Dermatopontin Influences the Development of Obesity-Associated Colon Cancer by Changes in the Expression of Extracellular Matrix Proteins
Abstract
1. Introduction
2. Results
2.1. Colon Cancer Decreases DPT Circulating Levels and Its mRNA Expression in the Colon
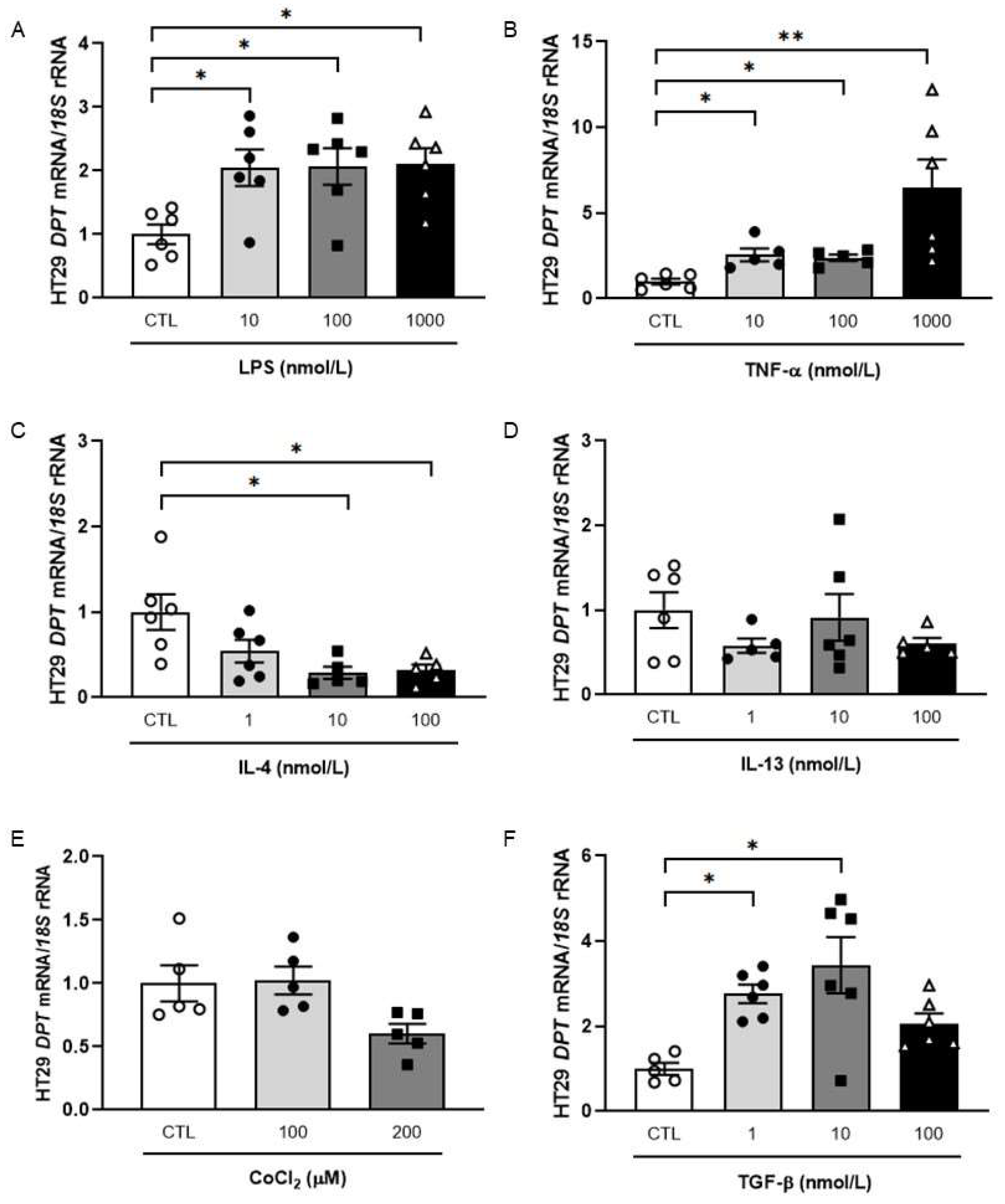
2.2. Influence of Inflammation-Related Factors in the Expression of DPT in HT-29 Cells
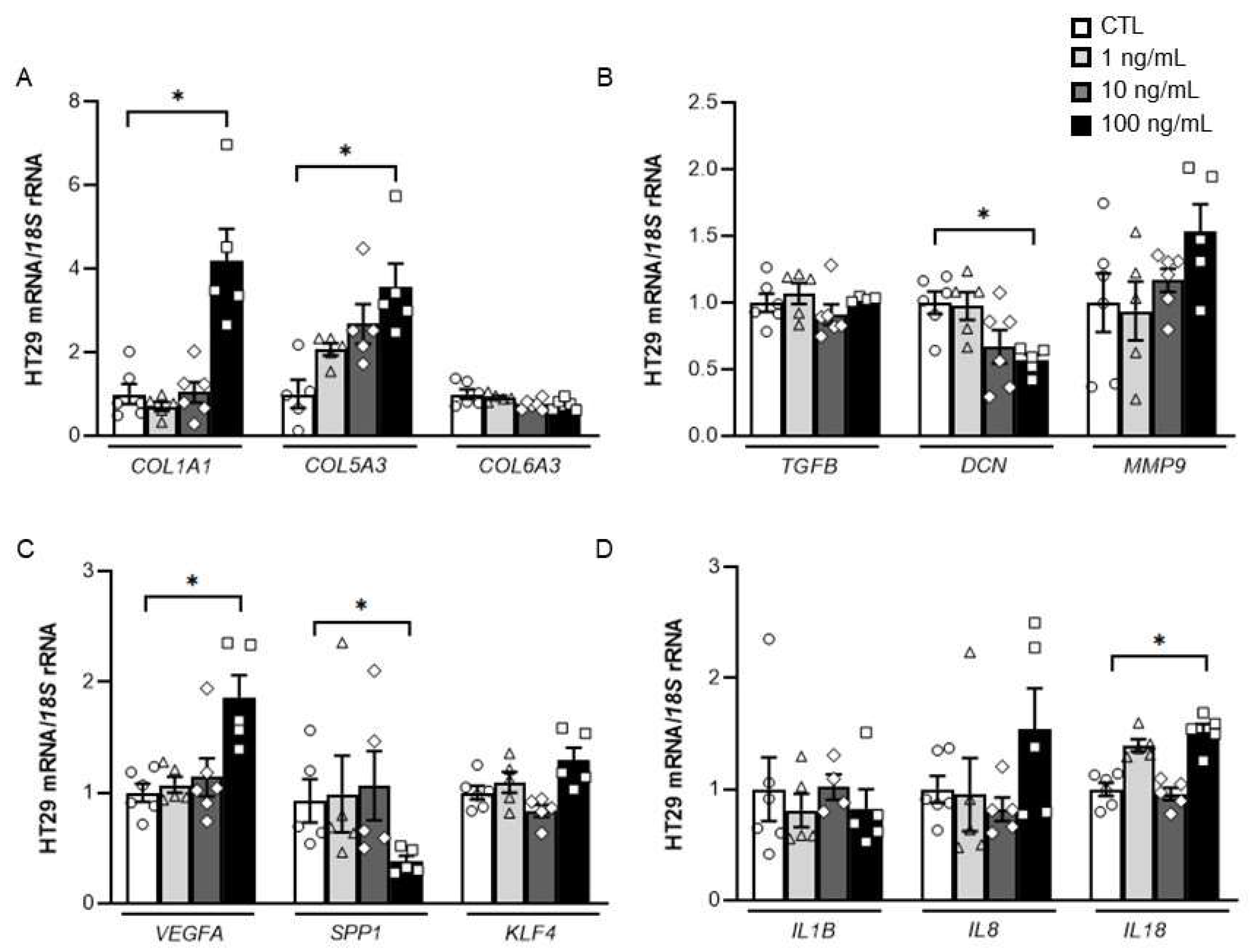
2.3. Impact of DPT on the Expression of Key ECM Remodelling-Related Genes in HT-29 Cells
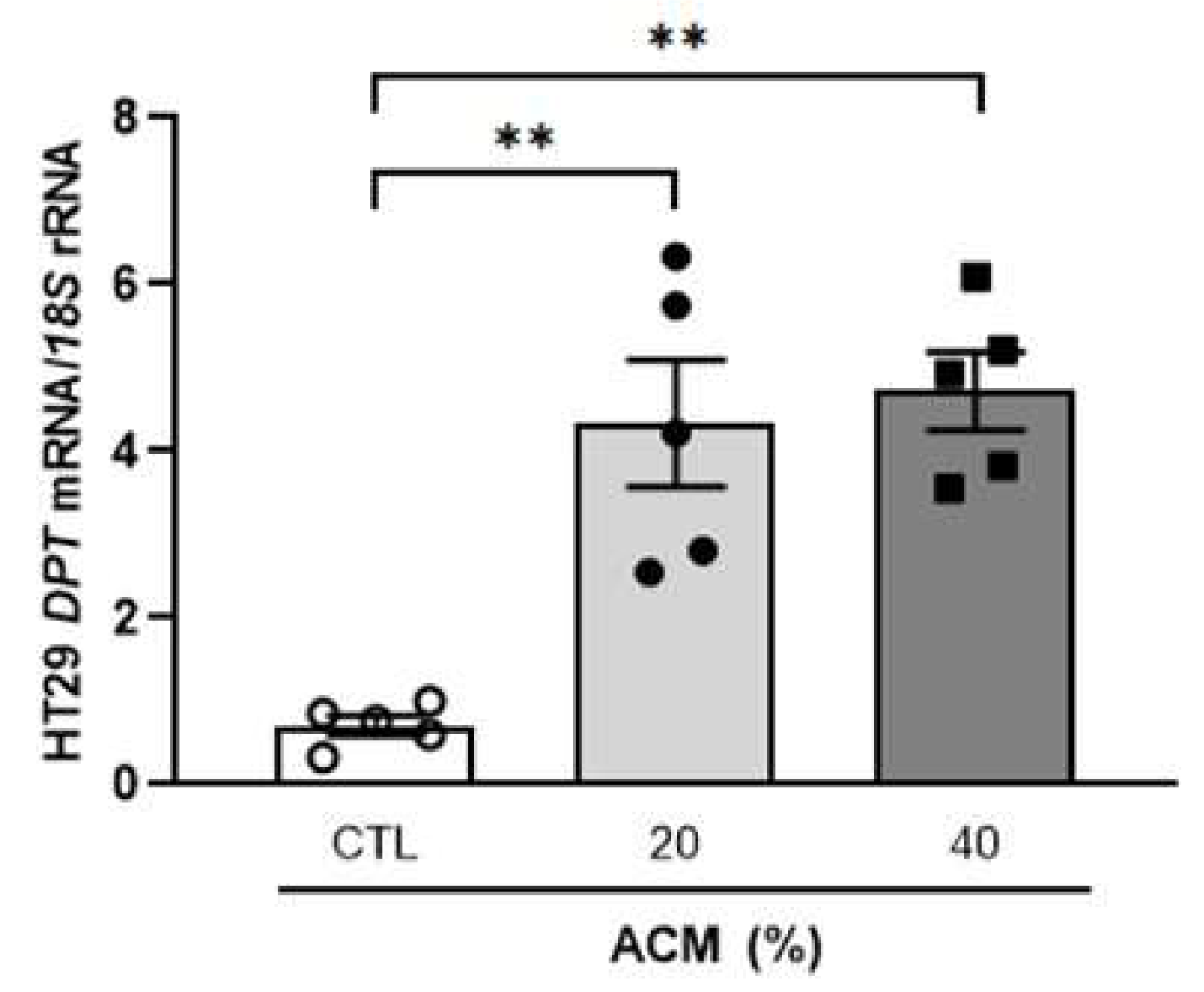
2.4. Adipocyte-Conditioned Media Upregulate DPT Expression Levels in Tumour Cells
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Analytical Measurements
4.3. RNA Isolation and Real-Time PCR
4.4. Cell Cultures
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, A.G.; Stevens, G.A.; Ezzati, M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015, 16, 36–46. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.C.C. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet Gastroenterol. Hepatol. 2019, 4, 913–933. [Google Scholar]
- Song, M.; Giovannucci, E. Preventable Incidence and Mortality of Carcinoma Associated With Lifestyle Factors Among White Adults in the United States. JAMA Oncol. 2016, 2, 1154–1161. [Google Scholar] [CrossRef]
- Fleming, C.A.; O’Connell, E.P.; Kavanagh, R.G.; O’Leary, D.P.; Twomey, M.; Corrigan, M.A.; Wang, J.H.; Maher, M.M.; O’Connor, O.J.; Redmond, H.P. Body Composition, Inflammation, and 5-Year Outcomes in Colon Cancer. JAMA Netw. Open 2021, 4, e2115274. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.; Park, J. Extracellular matrix remodeling facilitates obesity-associated cancer progression. Trends Cell Biol. 2022. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef]
- Herrera, J.; Henke, C.A.; Bitterman, P. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Catalán, V.; Gomez-Ambrosi, J.; Rodríguez, A.; Frühbeck, G. Role of extracellular matrix remodelling in adipose tissue pathophysiology: Relevance in the development of obesity. Histol. Histopathol. 2012, 27, 1515–1528. [Google Scholar]
- Unamuno, X.; Gómez-Ambrosi, J.; Becerril, S.; Álvarez-Cienfuegos, F.J.; Ramírez, B.; Rodríguez, A.; Ezquerro, S.; Valentí, V.; Moncada, R.; Mentxaka, A.; et al. Changes in mechanical properties of adipose tissue after bariatric surgery driven by extracellular matrix remodelling and neovascularization are associated with metabolic improvements. Acta Biomater. 2022, 141, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Kang, I.; Harten, I.A.; Gebe, J.A.; Chan, C.K.; Omer, M.; Alonge, K.M.; Hartigh, L.J.D.; Kjerulf, D.G.; Goodspeed, L.; et al. Adipocyte-Derived Versican and Macrophage-Derived Biglycan Control Adipose Tissue Inflammation in Obesity. Cell Rep. 2020, 31, 107818. [Google Scholar] [CrossRef] [PubMed]
- Hepler, C.; Shan, B.; Zhang, Q.; Henry, G.H.; Shao, M.; Vishvanath, L.; Ghaben, A.L.; Mobley, A.B.; Strand, D.; Hon, G.C.; et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 2018, 7, e39636. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Neame, P.J.; Choi, H.U.; Rosenberg, L.C. The Isolation and Primary Structure of a 22-kDa Extracellular Matrix Protein from Bovine Skin. J. Biol. Chem. 1989, 264, 5474–5479. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Ramírez, B.; Rodríguez, A.; Becerril, S.; Valentí, V.; Moncada, R.; Silva, C.; Salvador, J.; Frühbeck, G.; et al. Dermatopontin, A Novel Adipokine Promoting Adipose Tissue Extracellular Matrix Remodelling and Inflammation in Obesity. J. Clin. Med. 2020, 9, 1069. [Google Scholar] [CrossRef]
- Takeda, U.; Utani, A.; Wu, J.; Shinkai, H.; Adachi, E.; Koseki, H.; Taniguchi, M.; Matsumoto, T.; Ohashi, T.; Sato, M. Targeted Disruption of Dermatopontin Causes Abnormal Collagen Fibrillogenesis. J. Investig. Dermatol. 2002, 119, 678–683. [Google Scholar] [CrossRef]
- Lefebvre, P.; Lalloyer, F.; Bauge, E.; Pawlak, M.; Gheeraert, C.; Dehondt, H.; Vanhoutte, J.; Woitrain, E.; Hennuyer, N.; Mazuy, C.; et al. Interspecies NASH disease activity whole-genome profiling identifies a fibrogenic role of PPARa-regulated dermatopontin. JCI Insight 2017, 2, e92264. [Google Scholar] [CrossRef]
- Okamoto, O.; Fujiwara, S. Dermatopontin, a Novel Player in the Biology of the Extracellular Matrix. Connect. Tissue Res. 2006, 47, 177–189. [Google Scholar] [CrossRef]
- Okamoto, O.; Fujiwara, S.; Abe, M.; Sato, Y. Dermatopontin interacts with transforming growth factor b and enhances its biological activity. Biochem J. 1999, 337, 537–541. [Google Scholar] [CrossRef]
- Okamoto, O.; Hozumi, K.; Katagiri, F.; Takahashi, N.; Sumiyoshi, H.; Matsuo, N.; Yoshioka, H.; Nomizu, M.; Fujiwara, S. Dermatopontin promotes epidermal keratinocyte adhesion via a3b1 integrin and a proteoglycan receptor. Biochemistry 2010, 49, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Sadr-Nabavi, A.; Bouromand-Noughabi, S.; Tayebi-Meybodi, N.; Dadkhah, K.; Amini, N.; Meindl, A.; Abbaszadegan, M.R. Non-collagenous extracellular matrix protein dermatopontin may play a role as another component of trans-forming growth factor-b signaling pathway in colon carcinogenesis. Iran J. Basic Med. Sci. 2021, 24, 444–450. [Google Scholar] [PubMed]
- Guo, Y.; Li, H.; Guan, H.; Ke, W.; Liang, W.; Xiao, H.; Li, Y. Dermatopontin inhibits papillary thyroid cancer cell proliferation through MYC repression. Mol. Cell. Endocrinol. 2019, 480, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qiu, J.; He, G.; Geng, C.; He, W.; Liu, C.; Cai, D.; Pan, H.; Tian, Q. Dermatopontin inhibits WNT signaling pathway via CXXC finger protein 4 in hepatocellular carcinoma. J. Cancer 2020, 11, 6288–6298. [Google Scholar] [CrossRef]
- Dahl, E.; Sadr-Nabavi, A.; Klopocki, E.; Betz, B.; Grube, S.; Kreutzfeld, R.; Himmelfarb, M.; An, H.-X.; Gelling, S.; Klaman, I.; et al. Systematic identification and molecular characterization of genes differentially expressed in breast and ovarian cancer. J. Pathol. 2005, 205, 21–28. [Google Scholar] [CrossRef]
- Yamatoji, M.; Kasamatsu, A.; Kouzu, Y.; Koike, H.; Sakamoto, Y.; Ogawara, K.; Shiiba, M.; Tanzawa, H.; Uzawa, K. Dermatopontin: A potential predictor for metastasis of human oral cancer. Int. J. Cancer 2012, 130, 2903–2911. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Catalán, V.; Aviles-Olmos, I.; Rodríguez, A.; Becerril, S.; Fernández-Formoso, J.A.; Kiortsis, D.; Portincasa, P.; Gómez-Ambrosi, J.; Frühbeck, G. Time to consider the “Exposome Hypothesis” in the development of the obesity pandemic. Nutrients 2022, 14, 1597. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Alfano, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Burger, R.A.; Chlebowski, R.T.; Fabian, C.J.; Gucalp, A.; Hershman, D.L.; Hudson, M.M.; et al. American Society of Clinical Oncology Position Statement on Obesity and Cancer. J. Clin. Oncol. 2014, 32, 3568–3574. [Google Scholar] [CrossRef]
- Yarnoz-Esquiroz, P.; Olazaran, L.; Aguas-Ayesa, M.; Perdomo, C.M.; García-Goñi, M.; Silva, C.; Fernández-Formoso, J.A.; Escalada, J.; Montecucco, F.; Portincasa, P.; et al. ‘Obesities’: Position statement on a complex disease entity with multifaceted drivers. Eur. J. Clin. Invest. 2022, 52, e13811. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Frühbeck, G. Adipose tissue immunity and cancer. Front. Physiol. 2013, 4, 275. [Google Scholar] [CrossRef]
- Frühbeck, G.; Gómez-Ambrosi, J. Control of body weight: A physiologic and transgenic perspective. Diabetologia 2003, 46, 143–172. [Google Scholar] [CrossRef]
- Pérez-Hernandez, A.I.; Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Frühbeck, G. Mechanisms linking excess adiposity and carcinogenesis promotion. Front Endocrinol. 2014, 5, 65. [Google Scholar]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef]
- Li, X.; Feng, P.; Ou, J.; Luo, Z.; Dai, P.; Wei, D.; Zhang, C. Dermatopontin is expressed in human liver and is downregulated in hepatocellular carcinoma. Biochemistry 2009, 74, 979–985. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Derynck, R. TGF-b signaling in cancer-a double-edged sword. Trends Cell Biol. 2001, 11, S44–S51. [Google Scholar]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-b signaling in tumor suppression and cancer progression. Nat Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kramer, A.; Astuti, Y.; Elfstrum, A.; Lehrke, M.J.; Tolar, J.; Blazar, B.R.; Blake, A.; Taisto, M.; Furcich, J.W.; Nolan, E.E.; et al. An irradiated marrow niche reveals a small non-collagenous protein mediator of homing, dermatopontin. Blood Adv. 2021, 5, 3609–3622. [Google Scholar] [CrossRef]
- Kuroda, K.; Okamoto, O.; Shinkai, H. Dermatopontin expression is decreased in hypertrophic scar and systemic sclerosis skin fibroblasts and is regulated by transforming growth factor-b1, interleukin-4, and matrix collagen. J. Investig. Dermatol. 1999, 112, 706–710. [Google Scholar] [CrossRef][Green Version]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Okamoto, O.; Suzuki, Y.; Kimura, S.; Shinkai, H. Extracellular Matrix 22-kDa Protein Interacts with Decorin Core Protein and Is Expressed in Cutaneous Fibrosis. J. Biochem. 1996, 119, 106–114. [Google Scholar] [CrossRef]
- MacBeath, J.; Shackleton, D.; Hulmes, D. Tyrosine-rich acidic matrix protein (TRAMP) accelerates collagen fibril formation in vitro. J. Biol. Chem. 1993, 268, 19826–19832. [Google Scholar] [CrossRef]
- Vogel, K.G.; Paulsson, M.; Heinegård, D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem. J. 1984, 223, 587–597. [Google Scholar] [CrossRef]
- Mao, L.; Yang, J.; Yue, J.; Chen, Y.; Zhou, H.; Fan, D.; Zhang, Q.; Buraschi, S.; Iozzo, R.V.; Bi, X. Decorin deficiency promotes epithelial-mesenchymal transition and colon cancer metastasis. Matrix Biol. 2021, 95, 1–14. [Google Scholar] [CrossRef]
- Krishnaswamy, V.R.; Balaguru, U.M.; Chatterjee, S.; Korrapati, P.S. Dermatopontin augments angiogenesis and modulates the expression of transforming growth factor b1 and integrin a3b1 in endothelial cells. Eur. J. Cell Biol. 2017, 96, 266–275. [Google Scholar] [CrossRef]
- Amilca-Seba, K.; Sabbah, M.; Larsen, A.; Denis, J. Osteopontin as a Regulator of Colorectal Cancer Progression and Its Clinical Applications. Cancers 2021, 13, 3793. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 356. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Izaguirre, M.; Hernández-Lizoain, J.L.; Baixauli, J.; Martí, P.; Valentí, V.; Moncada, R.; et al. Increased obesity-associated circulating levels of the extracellular matrix proteins os-teopontin, chitinase-3 like-1 and tenascin C are associated with colon cancer. PLoS ONE 2016, 11, e0162189. [Google Scholar]
- Johnston, N.I.F.; Gunasekharan, V.K.; Ravindranath, A.; O’Connell, C.; Johnston, P.G.; El-Tanani, M.K. Osteopontin as a target for cancer therapy. Front. Biosci. 2008, 13, 4361–4372. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Silva, C.; Rotellar, F.; Hernández-Lizoain, J.L.; Baixauli, J.; Valentí, V.; Pardo, F.; et al. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. J. Nutr. Biochem. 2011, 22, 634–641. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef]
- Frühbeck, G.; Mentxaka, A.; Ahechu, P.; Gómez-Ambrosi, J.; Ramírez, B.; Becerril, S.; Rodríguez, A.; Unamuno, X.; Cienfuegos, J.A.; Casado, M.; et al. The Differential Expression of the Inflammasomes in Adipose Tissue and Colon Influences the Development of Colon Cancer in a Context of Obesity by Regulating Intestinal Inflammation. J. Inflamm. Res. 2021, 14, 6431–6446. [Google Scholar] [CrossRef]
- Pulido, M.R.; Diaz-Ruiz, A.; Jiménez-Gómez, Y.; Garcia-Navarro, S.; Gracia-Navarro, F.; Tinahones, F.; López-Miranda, J.; Frühbeck, G.; Vázquez-Martínez, R.; Malagón, M.M. Rab18 Dynamics in Adipocytes in Relation to Lipogenesis, Lipolysis and Obesity. PLoS ONE 2011, 6, e22931. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Silva, C.; Catalán, V.; Rodríguez, A.; Galofré, J.C.; Escalada, J.; Valentí, V.; Rotellar, F.; Romero, S.; Ramírez, B.; et al. Clinical Usefulness of a New Equation for Estimating Body Fat. Diabetes Care 2012, 35, 383–388. [Google Scholar] [CrossRef]
- Fortuño, A.; Rodríguez, A.; Gómez-Ambrosi, J.; Muniz, P.; Salvador, J.; Diez, J.; Frühbeck, G. Leptin inhibits an-giotensin II-induced intracellular calcium increase and vasoconstriction in the rat aorta. Endocrinology 2002, 143, 3555–3560. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Ortega, V.A.; Hernández-Lizoain, J.L.; Baixauli, J.; Becerril, S.; Rotellar, F.; Valentí, V.; et al. IL-32α-induced inflammation constitutes a link between obesity and colon cancer. OncoImmunology 2017, 6, e1328338. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gomez-Ambrosi, J.; Catalan, V.; Rotellar, F.; Valentí, V.; Silva, C.; Mugueta, M.D.C.; Pulido, M.R.; Vázquez, R.; Salvador, J.; et al. The ghrelin O-acyltransferase–ghrelin system reduces TNF-α-induced apoptosis and autophagy in human visceral adipocytes. Diabetologia 2012, 55, 3038–3050. [Google Scholar] [CrossRef]

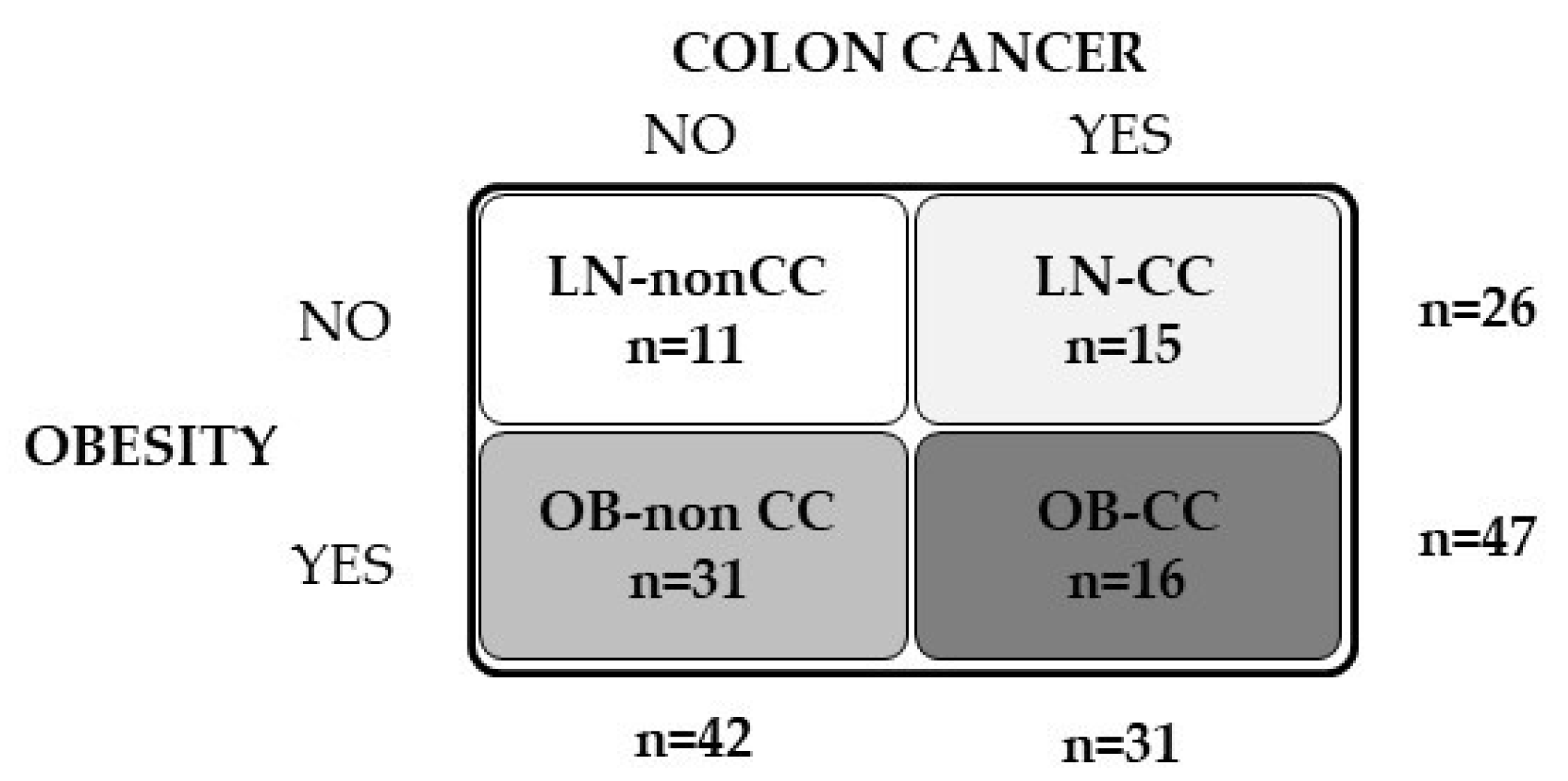
| Lean | OB | ||||||
|---|---|---|---|---|---|---|---|
| non-CC | CC | non-CC | CC | p OB | p CC | p OBxCC | |
| n (male, female) | 11 (7, 4) | 15 (8, 7) | 31 (19, 12) | 16 (9, 7) | |||
| Age (years) | 53 ± 3 | 63 ± 3 | 55 ± 1 | 62 ± 3 | 0.518 | <0.001 | 0.925 |
| Body weight (kg) | 65.4 ± 1.9 | 63.4 ± 1.8 | 84.9 ± 2.1 | 81.1 ± 2.9 | <0.001 | 0.303 | 0.753 |
| Body mass index (kg/m2) | 23.1 ± 0.2 | 22.5 ± 0.4 | 36.7 ± 0.7 | 34.2 ± 0.8 | <0.001 | 0.065 | 0.772 |
| Estimated body fat (%) | 27.8 ± 2.2 | 29.1 ± 1.4 | 36.7 ± 1.2 | 34.2 ± 1.8 | <0.001 | 0.268 | 0.369 |
| Waist circumference (cm) | 83 ± 2 | 80 ± 2 | 100 ± 2 | 115 ± 7 | <0.001 | 0.270 | 0.228 |
| Fasting glucose (mg/dL) | 103 ± 5 | 142 ± 12 | 113 ± 5 | 127 ± 11 | 0.775 | 0.020 | 0.221 |
| Free fatty acids (mg/dL) | 11.7 ± 1.5 | 26.5 ± 2.4 | 15.2 ±1.2 | 22.6 ± 2.1 | 0.893 | <0.001 | 0.086 |
| Triglycerides (mg/dL) | 93 ± 15 | 116 ± 11 | 118 ± 13 | 151 ± 28 | 0.079 | 0.398 | 0.324 |
| CRP (mg/L) | 0.90 ± 0.07 | 1.39 ± 0.82 | 1.63 ± 0.09 | 9.64 ± 1.49 *** | <0.001 | <0.001 | <0.001 |
| Fibrinogen (mg/dL) | 330 ± 18 | 273 ± 26 | 303 ± 20 | 447 ± 48 ‡ | 0.203 | 0.398 | 0.017 |
| CEA (ng/mL) | 1.58 ± 0.32 | 2.55 ± 0.44 | 1.68 ± 0.28 | 8.41 ± 2.60 | 0.267 | 0.021 | 0.401 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalán, V.; Domench, P.; Gómez-Ambrosi, J.; Ramírez, B.; Becerril, S.; Mentxaka, A.; Rodríguez, A.; Valentí, V.; Moncada, R.; Baixauli, J.; et al. Dermatopontin Influences the Development of Obesity-Associated Colon Cancer by Changes in the Expression of Extracellular Matrix Proteins. Int. J. Mol. Sci. 2022, 23, 9222. https://doi.org/10.3390/ijms23169222
Catalán V, Domench P, Gómez-Ambrosi J, Ramírez B, Becerril S, Mentxaka A, Rodríguez A, Valentí V, Moncada R, Baixauli J, et al. Dermatopontin Influences the Development of Obesity-Associated Colon Cancer by Changes in the Expression of Extracellular Matrix Proteins. International Journal of Molecular Sciences. 2022; 23(16):9222. https://doi.org/10.3390/ijms23169222
Chicago/Turabian StyleCatalán, Victoria, Paula Domench, Javier Gómez-Ambrosi, Beatriz Ramírez, Sara Becerril, Amaia Mentxaka, Amaia Rodríguez, Víctor Valentí, Rafael Moncada, Jorge Baixauli, and et al. 2022. "Dermatopontin Influences the Development of Obesity-Associated Colon Cancer by Changes in the Expression of Extracellular Matrix Proteins" International Journal of Molecular Sciences 23, no. 16: 9222. https://doi.org/10.3390/ijms23169222
APA StyleCatalán, V., Domench, P., Gómez-Ambrosi, J., Ramírez, B., Becerril, S., Mentxaka, A., Rodríguez, A., Valentí, V., Moncada, R., Baixauli, J., Silva, C., Escalada, J., & Frühbeck, G. (2022). Dermatopontin Influences the Development of Obesity-Associated Colon Cancer by Changes in the Expression of Extracellular Matrix Proteins. International Journal of Molecular Sciences, 23(16), 9222. https://doi.org/10.3390/ijms23169222








