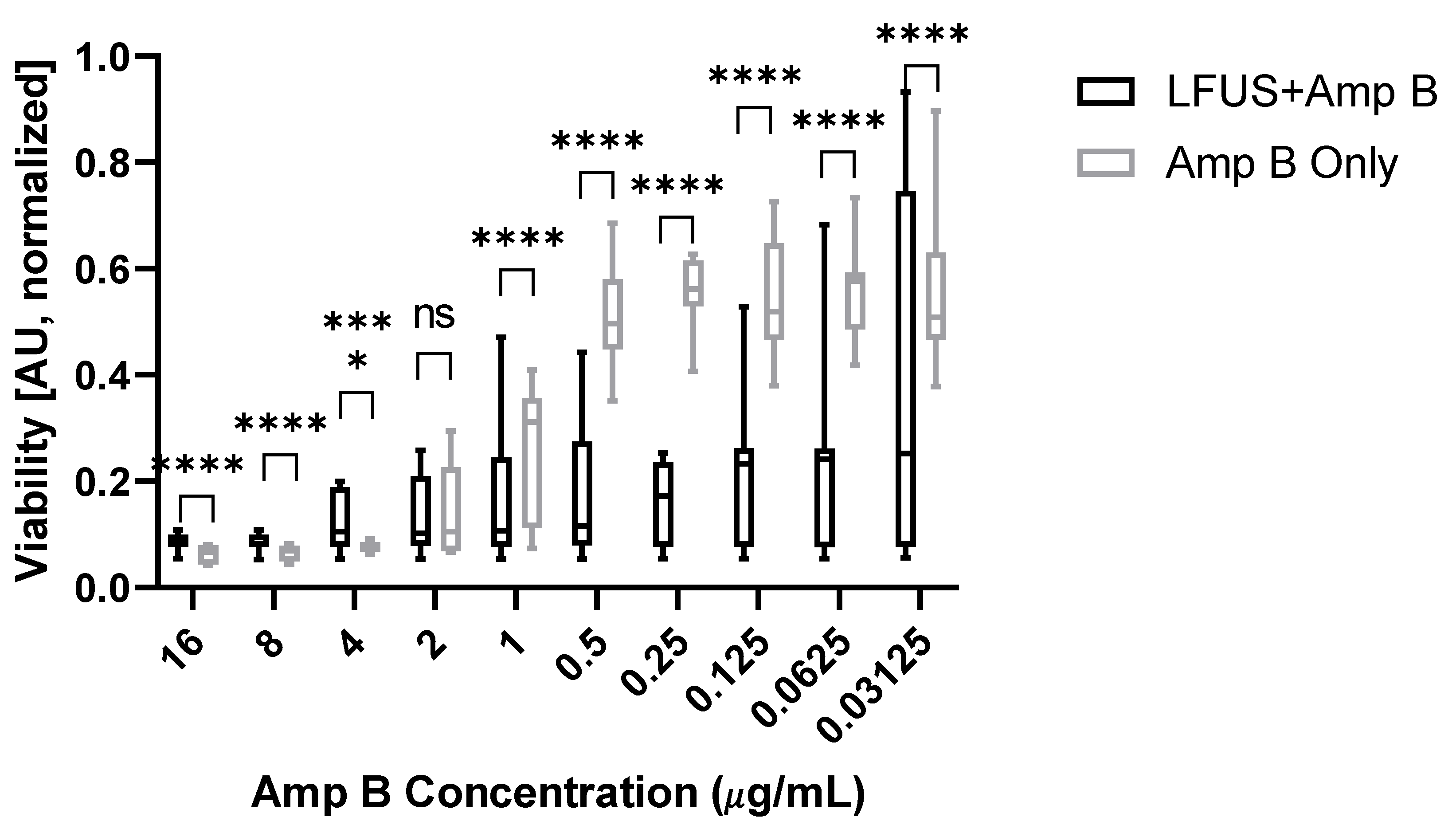
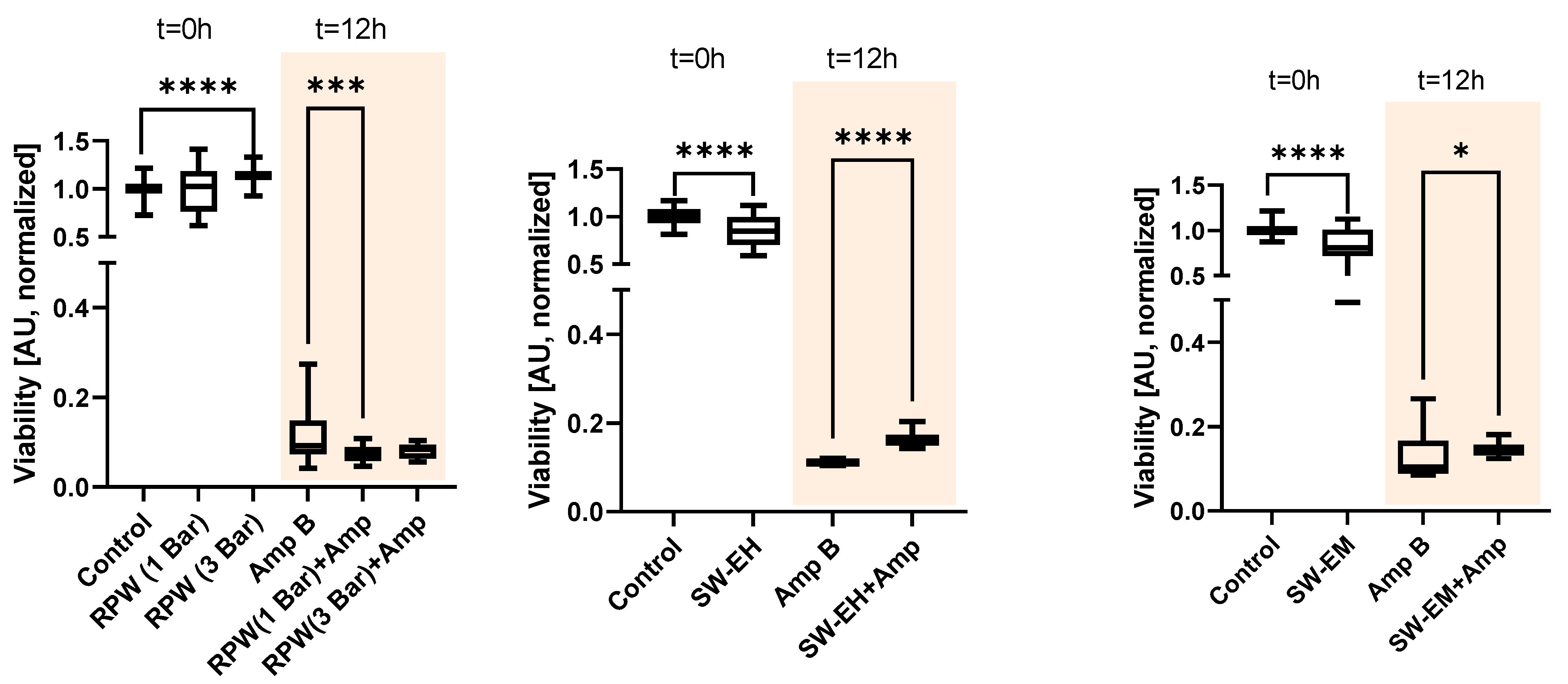
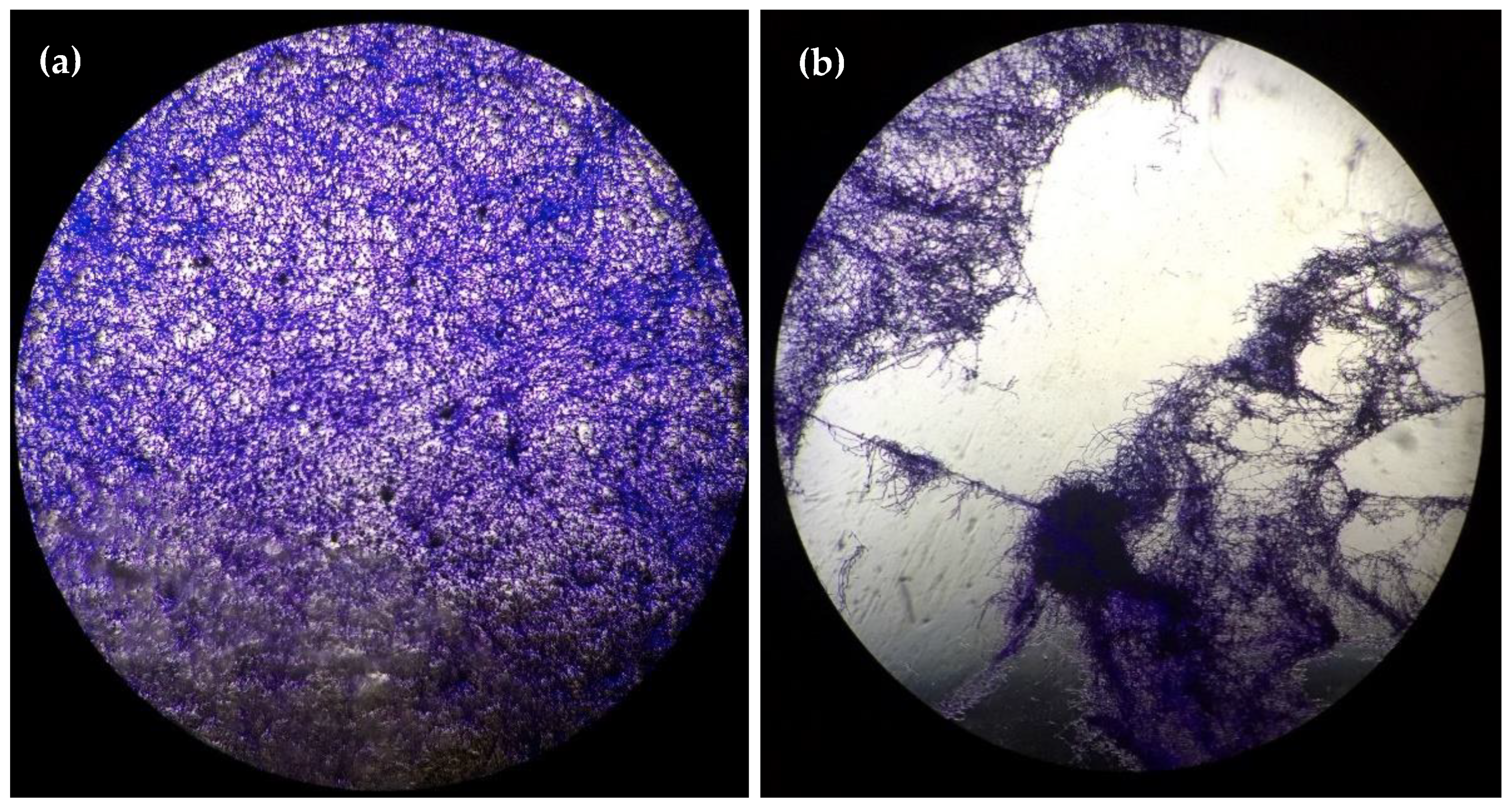
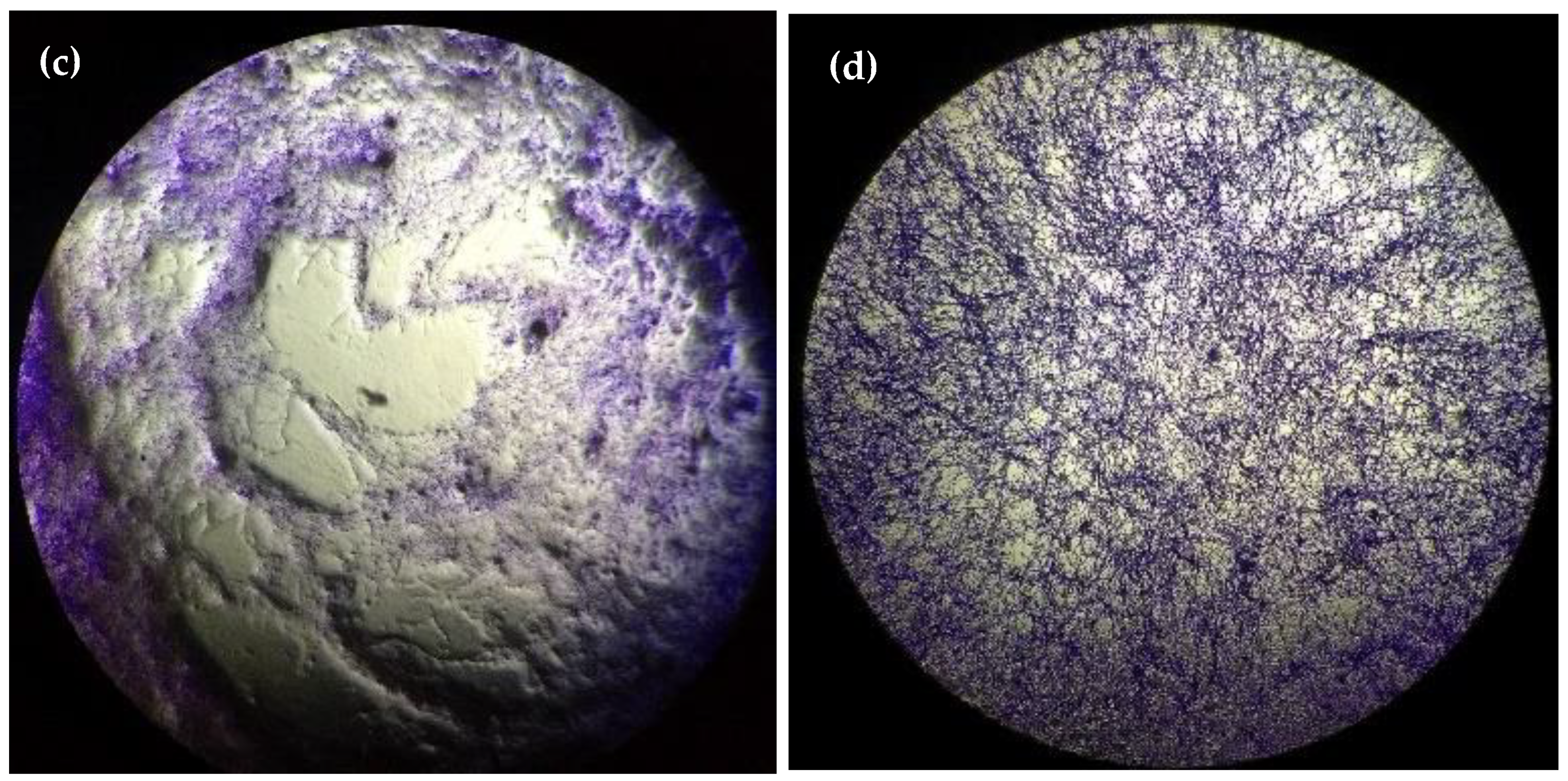
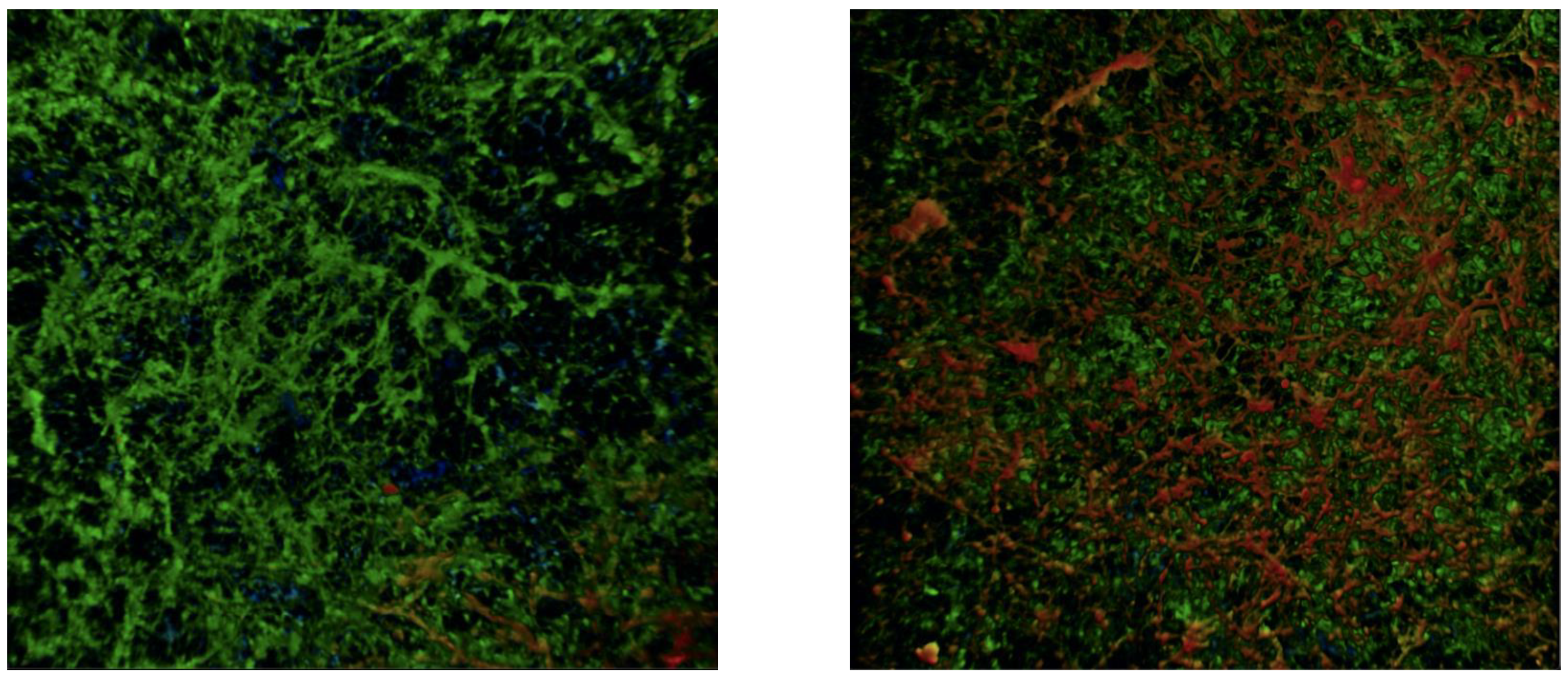
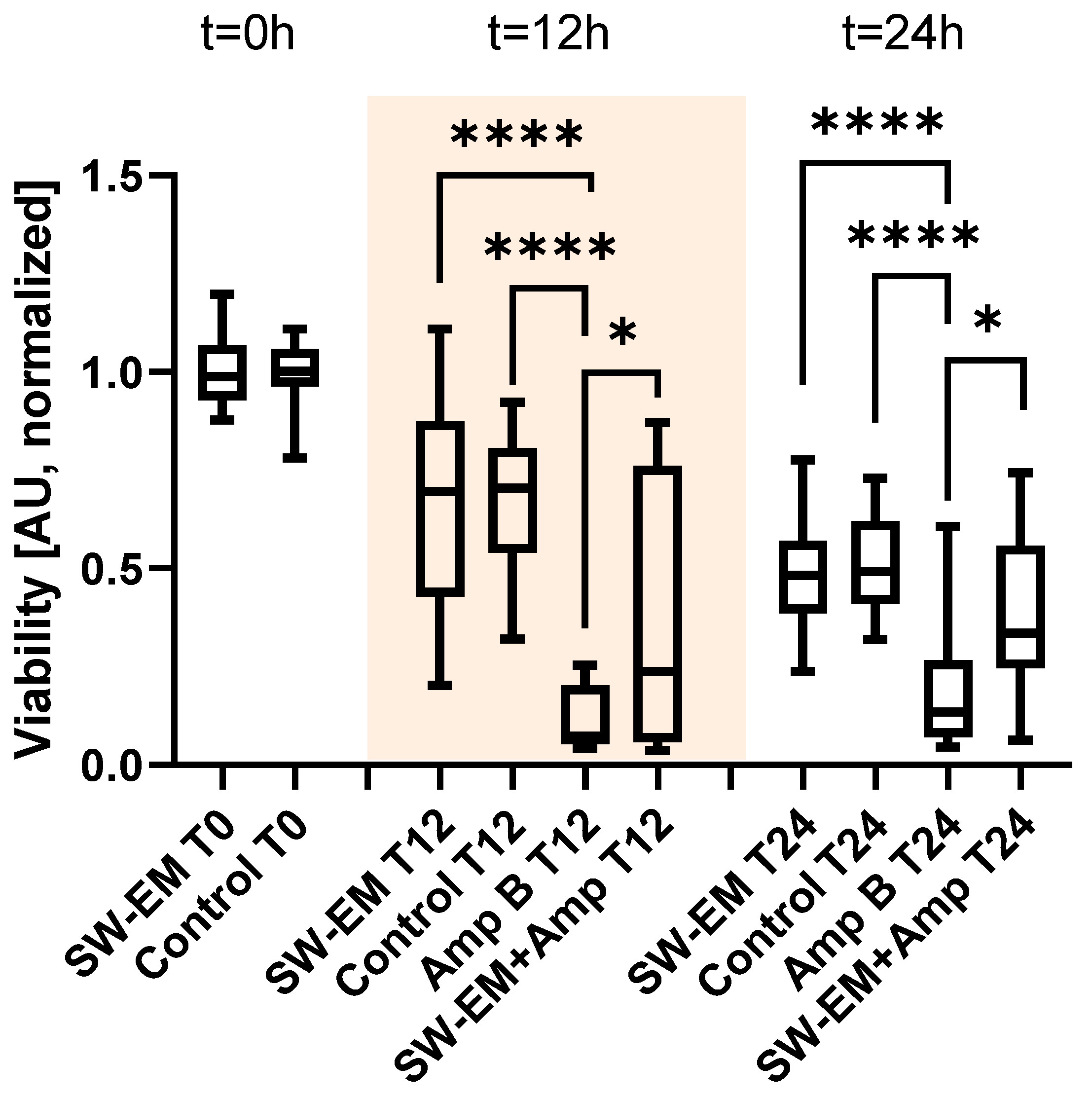
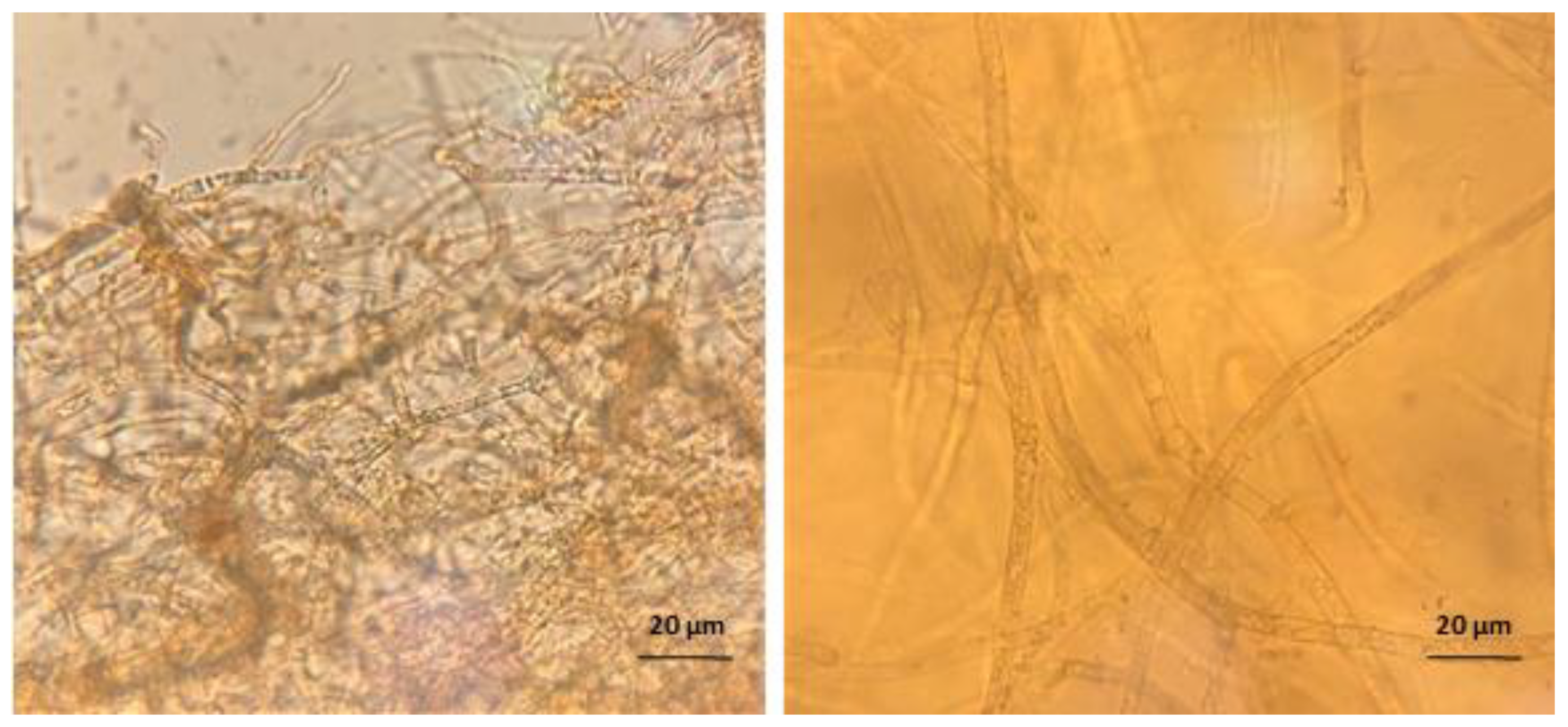
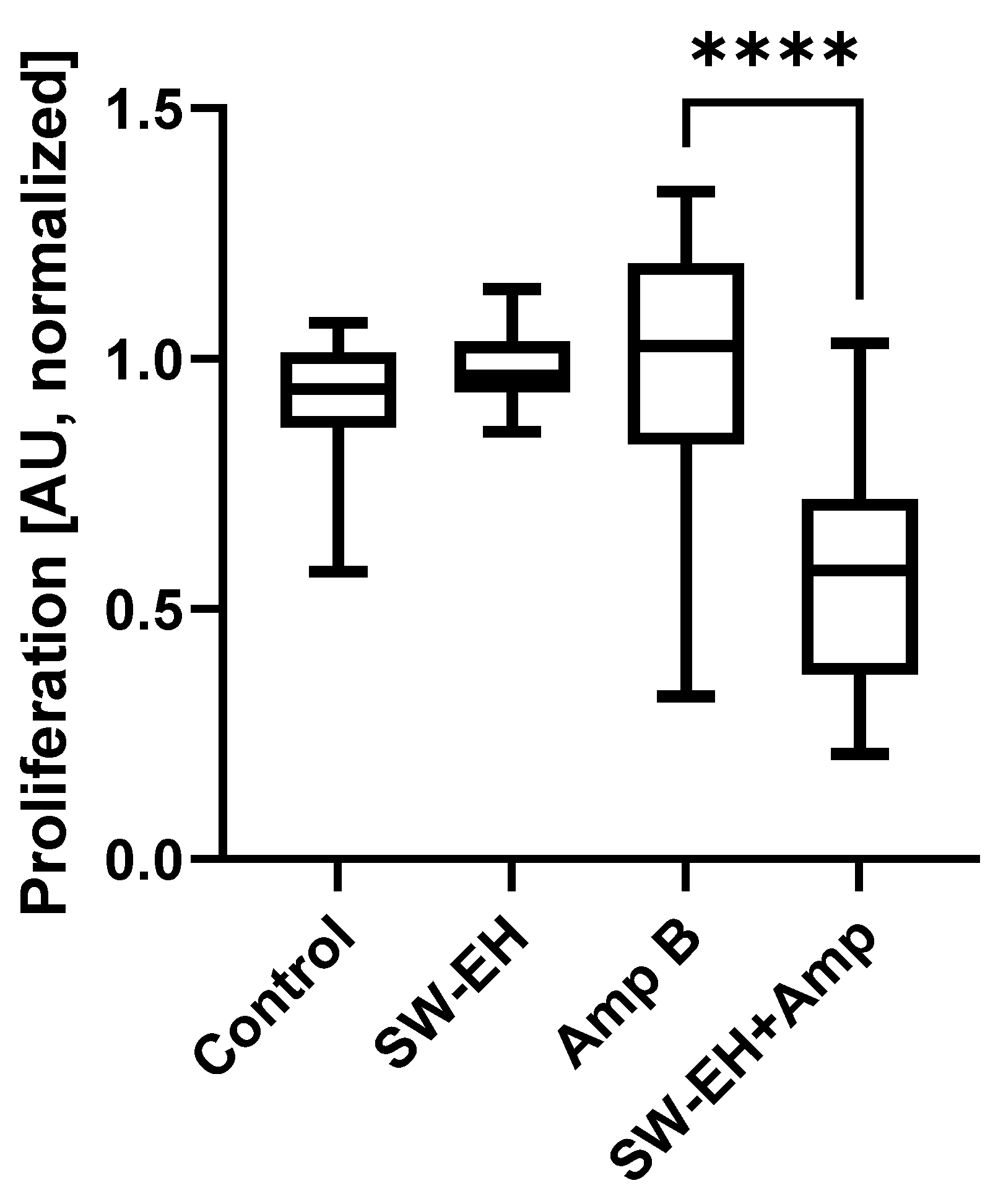
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Biofilm Formation
4.3. Ultrasound and Antifungal Treatment
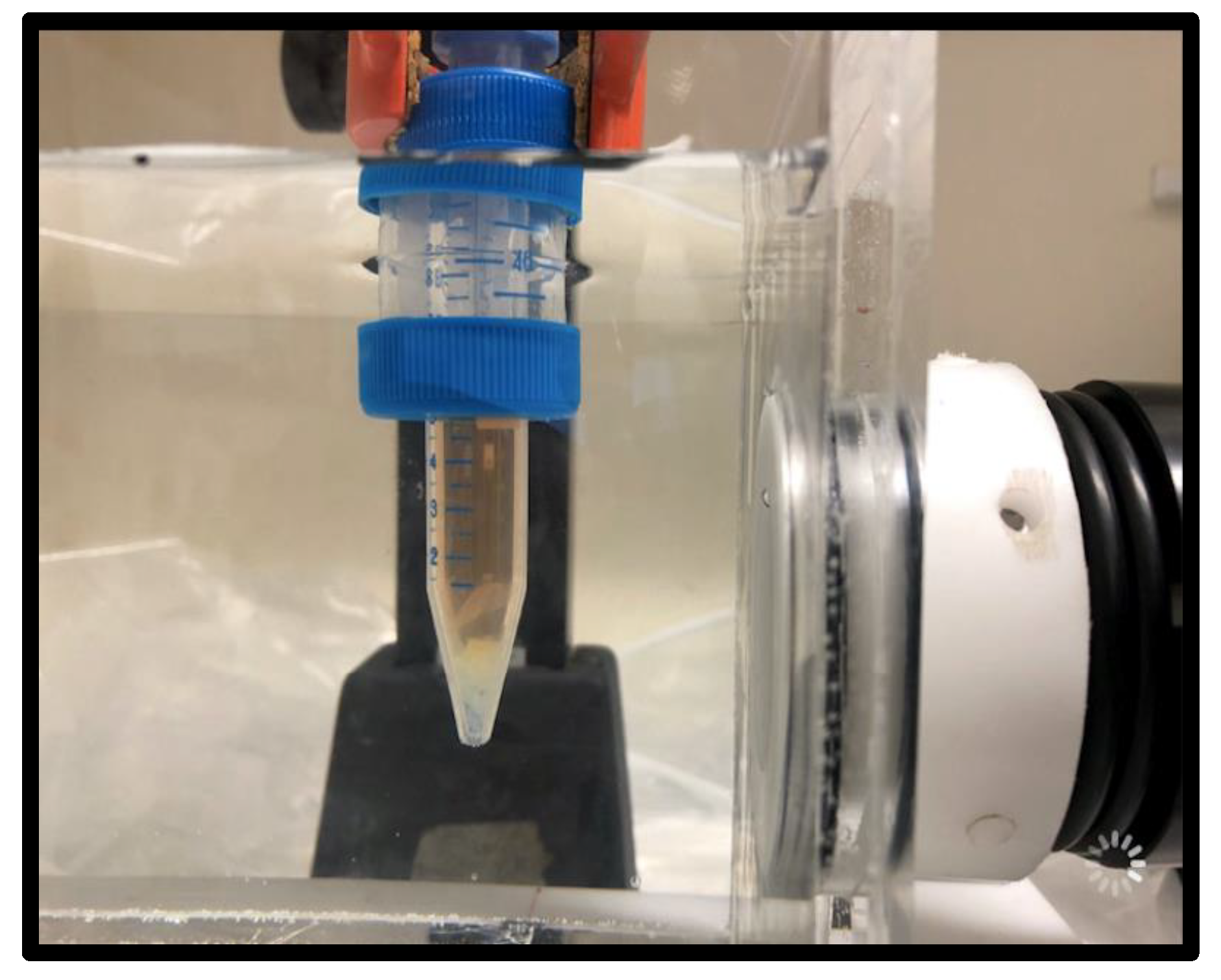
4.4. Shockwave or RPW and Antifungal Treatment
4.5. Biofilm Metabolic Assay
4.6. Biofilm Cell Proliferation Assay
4.7. Microscopy
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittermayr, R.; Antonic, V.; Hartinger, J.; Kaufmann, H.; Redl, H.; Téot, L.; Stojadinovic, A.; Schaden, W. Extracorporeal shock wave therapy (ESWT) for wound healing: Technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012, 20, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Taylor, Z.D.; Navarro, A.; Kealey, C.P.; Beenhouwer, D.; Haake, D.A.; Grundfest, W.S.; Gupta, V. Bacterial biofilm disruption using laser generated shockwaves. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 1028–1032. [Google Scholar] [CrossRef]
- Wanner, S.; Gstöttner, M.; Meirer, R.; Hausdorfer, J.; Fille, M.; Stöckl, B. Low-energy shock waves enhance the susceptibility of staphylococcal biofilms to antimicrobial agents in vitro. J. Bone Jt. Surg. Br. Vol. 2011, 93-B, 824–827. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ensing, G.T.; Roeder, B.L.; Nelson, J.L.; Horn, J.R.; der Mei, H.C.; Busscher, H.J.; Pitt, W.G. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J. Appl. Microbiol. 2005, 99, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Guggenheim, B.; Attin, T.; Marlinghaus, E.; Schmidlin, P.R. Potential of shock waves to remove calculus and biofilm. Clin. Oral Investig. 2011, 15, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.W.; Marques, L.L.R.; Howard, R.J.; Olson, M.E. Can filamentous fungi form biofilms? Trends Microbiol. 2009, 17, 475–480. [Google Scholar] [CrossRef]
- di Bonaventura, G.; Pompilio, A.; Picciani, C.; Iezzi, M.; D’Antonio, D.; Piccolomini, R. Biofilm Formation by the Emerging Fungal Pathogen Trichosporon asahii: Development, Architecture, and Antifungal Resistance. Antimicrob. Agents Chemother. 2006, 50, 3269–3276. [Google Scholar] [CrossRef]
- Ramage, G.; vande Walle, K.; Wickes, B.L.; López-Ribot, J.L. Standardized Method for In Vitro Antifungal Susceptibility Testing of Candida albicans Biofilms. Antimicrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef]
- Olson, M.E.; Ceri, H.; Morck, D.W. Interaction of Biofilms with Tissues. In Medical Biofilms; John Wiley & Sons, Ltd.: Chichester, UK, 2005; pp. 125–148. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Panda, N.; Shivaprakash, M.R.; Kaur, A.; Varma, S.C.; Singhi, S.; Bhansali, A.; Sakhuja, V. Invasive zygomycosis in India: Experience in a tertiary care hospital. Postgrad. Med. J. 2009, 85, 573–581. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Das, A.; Sharma, A.; Panda, N.; Das, S.; Gupta, K.L.; Sakhuja, V. Ten Years’ Experience in Zygomycosis at a Tertiary Care Centre in India. J. Infect. 2001, 42, 261–266. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Das, A.; Mandal, J.; Shivaprakash, M.R.; George, V.K.; Tarai, B.; Rao, P.; Panda, N.; Verma, S.C.; Sakhuja, V. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med. Mycol. 2006, 44, 335–342. [Google Scholar] [CrossRef]
- Burgess, J.L.; Birchall, R. Nephrotoxicity of amphotericin B, with emphasis on changes in tubular function. Am. J. Med. 1972, 53, 77–84. [Google Scholar] [CrossRef]
- Sabra, R.; Branch, R.A. Amphotericin B Nephrotoxicity. Drug Saf. 1990, 5, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.; Loesche, M.; Hodkinson, B.P.; Heilmann, K.; Ruthel, G.; Gardner, S.E.; Grice, E.A. Redefining the Chronic-Wound Microbiome: Fungal Communities Are Prevalent, Dynamic, and Associated with Delayed Healing. mBio 2016, 7, e01058-16. [Google Scholar] [CrossRef] [PubMed]
- Dowd, S.E.; Hanson, J.D.; Rees, E.; Wolcott, R.D.; Zischau, A.M.; Sun, Y.; White, J.; Smith, D.M.; Kennedy, J.; Jones, C.E. Survey of fungi and yeast in polymicrobial infections in chronic wounds. J. Wound Care 2011, 20, 40–47. [Google Scholar] [CrossRef]
- Kean, R.; Rajendran, R.; Haggarty, J.; Townsend, E.M.; Short, B.; Burgess, K.E.; Lang, S.; Millington, O.; Mackay, W.G.; Williams, C.; et al. Candida albicans Mycofilms Support Staphylococcus aureus Colonization and Enhances Miconazole Resistance in Dual-Species Interactions. Front. Microbiol. 2017, 8, 258. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal Protection of Staphylococcus aureus against Antimicrobials by Candida albicans Biofilm Matrix. mBio 2016, 7, e01365-16. [Google Scholar] [CrossRef]
- Kalan, L.; Grice, E.A. Fungi in the Wound Microbiome. Adv. Wound Care 2018, 7, 247–255. [Google Scholar] [CrossRef]
- Eckhard, M.; Lengler, A.; Liersch, J.; Bretzel, R.G.; Mayser, P. Fungal foot infections in patients with diabetes mellitus? results of two independent investigations. Mycoses 2007, 50, 14–19. [Google Scholar] [CrossRef]
- Weerasekera, M.M.; Kottahachchi, J.; Ranasinghe, K.N.P.; Dissanayake, M.S.S.; Prathapan, S.; Gunasekara, T.D.C.P.; Nagahawatte, A.; Guruge, L.D.; Bulugahapitiya, U.; Fernando, S.S.N.; et al. Proportion of lower limb fungal foot infections in patients with type 2 diabetes at a tertiary care hospital in Sri Lanka. Indian J. Endocrinol. Metab. 2014, 18, 63. [Google Scholar] [CrossRef]
- Seth, A.K.; Nguyen, K.T.; Geringer, M.R.; Hong, S.J.; Leung, K.P.; Mustoe, T.A.; Galiano, R.D. Noncontact, low-frequency ultrasound as an effective therapy {againstPseudomonas} aeruginosa-infected biofilm wounds. Wound Repair Regen. 2013, 21, 266–274. [Google Scholar] [CrossRef]
- Bharatula, L.D.; Marsili, E.; Rice, S.A.; Kwan, J.J. Influence of High Intensity Focused Ultrasound on the Microstructure and c-di-{GMP} Signaling of Pseudomonas aeruginosa Biofilms. Front. Microbiol. 2020, 11, 599407. [Google Scholar] [CrossRef] [PubMed]
- Durham, P.G.; Sidders, A.E.; Beam, J.E.; Kedziora, K.M.; Dayton, P.A.; Conlon, B.P.; Papadopoulou, V.; Rowe, S.E. Harnessing ultrasound-stimulated phase change contrast agents to improve antibiotic efficacy against methicillin-resistant Staphylococcus aureus biofilms. Biofilm 2021, 3, 100049. [Google Scholar] [CrossRef]
- Ennis, W.J.; Valdes, W.; Gainer, M.; Meneses, P. Evaluation of Clinical Effectiveness of {MIST} Ultrasound Therapy for the Healing of Chronic Wounds. Adv. Ski. Wound Care 2006, 19, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Fukumoto, Y.; Shimokawa, H. Extracorporeal Shock Wave Therapy as a New and Non-invasive Angiogenic Strategy. Tohoku J. Exp. Med. 2009, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Djedovic, G.; Kamelger, F.S.; Jeschke, J.; Piza-Katzer, H. Effect of Extracorporeal Shock Wave Treatment on Deep Partial-Thickness Burn Injury in Rats: A Pilot Study. Plast. Surg. Int. 2014, 2014, 495967. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Jiang, Z.; Han, L. Effect of low-energy extracorporeal shock wave on vascular regeneration after spinal cord injury and the recovery of motor function. Neuropsychiatr. Dis. Treat. 2016, 12, 2189–2198. [Google Scholar] [CrossRef]
- Slezak, C.; Rose, R.; Jilge, J.M.; Nuster, R.; Hercher, D.; Slezak, P. Physical Considerations for In Vitro ESWT Research Design. Int. J. Mol. Sci. 2021, 23, 313. [Google Scholar] [CrossRef]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Ohl, S.-W.; Klaseboer, E.; Khoo, B.C. Bubbles with shock waves and ultrasound: A review. Interface Focus 2015, 5, 20150019. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, M.; Jiang, H.; Li, D.; Du, Y. Effects of low-intensity and low-frequency ultrasound combined with tobramycin on biofilms of extended-spectrum beta-lactamases (ESBLs) Escherichia coli. FEMS Microbiol. Lett. 2019, 366, fnz026. [Google Scholar] [CrossRef]
- Su, H.; Li, Z.; Dong, Y.; Jiang, H.-X.; Zheng, H.-M.; Du, Y.-H.; Wu, J.; Wang, Z.-B. Damage Effects on Bacille Calmette-Guérin by Low-Frequency, Low-Intensity Ultrasound. J. Ultrasound Med. 2016, 35, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Coussios, C.C.; Farny, C.H.; ter Haar, G.; Roy, R.A. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU). Int. J. Hyperth. 2007, 23, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Ueda, M.; Kanzaki, H.; Tada, J.; Arata, J. Biofilm formation of Staphylococcus aureus strains isolated from impetigo and furuncle: Role of fibrinogen and fibrin. J. Dermatol. Sci. 1997, 16, 2–10. [Google Scholar] [CrossRef]
- Souza, M.C.; dos Santos, L.S.; Sousa, L.P.; Faria, Y.V.; Ramos, J.N.; Sabbadini, P.S.; da Santos, C.S.; Nagao, P.E.; Vieira, V.V.; Gomes, D.L.R.; et al. Biofilm formation and fibrinogen and fibronectin binding activities by Corynebacterium pseudodiphtheriticum invasive strains. Antonie Van Leeuwenhoek 2015, 107, 1387–1399. [Google Scholar] [CrossRef]
- Thaarup, I.C.; Bjarnsholt, T. Current In Vitro Biofilm-Infected Chronic Wound Models for Developing New Treatment Possibilities. Adv. Wound Care 2021, 10, 91–102. [Google Scholar] [CrossRef]
- Bouchara, J.P.; Bouali, A.; Tronchin, G.; Robert, R.; Chabasse, D.; Senet, J.M. Binding of fibrinogen to the pathogenic Aspergillus species. J. Med. Vet. Mycol. 1988, 26, 327–334. [Google Scholar] [CrossRef]
- Bertesteanu, S.; Triaridis, S.; Stankovic, M.; Lazar, V.; Chifiriuc, M.C.; Vlad, M.; Grigore, R. Polymicrobial wound infections: Pathophysiology and current therapeutic approaches. Int. J. Pharm. 2014, 463, 119–126. [Google Scholar] [CrossRef]
- Spotnitz, W.D. Fibrin sealant: Past, present, and future: A brief review. World J. Surg. 2010, 34, 632–634. [Google Scholar] [CrossRef]
- Jawhara, S. How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response. Antibiotics 2020, 9, 385. [Google Scholar] [CrossRef]
- Gollmann-Tepeköylü, C.; Pölzl, L.; Graber, M.; Hirsch, J.; Nägele, F.; Lobenwein, D.; Hess, M.W.; Blumer, M.J.; Kirchmair, E.; Zipperle, J.; et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc. Res. 2020, 116, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, L.; Wei, Y.; Krishnamurthy, P.; Walcott, G.P.; Menasché, P.; Zhang, J. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci. Transl. Med. 2020, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Nicolás-Boluda, A.; Mulens-Arias, V.; Richard, S.; Rahmi, G.; Gazeau, F.; Wilhelm, C.; Silva, A.K.A. Extracellular vesicles for personalized medicine: The input of physically triggered production, loading and theranostic properties. Adv. Drug Deliv. Rev. 2019, 138, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bruni, G.O.; Taylor, C.M.; Zhang, Z.; Wang, P. Comparative genome-wide analysis of extracellular small RNAs from the mucormycosis pathogen Rhizopus delemar. Sci. Rep. 2018, 8, 5243. [Google Scholar] [CrossRef]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Février, B.; Raposo, G. Exosomes: Endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004, 16, 415–421. [Google Scholar] [CrossRef]
- Weihs, A.M.; Fuchs, C.; Teuschl, A.H.; Hartinger, J.; Slezak, P.; Mittermayr, R.; Redl, H.; Junger, W.G.; Sitte, H.H.; Rünzler, D. Shock Wave Treatment Enhances Cell Proliferation and Improves Wound Healing by ATP Release-coupled Extracellular Signal-regulated Kinase (ERK) Activation. J. Biol. Chem. 2014, 289, 27090–27104. [Google Scholar] [CrossRef]
- Liao, Y.; Gose, J.W.; Arruda, E.M.; Liu, A.P.; Merajver, S.D.; Young, Y.L. Shock wave impact on the viability of MDA-MB-231 cells. PLoS ONE 2020, 15, e0234138. [Google Scholar] [CrossRef]
- Li, W.; Ma, H.; He, R.; Ren, X.; Zhou, C. Prospects and application of ultrasound and magnetic fields in the fermentation of rare edible fungi. Ultrason. Sonochem. 2021, 76, 105613. [Google Scholar] [CrossRef]
- Ito, Y.; Veysset, D.; Kooi, S.E.; Martynowych, D.; Nakagawa, K.; Nelson, K.A. Interferometric and fluorescence analysis of shock wave effects on cell membrane. Commun. Phys. 2020, 3, 124. [Google Scholar] [CrossRef]
- Bozza, S.; Clavaud, C.; Giovannini, G.; Fontaine, T.; Beauvais, A.; Sarfati, J.; D’Angelo, C.; Perruccio, K.; Bonifazi, P.; Zagarella, S.; et al. Immune Sensing of Aspergillus fumigatus Proteins, Glycolipids, and Polysaccharides and the Impact on Th Immunity and Vaccination. J. Immunol. 2009, 183, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J. 30 years of battling the cell wall. Med. Mycol. 2017, 55, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Balzer, J.; Heuer, K.; Demir, E.; Hoffmanns, M.A.; Baldus, S.; Fuchs, P.C.; Awakowicz, P.; Suschek, C.V.; Opländer, C. Non-Thermal Dielectric Barrier Discharge (DBD) Effects on Proliferation and Differentiation of Human Fibroblasts Are Primary Mediated by Hydrogen Peroxide. PLoS ONE 2015, 10, e0144968. [Google Scholar] [CrossRef] [PubMed]
- Quent, V.M.C.; Loessner, D.; Friis, T.; Reichert, J.C.; Hutmacher, D.W. Discrepancies between metabolic activity and DNA content as tool to assess cell proliferation in cancer research. J. Cell. Mol. Med. 2010, 14, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Holfeld, J.; Tepeköylü, C.; Kozaryn, R.; Urbschat, A.; Zacharowski, K.; Grimm, M.; Paulus, P. Shockwave Therapy Differentially Stimulates Endothelial Cells: Implications on the Control of Inflammation via Toll-Like Receptor 3. Inflammation 2014, 37, 65–70. [Google Scholar] [CrossRef]
- Williams, C. Hydrosoluble formazan XTT: Its application to natural products drug discovery for Leishmania. J. Microbiol. Methods 2003, 55, 813–816. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slezak, C.; Anderson, K.; Hillock, T.; Miller, M.; Dungel, P.; Kopp, O.; Sterflinger, K.; Slezak, P. Shockwaves Increase In Vitro Resilience of Rhizopus oryzae Biofilm under Amphotericin B Treatment. Int. J. Mol. Sci. 2022, 23, 9226. https://doi.org/10.3390/ijms23169226
Slezak C, Anderson K, Hillock T, Miller M, Dungel P, Kopp O, Sterflinger K, Slezak P. Shockwaves Increase In Vitro Resilience of Rhizopus oryzae Biofilm under Amphotericin B Treatment. International Journal of Molecular Sciences. 2022; 23(16):9226. https://doi.org/10.3390/ijms23169226
Chicago/Turabian StyleSlezak, Cyrill, Karaleen Anderson, Tyson Hillock, Mariel Miller, Peter Dungel, Olga Kopp, Katja Sterflinger, and Paul Slezak. 2022. "Shockwaves Increase In Vitro Resilience of Rhizopus oryzae Biofilm under Amphotericin B Treatment" International Journal of Molecular Sciences 23, no. 16: 9226. https://doi.org/10.3390/ijms23169226
APA StyleSlezak, C., Anderson, K., Hillock, T., Miller, M., Dungel, P., Kopp, O., Sterflinger, K., & Slezak, P. (2022). Shockwaves Increase In Vitro Resilience of Rhizopus oryzae Biofilm under Amphotericin B Treatment. International Journal of Molecular Sciences, 23(16), 9226. https://doi.org/10.3390/ijms23169226






