Human Pluripotent Stem Cell-Derived Alveolar Organoid with Macrophages
Abstract
:1. Introduction
2. Results
2.1. Construction of 3D Lung Organoid Differentiation
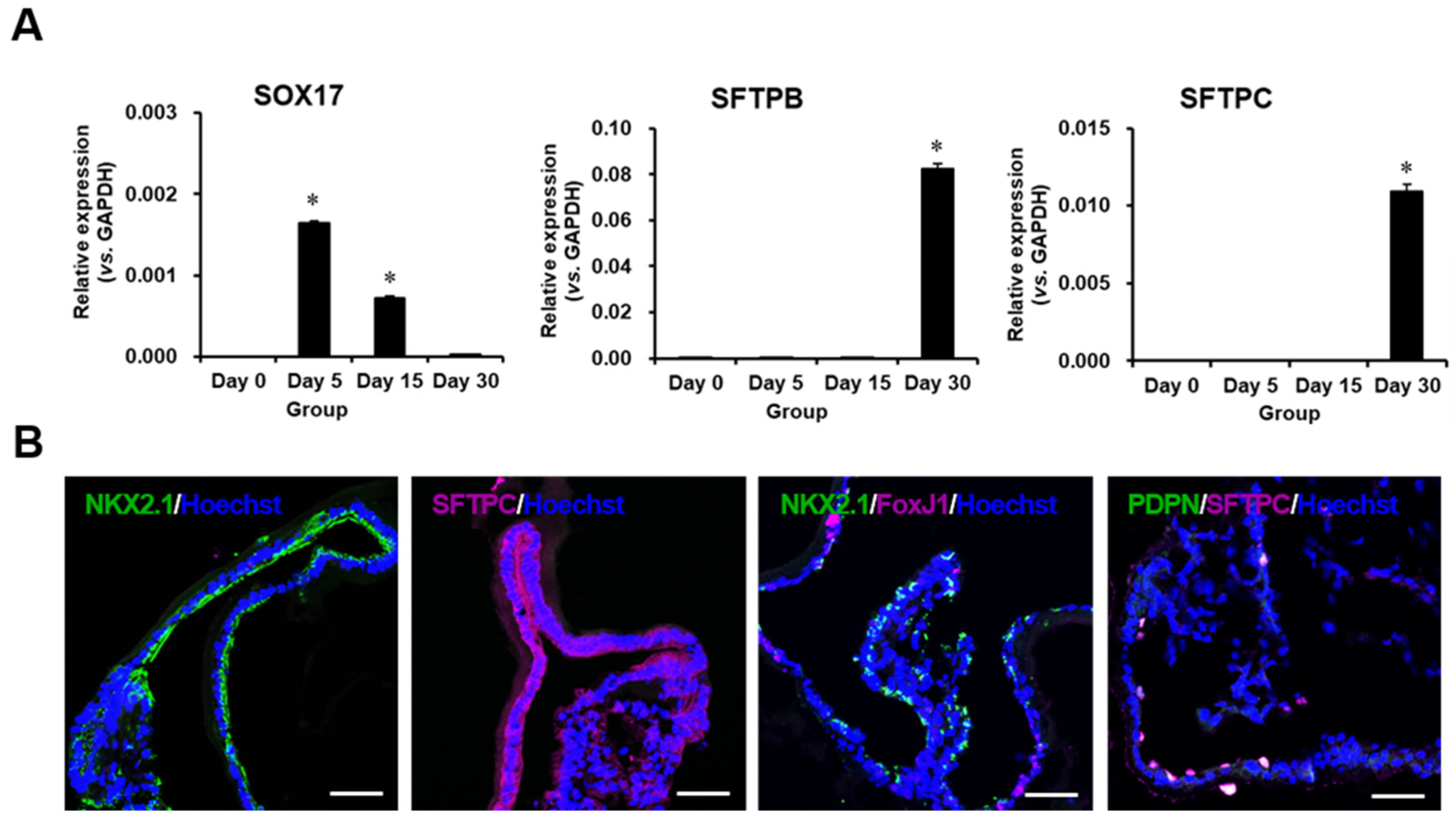
2.2. Characterization of Differentiated Lung Organoids
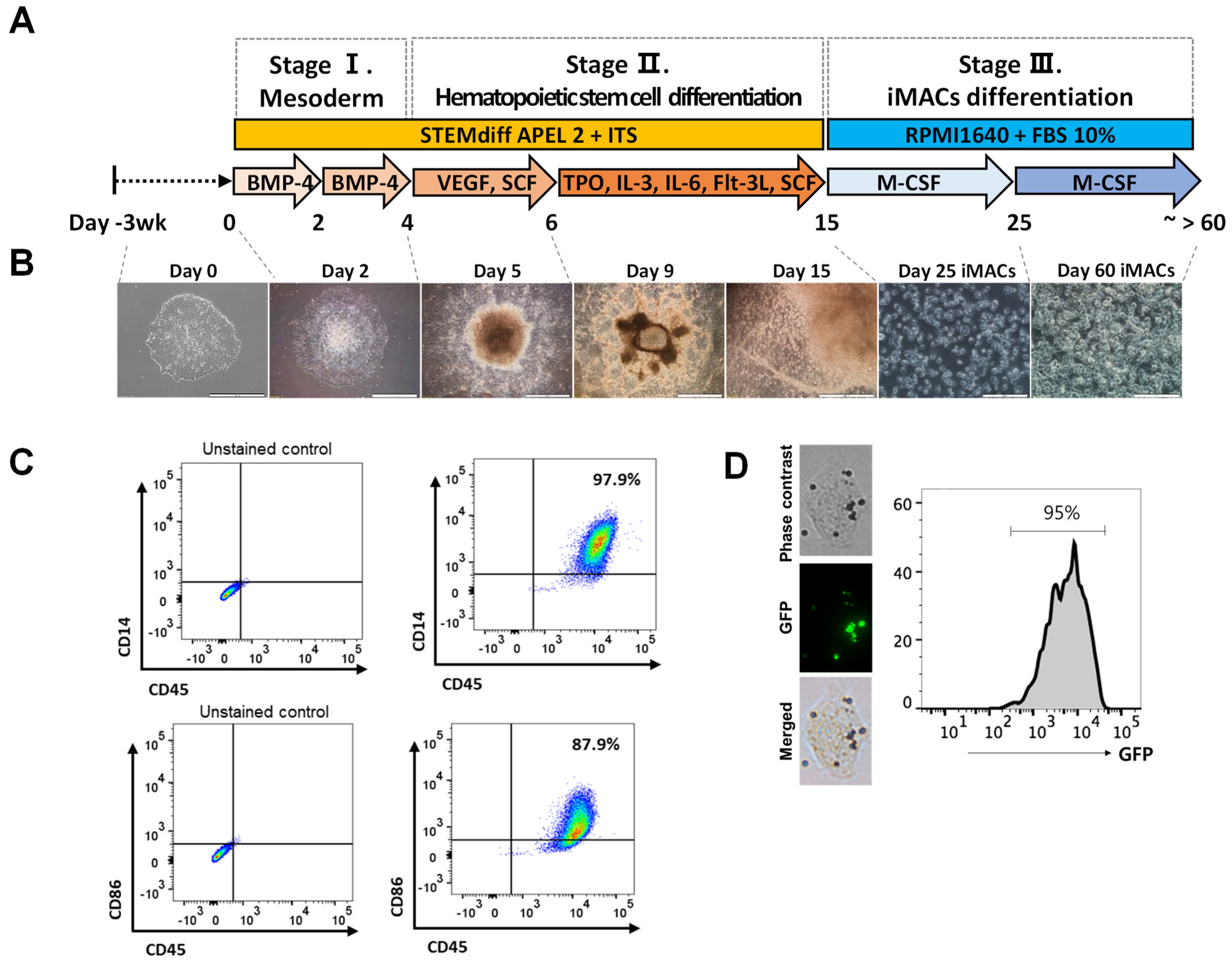
2.3. Differentiation of iMACs from the hPSCs
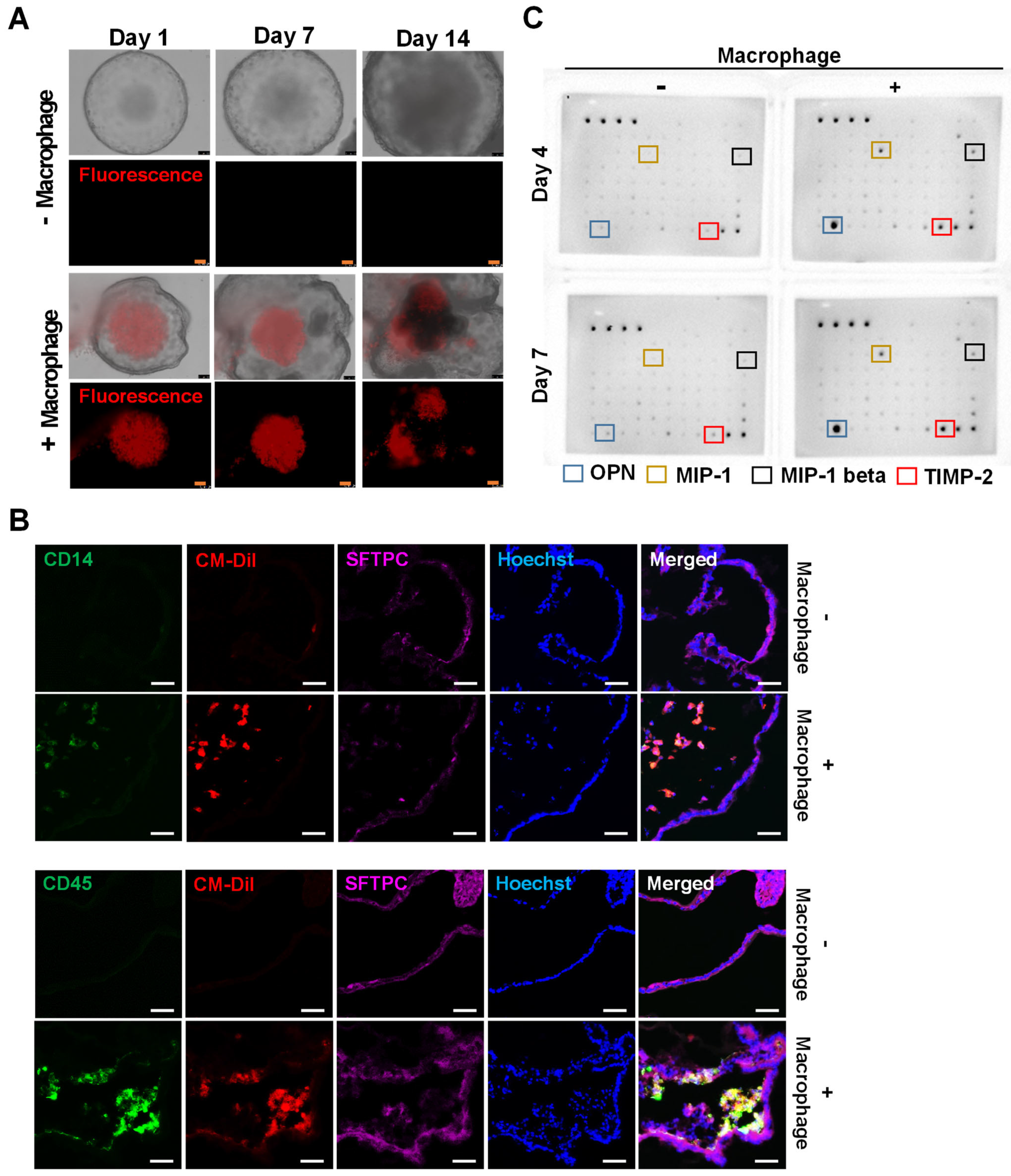
2.4. Introduction of iMACs into the Lung Organoid
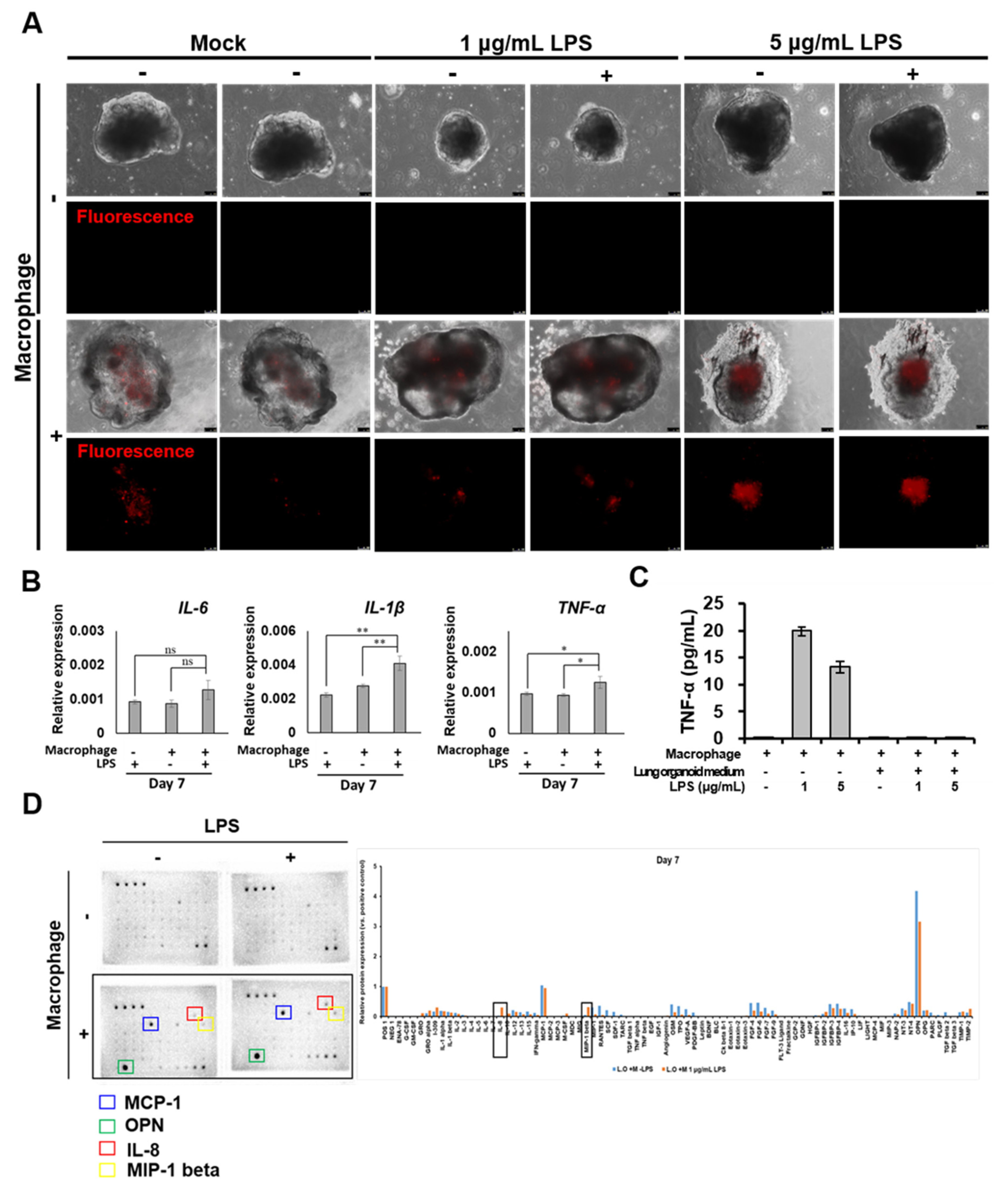
2.5. Morphological and Molecular Changes of Lung Organoids with iMACs
3. Discussion
4. Materials and Methods
4.1. hPSCs Culture and Maintenance
4.2. hPSCs Differentiation into Alveolar Organoids
4.3. hESCs Differentiation into Macrophages (iMAC)
4.4. Phagocytosis Assay
4.5. Generation of iMACs Containing AOs
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Immunofluorescence Staining
4.8. Flow Cytometry
4.9. Cytokine Array
4.10. Statistics
5. Conclusions
- A method for lung organoid differentiation has been successfully established;
- To verify the morphological changes, iMACs were injected directly into the lung organoids;
- For 14 days, iMACs coexisted in the lung organoids;
- IL-8 and MIP-1 release in lung organoid-embedded iMACs increased when the inflammatory response was triggered.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Susaki, E.A.; Takasato, M. Perspective: Extending the Utility of Three-Dimensional Organoids by Tissue Clearing Technologies. Front. Cell Dev. Biol. 2021, 9, 679226. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Choi, K.M.; Sicard, D.; Tschumperlin, D.J. Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials 2017, 113, 118–132. [Google Scholar] [CrossRef]
- Zorn, A.M.; Wells, J.M. Vertebrate Endoderm Development and Organ Formation. Annu. Rev. Cell Dev. Biol. 2009, 25, 221–251. [Google Scholar] [CrossRef] [PubMed]
- Sasai, Y. Next-Generation Regenerative Medicine: Organogenesis from Stem Cells in 3D Culture. Cell Stem Cell 2013, 12, 520–530. [Google Scholar] [CrossRef]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.H.; Tsai, Y.-H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015, 4, e05098. [Google Scholar] [CrossRef] [PubMed]
- Tamo, L.; Hibaoui, Y.; Kallol, S.; Alves, M.P.; Albrecht, C.; Hostettler, K.E.; Feki, A.; Rougier, J.-S.; Abriel, H.; Knudsen, L.; et al. Generation of an alveolar epithelial type II cell line from induced pluripotent stem cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L921–L932. [Google Scholar] [CrossRef] [PubMed]
- Barkauskas, C.E.; Chung, M.-I.; Fioret, B.; Gao, X.; Katsura, H.; Hogan, B.L.M. Lung organoids: Current uses and future promise. Development 2017, 144, 986–997. [Google Scholar] [CrossRef]
- McCauley, K.B.; Hawkins, F.; Serra, M.; Thomas, D.C.; Jacob, A.; Kotton, D.N. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell 2017, 20, 844–857.e6. [Google Scholar] [CrossRef]
- Jacob, A.; Morley, M.; Hawkins, F.; McCauley, K.B.; Jean, J.; Heins, H.; Na, C.-L.; Weaver, T.E.; Vedaie, M.; Hurley, K.; et al. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell 2017, 21, 472–488.e10. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef]
- Yamasaki, K.; Eeden, S.F.V. Lung Macrophage Phenotypes and Functional Responses: Role in the Pathogenesis of COPD. Int. J. Mol. Sci. 2018, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.R.; Hong, S.H. Generation of macrophage containing alveolar organoids derived from human pluripotent stem cells for pulmonary fibrosis modeling and drug efficacy testing. Cell Biosci. 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Q.; Mei, X.D.; Feng, D. Air pollution and chronic airway diseases: What should people know and do? J. Thorac. Dis. 2016, 8, E31–E40. [Google Scholar] [PubMed]
- Weisberg, S.P.; Ural, B.B.; Farber, D.L. Tissue-specific immunity for a changing world. Cell 2021, 184, 1517–1529. [Google Scholar] [CrossRef]
- Baharom, F.; Rankin, G.; Blomberg, A.; Smed-Sörensen, A. Human Lung Mononuclear Phagocytes in Health and Disease. Front. Immunol. 2017, 8, 499. [Google Scholar] [CrossRef]
- Han, H.-W.; Seo, H.-H.; Jo, H.-Y.; Han, H.-J.; Falcão, V.C.A.; Delorme, V.; Heo, J.; Shum, D.; Choi, J.-H.; Lee, J.-M.; et al. Drug Discovery Platform Targeting M. tuberculosis with Human Embryonic Stem Cell-Derived Macrophages. Stem Cell Rep. 2019, 13, 980–991. [Google Scholar] [CrossRef]
- Jo, H.Y.; Seo, H.H.; Gil, D.; Park, Y.; Han, H.J.; Han, H.W.; Thimmulappa, R.K.; Kim, S.C.; Kim, J.H. Single-Cell RNA Sequencing of Human Pluripotent Stem Cell-Derived Macrophages for Quality Control of The Cell Therapy Product. Front Genet 2022, 31, 658862. [Google Scholar] [CrossRef]
- Rittling, S.R. Osteopontin in macrophage function. Expert Rev. Mol. Med. 2011, 13, e15. [Google Scholar] [CrossRef]
- Yla-Herttuala, S.; Lipton, B.A.; Rosenfeld, M.E.; Sarkioja, T.; Yoshimura, T.; Leonard, E.J.; Witztum, J.L.; Steinberg, D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 1991, 88, 5252–5256. [Google Scholar] [CrossRef]
- Sherry, B.; Tekamp-Olson, P.; Gallegos, C.; Bauer, D.; Davatelis, G.; Wolpe, S.D.; Masiarz, F.; Coit, D.; Cerami, A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 beta. J. Exp. Med. 1988, 168, 2251–2259. [Google Scholar] [CrossRef]
- Newby, A.C. Mettaloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arter. Thromb Vasc. Biol. 2008, 28, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Steele, L.; Errington, F.; Prestwich, R.; Ilett, E.; Harrington, K.; Pandha, H.; Coffey, M.; Selby, P.; Vile, R.; Melcher, A. Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NF-kappaB mediated and supports innate and adaptive anti-tumour immune priming. Mol. Cancer 2011, 10, 20. [Google Scholar] [CrossRef]
- Skov, L.; Beurskens, F.J.; Zachariae, C.O.C.; Reitamo, S.; Teeling, J.; Satijn, D.; Knudsen, K.M.; Boot, E.P.J.; Hudson, D.; Baadsgaard, O.; et al. IL-8 as Antibody Therapeutic Target in Inflammatory Diseases: Reduction of Clinical Activity in Palmoplantar Pustulosis. J. Immunol. 2008, 181, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Widmer, U.; Manogue, K.R.; Cerami, A.; Sherry, B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J. Immunol. 1993, 150, 4996–5012. [Google Scholar] [PubMed]
- Philpott, D.J.; Yamaoka, S.; Israel, A.; Sansonetti, P.J. Invasive Shigella flexneri activates NF-kappa B through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J. Immunol. 2000, 165, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Bordag, N.; Klie, S.; Jürchott, K.; Vierheller, J.; Schiewe, H.; Albrecht, V.; Tonn, J.-C.; Schwartz, C.; Schichor, C.; Selbig, J. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci. Rep. 2015, 5, 15954. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Marques, L.J.; Zheng, L.; Poulakis, N.; Guzman, J.; Costabel, U. Pentoxifylline Inhibits TNF- α Production from Human Alveolar Macrophages. Am. J. Respir. Crit. Care Med. 1999, 159, 508–511. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Canetti, C.; Mancuso, P.; Coffey, M.J. Leukotrienes: Underappreciated mediators of innate immune responses. J. Immunol. 2005, 174, 589–594. [Google Scholar] [CrossRef]
- Matera, M.G.; Page, C.; Cazzola, M. PDE inhibitors currently in early clinical trials for the treatment of asthma. Expert Opin. Investig. Drugs 2014, 23, 1267–1275. [Google Scholar] [CrossRef]
- Lee, C.D.; Choi, W.S.; Choi, Y.G.; Kang, H.S.; Lee, W.T.; Kim, H.J.; Lee, J.-Y. Inhibition of phosphodiesterase suppresses allergic lung inflammation by regulating MCP-1 in an OVA-induced asthma murine model with co-exposure to lipopolysaccharide. J. Int. Med. Res. 2020, 48, 300060520903663. [Google Scholar] [CrossRef] [PubMed]
- Serezani, C.H.; Ballinger, M.N.; Aronoff, D.M.; Peters-Golden, M. Cyclic AMP: Master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 2008, 39, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Gao, L.-N.; Cui, Y.-L.; Zhang, Y.; Zhou, X. The cyclic AMP signaling pathway: Exploring targets for successful drug discovery (Review). Mol. Med. Rep. 2016, 13, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Vunta, H.; Davis, F.; Palempalli, U.D.; Bhat, D.; Arner, R.J.; Thompson, J.T.; Peterson, D.G.; Reddy, C.C.; Prabhu, K.S. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta 12,14-prostaglandin J2 in macrophages. J. Bio. Chem. 2007, 282, 17964–17973. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Huang, S.X.; De Carvalho, A.L.R.T.; Ho, S.-H.; Islam, M.N.; Volpi, S.; Notarangelo, L.D.; Ciancanelli, M.; Casanova, J.-L.; Bhattacharya, J.; et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 2017, 19, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Porotto, M.; Ferren, M.; Chen, Y.-W.; Siu, Y.; Makhsous, N.; Rima, B.; Briese, T.; Greninger, A.L.; Snoeck, H.-W.; Moscona, A. Authentic Modeling of Human Respiratory Virus Infection in Human Pluripotent Stem Cell-Derived Lung Organoids. mBio 2019, 10, e00723-19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gotoh, S.; Korogi, Y.; Seki, M.; Konishi, S.; Ikeo, S.; Sone, N.; Nagasaki, T.; Matsumoto, H.; Muro, S.; et al. Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat. Methods 2017, 14, 1097–1106. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.-R.; Han, H.-J.; Lee, Y.; Noh, Y.-W.; Cho, S.-J.; Kim, J.-H. Human Pluripotent Stem Cell-Derived Alveolar Organoid with Macrophages. Int. J. Mol. Sci. 2022, 23, 9211. https://doi.org/10.3390/ijms23169211
Seo H-R, Han H-J, Lee Y, Noh Y-W, Cho S-J, Kim J-H. Human Pluripotent Stem Cell-Derived Alveolar Organoid with Macrophages. International Journal of Molecular Sciences. 2022; 23(16):9211. https://doi.org/10.3390/ijms23169211
Chicago/Turabian StyleSeo, Ha-Rim, Hyeong-Jun Han, Youngsun Lee, Young-Woock Noh, Seung-Ju Cho, and Jung-Hyun Kim. 2022. "Human Pluripotent Stem Cell-Derived Alveolar Organoid with Macrophages" International Journal of Molecular Sciences 23, no. 16: 9211. https://doi.org/10.3390/ijms23169211
APA StyleSeo, H.-R., Han, H.-J., Lee, Y., Noh, Y.-W., Cho, S.-J., & Kim, J.-H. (2022). Human Pluripotent Stem Cell-Derived Alveolar Organoid with Macrophages. International Journal of Molecular Sciences, 23(16), 9211. https://doi.org/10.3390/ijms23169211





