Glymphatic System a Window on TBI Pathophysiology: A Systematic Review
Abstract
:1. Introduction
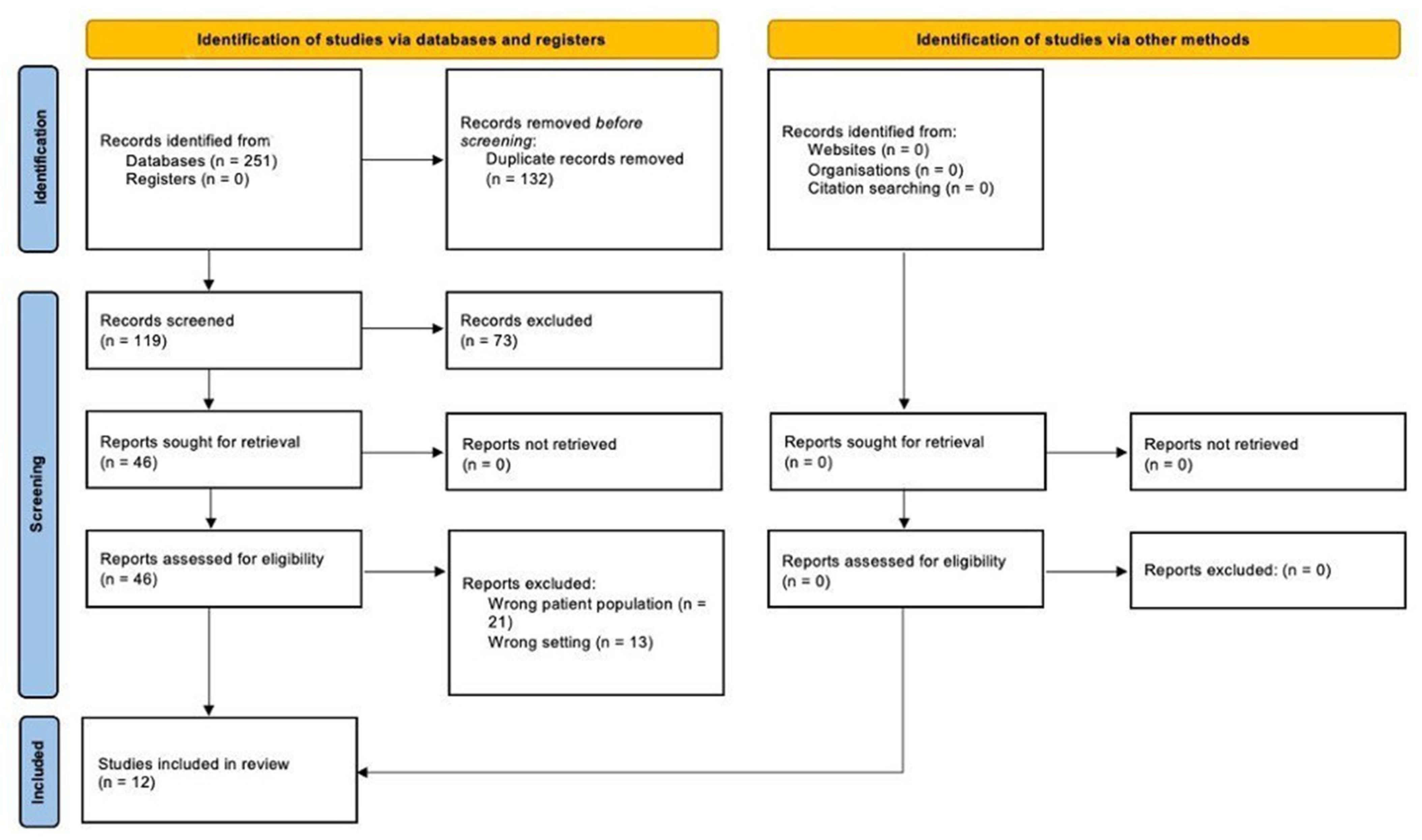
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Quality Assessment and Critical Appraisal
2.3. Risk of Bias
2.4. Characteristics of Eligible Studies
3. Results and Discussion
3.1. Alteration of Glymphatic Pathways after TBI
3.2. Cellular Factors Released after TBI
3.3. Post-Mortem Investigations
3.4. Neurological and Behavioral Alterations
- Sampling and fixation in formolic solution of the brain as a whole in a study group (subjects who died of head trauma) and a control group (subjects who died of other causes, excluding subjects suffering from encephalopathies);
- Sampling of the following brain areas: periventricular spaces, as well as of the hypothalamus, thalamus, hippocampus, amygdala, olfactory bulb, and cortex;
- Standard H&E and immunohistochemical staining with anti-AQP4 antibodies, anti-S100b, anti-GFAP, anti-NSE, anti-Tau, anti-CD68, anti-CD34, anti-GFAP, and anti-ß-amyloid;
- Blood and CSF collection for MAPT evaluation using ELISA (enzyme-linked immunosorbent assay) [50].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol. Res. Pract. 2021, 3, 5. [Google Scholar] [CrossRef]
- Peng, W.; Achariyar, T.M.; Li, B.; Liao, Y.; Mestre, H.; Hitomi, E.; Regan, S.; Kasper, T.; Peng, S.; Ding, F.; et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016, 93, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Pu, T.; Feng, W.; Lu, M.; Zheng, Y.; Du, R.; Xiao, M.; Hu, G. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl. Neurodegener. 2019, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Christensen, J.; Yamakawa, G.R.; Shultz, S.R.; Mychasiuk, R. Is the glymphatic system the missing link between sleep impairments and neurological disorders? Examining the implications and uncertainties. Prog. Neurobiol. 2021, 198, 101917. [Google Scholar] [CrossRef]
- Wostyn, P.; de Groot, V.; van Dam, D.; Audenaert, K.; de Deyn, P.P.; Killer, H.E. The Glymphatic System: A New Player in Ocular Diseases? Investig. Ophthalmol. Vis. Sci. 2016, 57, 5426–5427. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Walker, A.K.; Rollo, B.; Ayers, K.L.; Farah, R.; O’Brien, T.J.; Wright, D.K. Impaired glymphatic function in the early stages of disease in a TDP-43 mouse model of amyotrophic lateral sclerosis. Transl. Neurodegener. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Sullan, M.J.; Asken, B.M.; Jaffee, M.S.; DeKosky, S.T.; Bauer, R.M. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci. Biobehav. Rev. 2018, 84, 316–324. [Google Scholar] [CrossRef]
- Piantino, J.; Lim, M.M.; Newgard, C.D.; Iliff, J. Linking Traumatic Brain Injury, Sleep Disruption and Post-Traumatic Headache: A Potential Role for Glymphatic Pathway Dysfunction. Curr. Pain Headache Rep. 2019, 23, 1–11. [Google Scholar] [CrossRef]
- Iliff, J.J.; Goldman, S.A.; Nedergaard, M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015, 14, 977–979. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Betrus, C.; Kreipke, C.W. Historical perspectives in understanding traumatic brain injury and in situating disruption in CBF in the pathotrajectory of head trauma. In Cerebral Blood Flow, Metabolism and Head Trauma: The Pathotrajectory of Traumatic Brain Injury; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–27. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.P.; Gao, Z.; Shields, L.B.E.; Li, F.; Chu, T.; Lv, H.; Moriarty, T.; Xu, X.M.; Yang, X.; et al. Pathophysiological and behavioral deficits in developing mice following rotational acceleration-deceleration traumatic brain injury. DMM Dis. Models Mech. 2018, 11, dmm030387. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Frati, A.; Santoro, A.; Frati, P.; Fineschi, V.; Pesce, A. Diffuse Axonal Injury: Clinical Prognostic Factors, Molecular Experimental Models and the Impact of the Trauma Related Oxidative Stress. An Extensive Review Concerning Milestones and Advances. Int. J. Mol. Sci. 2021, 22, 10865. [Google Scholar] [CrossRef] [PubMed]
- Maiese, A.; Iannaccone, F.; Scatena, A.; Del Fante, Z.; Oliva, A.; Frati, P.; Fineschi, V. Pediatric Abusive Head Trauma: A Systematic Review. Diagnostics 2021, 11, 734. [Google Scholar] [CrossRef]
- Martinez, A.P.; Scherer, M.J.; Tozser, T. Traumatic Brain Injury (TBI) in School-Based Populations: Common Sequelae and Assistive Technology Interventions. Adv. Neurodev. Disord. 2018, 2, 310–321. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Center for Health Statistics: Mortality Data on CDC WONDER. Available online: https://wonder.cdc.gov/mcd.html (accessed on 23 March 2022).
- Alkhier Ahmed, A.; Najb Almulla, N.; Ali Ahli, M.; Abdelrahman Alzarouni, A. Pituitary dysfunction following a traumatic brain injury (TBI) at the desk of a General Practitioner. World Fam. Med. 2021, 19, 92–97. [Google Scholar] [CrossRef]
- Laeke, T.; Tirsit, A.; Kassahun, A.; Sahlu, A.; Debebe, T.; Yesehak, B.; Masresha, S.; Deyassa, N.; Moen, B.E.; Lund-Johansen, M.; et al. Prospective Study of Surgery for Traumatic Brain Injury in Addis Ababa, Ethiopia: Trauma Causes, Injury Types, and Clinical Presentation. World Neurosurg. 2021, 149, e460–e468. [Google Scholar] [CrossRef] [PubMed]
- Iarussi, F.; Cipolloni, L.; Bertozzi, G.; Sasso, L.; Ferrara, M.; Salerno, M.; Rubino, G.T.R.; Maglietta, F.; Dinisi, A.; Albano, D.; et al. Dog-bite-related attacks: A new forensic approach. Forensic Sci. Int. 2020, 310, 110254. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Rus, R.M.; Chan, K.H.; Martin, A.G.; Awang, M.S. Prevalence of Postconcussion Syndrome after Mild Traumatic Brain Injury in Young Adults from a Single Neurosurgical Center in East Coast of Malaysia. Asian J. Neurosurg. 2019, 14, 201. [Google Scholar] [CrossRef]
- Langlois, J.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef]
- Ghajar, J. Traumatic brain injury. Lancet 2000, 356, 923–929. [Google Scholar] [CrossRef]
- Neri, M.; Büttner, A.; Fineschi, V. Brain Injury due to Mechanical Trauma and Ischemic-Hypoxic Insult: Biomarkers of Brain Injury and Oxidative Stress. Oxid. Med. Cell. Longev. 2017, 2017, 8923472. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Frati, A.; Cerretani, D.; Fiaschi, A.I.; Frati, P.; Gatto, V.; La Russa, R.; Pesce, A.; Pinchi, E.; Santurro, A.; Fraschetti, F.; et al. Diffuse Axonal Injury and Oxidative Stress: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 2600. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2017, 16, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, M.; Maiese, A.; De Matteis, A.; Viola, R.V.; Arcangeli, M.; La Russa, R.; Fineschi, V. Traumatic brain injury: Estimate of the age of the injury based on neuroinflammation, endothelial activation markers and adhesion molecules. Histol. Histopathol. 2021, 36, 795–806. [Google Scholar] [CrossRef] [PubMed]
- La Russa, R.; Maiese, A.; Cipolloni, L.; Di Fazio, N.; Delogu, G.; De Matteis, A.; Del Fante, Z.; Manetti, F.; Frati, P.; Fineschi, V. Diagnostic assessment of traumatic brain injury by vacuum extraction in newborns: Overview on forensic perspectives and proposal of operating procedures. Front. Biosci. 2022, 27, 79. [Google Scholar] [CrossRef]
- Cafarelli, F.P.; Grilli, G.; Zizzo, G.; Bertozzi, G.; Giuliani, N.; Mahakkanukrauh, P.; Pinto, A.; Guglielmi, G. Postmortem Imaging: An Update. Semin. Ultrasound CT MRI 2019, 40, 86–93. [Google Scholar] [CrossRef]
- Ferrara, M.; Sessa, F.; Rendine, M.; Spagnolo, L.; De Simone, S.; Riezzo, I.; Ricci, P.; Pascale, N.; Salerno, M.; Bertozzi, G.; et al. A multidisciplinary approach is mandatory to solve complex crimes: A case report. Egypt. J. Forensic Sci. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Aromatario, M.; Torsello, A.; D’errico, S.; Bertozzi, G.; Sessa, F.; Cipolloni, L.; Baldari, B. Traumatic Epidural and Subdural Hematoma: Epidemiology, Outcome, and Dating. Medicina 2021, 57, 125. [Google Scholar] [CrossRef]
- Zanza, C.; Piccolella, F.; Racca, F.; Romenskaya, T.; Longhitano, Y.; Franceschi, F.; Savioli, G.; Bertozzi, G.; De Simone, S.; Cipolloni, L.; et al. Ketamine in Acute Brain Injury: Current Opinion Following Cerebral Circulation and Electrical Activity. Healthcare 2022, 10, 566. [Google Scholar] [CrossRef]
- La Russa, R.; Maiese, A.; Di Fazio, N.; Morano, A.; Di Bonaventura, C.; De Matteis, A.; Fazio, V.; Frati, P.; Fineschi, V. Post-Traumatic Meningitis Is a Diagnostic Challenging Time: A Systematic Review Focusing on Clinical and Pathological Features. Int. J. Mol. Sci. 2020, 21, 4148. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Frati, A.; Turillazzi, E.; Cantatore, S.; Cipolloni, L.; Di Paolo, M.; Frati, P.; La Russa, R.; Maiese, A.; Scopetti, M.; et al. Immunohistochemical Evaluation of Aquaporin-4 and its Correlation with CD68, IBA-1, HIF-1α, GFAP, and CD15 Expressions in Fatal Traumatic Brain Injury. Int. J. Mol. Sci. 2018, 19, 3544. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, V.; Viola, R.V.; La Russa, R.; Santurro, A.; Frati, P. A Controversial Medicolegal Issue: Timing the Onset of Perinatal Hypoxic-Ischemic Brain Injury. Mediat. Inflamm. 2017, 2017, 6024959. [Google Scholar] [CrossRef] [PubMed]
- Turillazzi, E.; Karch, S.B.; Neri, M.; Pomara, C.; Riezzo, I.; Fineschi, V. Confocal laser scanning microscopy. Using new technology to answer old questions in forensic investigations. Int. J. Leg. Med. 2008, 122, 173–177. [Google Scholar] [CrossRef]
- Pinchi, E.; Frati, A.; Cipolloni, L.; Aromatario, M.; Gatto, V.; La Russa, R.; Pesce, A.; Santurro, A.; Fraschetti, F.; Frati, P.; et al. Clinical-pathological study on β-APP, IL-1β, GFAP, NFL, Spectrin II, 8OHdG, TUNEL, miR-21, miR-16, miR-92 expressions to verify DAI-diagnosis, grade and prognosis. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar]
- Falavigna, A.; Blauth, M.; Kates, S.L. Critical review of a scientific manuscript: A practical guide for reviewers. J. Neurosurg. 2017, 128, 312–321. [Google Scholar] [CrossRef]
- Christensen, J.; Wright, D.K.; Yamakawa, G.R.; Shultz, S.R.; Mychasiuk, R. Repetitive Mild Traumatic Brain Injury Alters Glymphatic Clearance Rates in Limbic Structures of Adolescent Female Rats. Sci. Rep. 2020, 10, 6254. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chopp, M.; Ding, G.; Davoodi-Bojd, E.; Zhang, L.; Li, Q.; Zhang, Y.; Xiong, Y.; Jiang, Q. MRI detection of impairment of glymphatic function in rat after mild traumatic brain injury. Brain Res. 2020, 1747, 147062. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, Y.; Wan, X.; Wu, J.; Fan, Z.; Yang, L. Protective Effects of Aquaporin-4 Deficiency on Longer-term Neurological Outcomes in a Mouse Model. Neurochem. Res. 2021, 46, 1380–1389. [Google Scholar] [CrossRef]
- Liu, E.; Sun, L.; Zhang, Y.; Wang, A.; Yan, J. Aquaporin4 Knockout Aggravates Early Brain Injury Following Subarachnoid Hemorrhage Through Impairment of the Glymphatic System in Rat Brain. Acta Neurochir. Suppl. 2020, 127, 59–64. [Google Scholar] [CrossRef]
- Zhang, E.; Wan, X.; Yang, L.; Wang, D.; Chen, Z.; Chen, Y.; Liu, M.; Zhang, G.; Wu, J.; Han, H.; et al. Omega-3 Polyunsaturated Fatty Acids Alleviate Traumatic Brain Injury by Regulating the Glymphatic Pathway in Mice. Front. Neurol. 2020, 11, 707. [Google Scholar] [CrossRef]
- Plog, B.A.; Dashnaw, M.L.; Hitomi, E.; Peng, W.; Liao, Y.; Lou, N.; Deane, R.; Nedergaard, M. Biomarkers of Traumatic Injury Are Transported from Brain to Blood via the Glymphatic System. J. Neurosci. 2015, 35, 518. [Google Scholar] [CrossRef]
- Opel, R.A.; Christy, A.; Boespflug, E.L.; Weymann, K.B.; Case, B.; Pollock, J.M.; Silbert, L.C.; Lim, M.M. Effects of traumatic brain injury on sleep and enlarged perivascular spaces. J. Cereb. Blood Flow Metab. 2019, 39, 2258–2267. [Google Scholar] [CrossRef]
- Piantino, J.; Schwartz, D.L.; Luther, M.; Newgard, C.; Silbert, L.; Raskind, M.; Pagulayan, K.; Kleinhans, N.; Iliff, J.; Peskind, E. Link between Mild Traumatic Brain Injury, Poor Sleep, and Magnetic Resonance Imaging: Visible Perivascular Spaces in Veterans. J. Neurotrauma 2021, 38, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Iliff, J.J.; Yang, L.; Yang, J.; Chen, X.; Chen, M.J.; Giese, R.N.; Wang, B.; Shi, X.; Nedergaard, M. “Hit & Run” model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 2013, 33, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Olczak, M.; Niderla-Bielińska, J.; Kwiatkowska, M.; Samojłowicz, D.; Tarka, S.; Wierzba-Bobrowicz, T. Tau protein (MAPT) as a possible biochemical marker of traumatic brain injury in postmortem examination. Forensic Sci. Int. 2017, 280, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, C.; Nelson, D.W.; Zeiler, F.A.; Ercole, A.; Ghatan, P.H.; Von Horn, H.; Risling, M.; Svensson, M.; Agoston, D.V.; Bellander, B.M.; et al. Influence of Blood–Brain Barrier Integrity on Brain Protein Biomarker Clearance in Severe Traumatic Brain Injury: A Longitudinal Prospective Study. J. Neurotrauma 2020, 37, 1381. [Google Scholar] [CrossRef] [PubMed]
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. 2018, 13, 379–394. [Google Scholar] [CrossRef]
- Mestre, H.; Mori, Y.; Nedergaard, M. The Brain’s Glymphatic System: Current Controversies. Trends Neurosci. 2020, 43, 458–466. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System—A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.H.; Liu, X.; Zhang, Z.; Gu, X.; Yu, S.P.; Keene, C.D.; Cheng, L.; Ye, K. Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog. Neurobiol. 2020, 185, 101730. [Google Scholar] [CrossRef]
- Graham, N.S.N.; Sharp, D.J. Understanding neurodegeneration after traumatic brain injury: From mechanisms to clinical trials in dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; Bertozzi, G.; Zanza, C.; Longhitano, Y.; Piccolella, F.; Lauritano, C.E.; Volonnino, G.; Manetti, A.C.; Maiese, A.; La Russa, R. Traumatic Brain Injury and Gut Brain Axis: The Disruption of an Alliance. Rev. Recent Clin. Trials 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, G.; Maglietta, F.; Sessa, F.; Scoto, E.; Cipolloni, L.; Di Mizio, G.; Salerno, M.; Pomara, C. Traumatic Brain Injury: A Forensic Approach: A Literature Review. Curr. Neuropharmacol. 2020, 18, 538. [Google Scholar] [CrossRef]
- Tomura, S.; Nawashiro, H.; Otani, N.; Uozumi, Y.; Toyooka, T.; Ohsumi, A.; Shima, K. Effect of decompressive craniectomy on aquaporin-4 expression after lateral fluid percussion injury in rats. J. Neurotrauma 2011, 28, 237–243. [Google Scholar] [CrossRef]
- Planel, E.; Bretteville, A.; Liu, L.; Virag, L.; Du, A.L.; Yu, W.H.; Dickson, D.W.; Whittington, R.A.; Duff, K.E. Acceleration and persistence of neurofibrillary pathology in a mouse model of tauopathy following anesthesia. FASEB J. 2009, 23, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
| Christensen et al. [43] | Iliff et al. [44] | Li et al. [45] | Liu et al. [46] | Liu et al. [47] | Zhang et al. [48] | Plog et al. [49] | Opel et al. [50] | Piantino et al. [51] | Ren et al. [52] | Olczak et al. [53] | Lindblad et al. [54] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Explicit study question | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Innovative or relevant | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Review of the literature | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Potential to advance scientific knowledge | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Clearly written and well-organized | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Eligibility criteria for inclusion and exclusion of studied subjects clearly stated | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ethics approval and/or informed consent obtained | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Conflicts of interests declared | Yes | Not specified | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Sample size | The exact number was not specified | The exact number was not specified | Yes | Yes | Yes | The exact number was not specified | The exact number was not specified | Yes | Yes | The exact number was not specified | Yes | The exact number was not specified |
| Randomization process and/or blinding techniques described | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Statistical analysis and/or softwares used | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bias and limitation mentioned | Yes | Not specified | Yes | Yes | Not specified | Yes | Yes | Yes | Yes | Not specified | Not specified | Yes |
| Reference | Research Subjects | Aim of the Study | TBI Model and Severity | Findings and Observed Neurological/Behavioral Investigations. |
|---|---|---|---|---|
| Christensen et al. [43] | Mice | To examine glymphatic function after repetitive mild TBI (RmTBI) evaluating signal intensity of Gadovist, previously injected in the cisterna magna, using in vivo MRI. | Three RmTBI using a lateral impact device (50 g weight propelled at an average speed of 9.02 ± 0.18 m/s), without eye or head lacerations. | In hypothalamus, hippocampus, amygdala, and olfactory bulb after RmTBI the glymphatic influx was increased while the efflux was slower. Behavioral test used:
|
| Iliff et al. [44] | Mice | To evaluate if AQP4 gene deletion is associated to the buildup of phosphorylated tau protein after TBI. | ‘Hit & Run” traumatic brain injury model to induce moderate TBI by using a pneumatic-controlled, cortical impact device (using a strike depth of 10 mm and 0.1 time of impact with an impact velocity of 5.2 m/s). | Twenty-eight days after TBI, in wild-type mice mild P-tau immunoreactivity was evident in the cortex surrounding the traumatic lesion, while in Aqp4-/- mice P-tau immunoreactivity was pronounced. Behavioral test used:
|
| Li et al. [45] | Mice | To investigate mild TBI effect on the glymphatic system using contrast-enhanced MRI. | mTBI induced before exposing the skull, by dropping a cylindrical column of segmented brass (450 g) from a height of 1 m. | mTBI determines lower infusion and clearance rate of contrast agent in cortex, hippocampus and thalamus than in hypothalamus, olfactory bulb and cerebellum. No behavioral/neurological test were performed. |
| Liu et al. [46] | Mice | To study the role of AQP4 in progression of TBI in knockout mice. | Cortical impact injury (CCI). TBI severity not specified. | AQP4 absence improve the symptoms of TBI, protect the integrity of the brain-blood barrier (BBB), promote the clearance of brain amyloid beta, and inhibit the inflammatory response in the brain. Behavioral test used:
|
| Liu et al. [47] | Mice | To explore the role of AQP4 using MRI after subarachnoid hemorrhage (SAH). | SAH established using endovascular perforation method (anesthetized rat in which a nylon suture was inserted through the right internal carotid artery to perforate the junction of the middle and anterior cerebral arteries). | After SAH, the diffusion of Gd-DTPA injected into the cisterna magna was markedly blocked in Aqp4−/−rats, which showed more severe brain edema aggravating neurological deficits. Neurological functions tests used: spontaneous activity, symmetry of the limb movement, forepaw outstretching, climbing, body proprioception, and whisker stimulation response. AQP4 played important roles in early brain injury following SAH. |
| Zhang et al. [48] | Mice | To investigate the effects of Omega-3 in promoting waste clearance thorough the glymphatic system in induced TBI model. | TBI induced by CCI apparatus. After making an incision to expose the skull; a hole of 3.5 mm in diameter was drilled on the right hemisphere 2.0 mm lateral to the midline and 2.0 mm from Bregma; impact applicator was applied at a depth of 2 mm at 4.5 m/s for 200 ms. | Administering fish oil (rich in Omega-3) for a period of two months before determining a TBI significantly improves neuronal function in mouse models, favors the clearance of radiolabeled tracers and prevents the accumulation of Aß by partially restoring the expression and depolarization of AQP4, impaired by TBI. Neurological functions tests used:
|
| Plog et al. [49] | Mice | To alter the glymphatic function through four different mechanisms: the deletion of AQP4; the administration of acetazolamide (which inhibits the production of cerebrospinal fluid at the level of the choroid plexuses); the execution of a cysternotomy of the cisterna magna (which promotes cerebrospinal fluid drainage and reduces glymphatic system function); and sleep deprivation (which has been shown to reduce glymphatic efflux). | “Hit & Run” TBI model: a CCI device was employed using an impactor velocity of 4.7 m/s, an impact depth of 10 mm, and impact duration of 100 ms. The location of the impact was the point 4.5 mm lateral to midline and 4.5 mm posterior to the left orbit. | By cortical administration of a protein tracer, it was observed that these four mechanisms cause a reduction in clearance compared to the control group. The clearance of specific markers for TBI such as S100b, GFAP, and NSE was evaluated, showing that in wild-type mice there was a significant increase in serum levels of these markers after TBI, while, in AQP4 knockout mice and mice undergoing cysternotomy, acetazolamide administration, and sleep deprivation, the serum levels were similar to those of wild-type mice not subjected to the trauma. No behavioral/neurological test were performed. |
| Opel et al. [50] | Humans | To evaluate the relationship between TBI and enlargement of the perivascular spaces (ePVS) using brain MRI and overnight polysomnography. | TBI severity not specified. | The enlargement of the perivascular spaces is independently related to a reduction in sleep time. However, this correlation was shown to be more significant in subjects who had undergone a TBI than in the group that did have TBI history. The results suggest that ePVS may be modulated by sleep and TBI and that there may be a causative relationship between loss of glymphatic function and dilation of the perivascular space. |
| Piantino et al. [51] | Humans | To determine the effect of mTBI on visible perivascular spaces using MRI in military veterans. | Military blast mTBI. | A significant correlation was found between mild TBI and PVS burden, which suggest waste clearance dysfunction. |
| Ren et al. [52] | Mice | To characterize changes in aquaporin-4 (AQP4) expression and localization after mild and moderate TBI. | “Hit & Run” TBI model using a strike depth of 10 mm, and 0.1 s of contact time, with a velocity of 4.8 m/s for mild and 5.2 m/s for moderate TBI. | After TBI, AQP4 expression was increased, with a loss of polarized localization at the end foot of reactive astrocytes, suggesting that changes in AQP4 may not contribute to cerebral edema formation, but may represent a compensatory mechanism to facilitate its resolution. Neurological functions tests used:
|
| Olczak et al. [53] | Humans | To check the relevance of MAPT examination for forensic purposes in head injury death. | Severe head injury. | MAPT serum and cerebrospinal fluid levels should be considered a TBI marker in postmortem examination. |
| Lindblad et al. [54] | Humans | To assess if and how clearance of S100 B and NSE (brain enriched proteins) from brain to blood is affected by BBB disruption in severe TBI patients. | Severe TBI. | Intracranial NSE accumulates and is cleared through a route other than the BBB, which may be the glymphatic system. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, M.; Bertozzi, G.; Volonnino, G.; Di Fazio, N.; Frati, P.; Cipolloni, L.; La Russa, R.; Fineschi, V. Glymphatic System a Window on TBI Pathophysiology: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9138. https://doi.org/10.3390/ijms23169138
Ferrara M, Bertozzi G, Volonnino G, Di Fazio N, Frati P, Cipolloni L, La Russa R, Fineschi V. Glymphatic System a Window on TBI Pathophysiology: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(16):9138. https://doi.org/10.3390/ijms23169138
Chicago/Turabian StyleFerrara, Michela, Giuseppe Bertozzi, Gianpietro Volonnino, Nicola Di Fazio, Paola Frati, Luigi Cipolloni, Raffaele La Russa, and Vittorio Fineschi. 2022. "Glymphatic System a Window on TBI Pathophysiology: A Systematic Review" International Journal of Molecular Sciences 23, no. 16: 9138. https://doi.org/10.3390/ijms23169138
APA StyleFerrara, M., Bertozzi, G., Volonnino, G., Di Fazio, N., Frati, P., Cipolloni, L., La Russa, R., & Fineschi, V. (2022). Glymphatic System a Window on TBI Pathophysiology: A Systematic Review. International Journal of Molecular Sciences, 23(16), 9138. https://doi.org/10.3390/ijms23169138






