Renin as a Marker of Tissue Perfusion, Septic Shock and Mortality in Septic Patients: A Prospective Observational Study
Abstract
:1. Introduction
2. Results
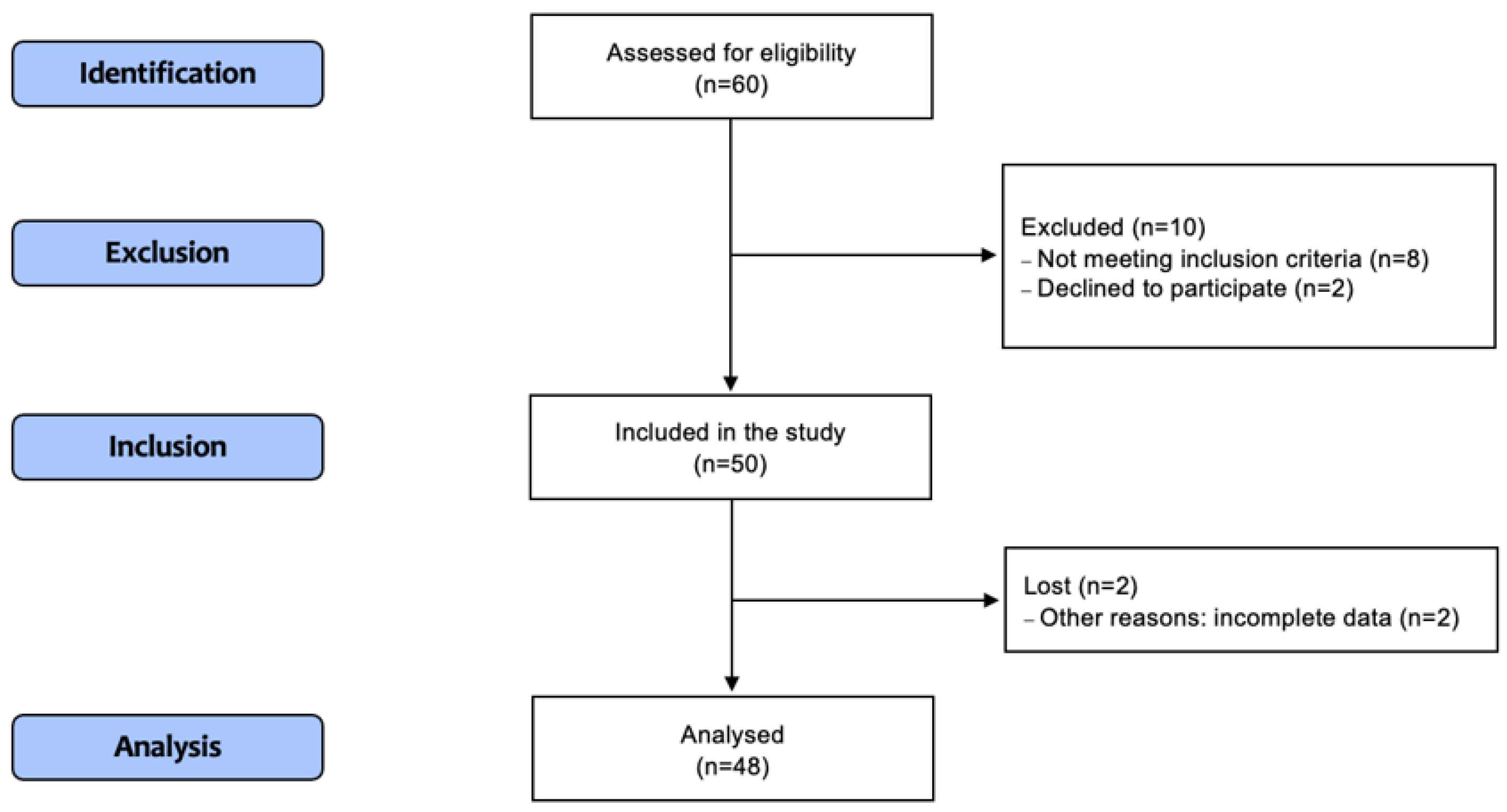
2.1. Patients’ Characteristics
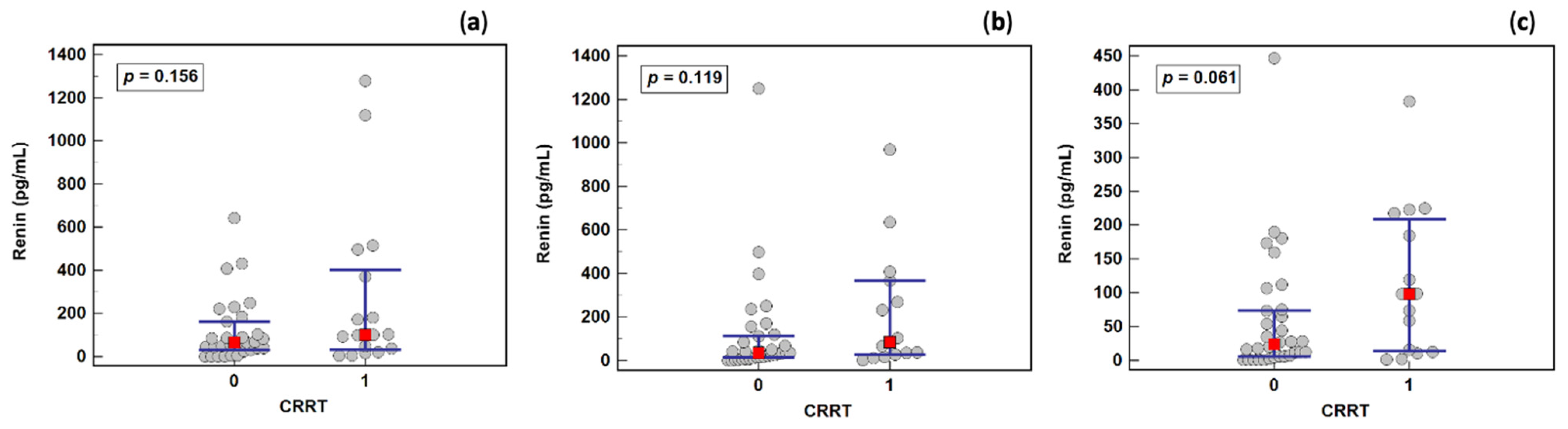
2.2. Removal of Renin by Continuous Renal Replacement Therapy (CRRT)
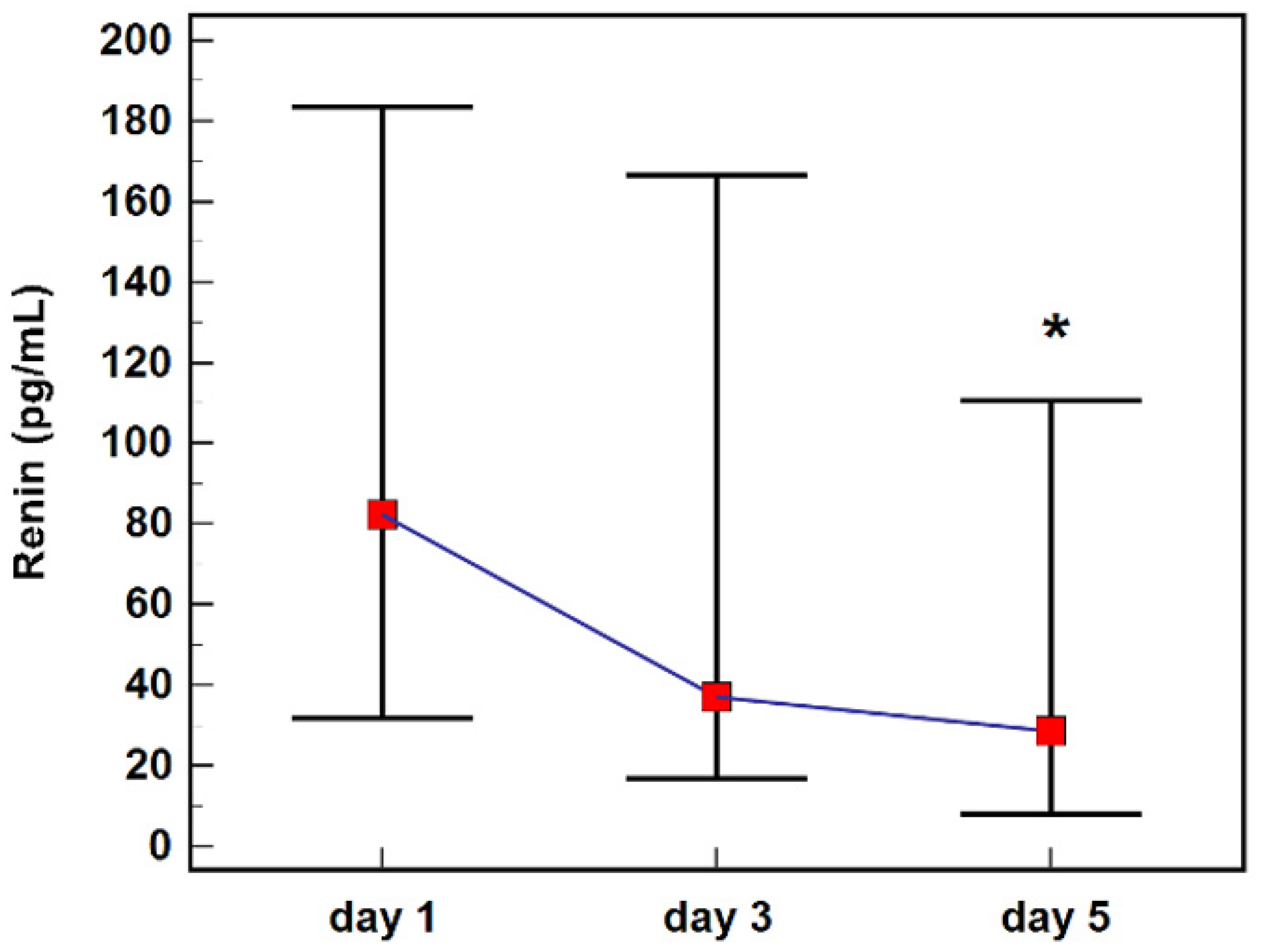
2.3. Renin Concentration during Follow-Up
2.4. Renin Association with Biochemical and Clinical Parameters and Follow-Up Treatment
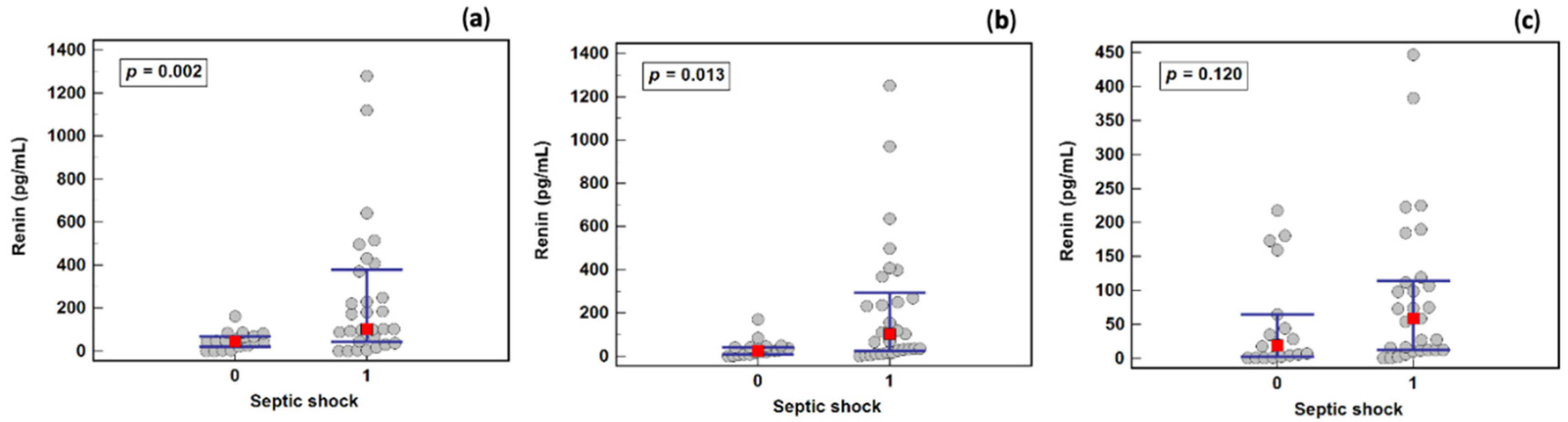
2.5. Renin as a Septic Shock Marker
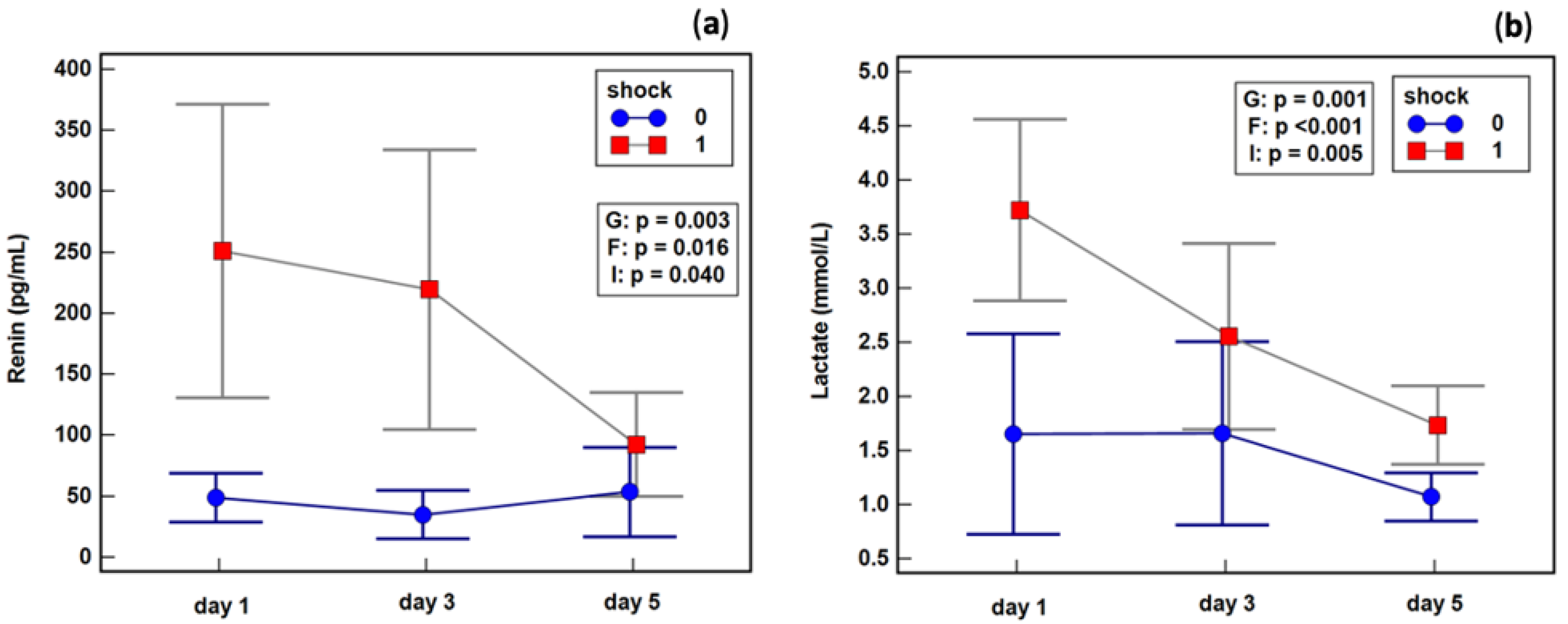
2.6. Effect of Septic Shock on Renin Dynamics
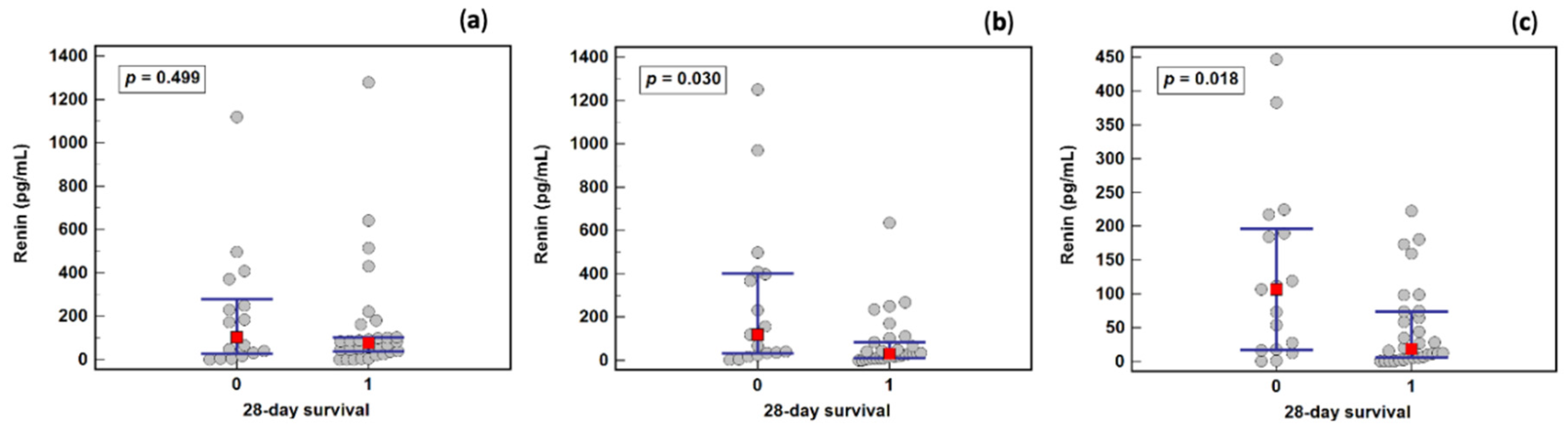
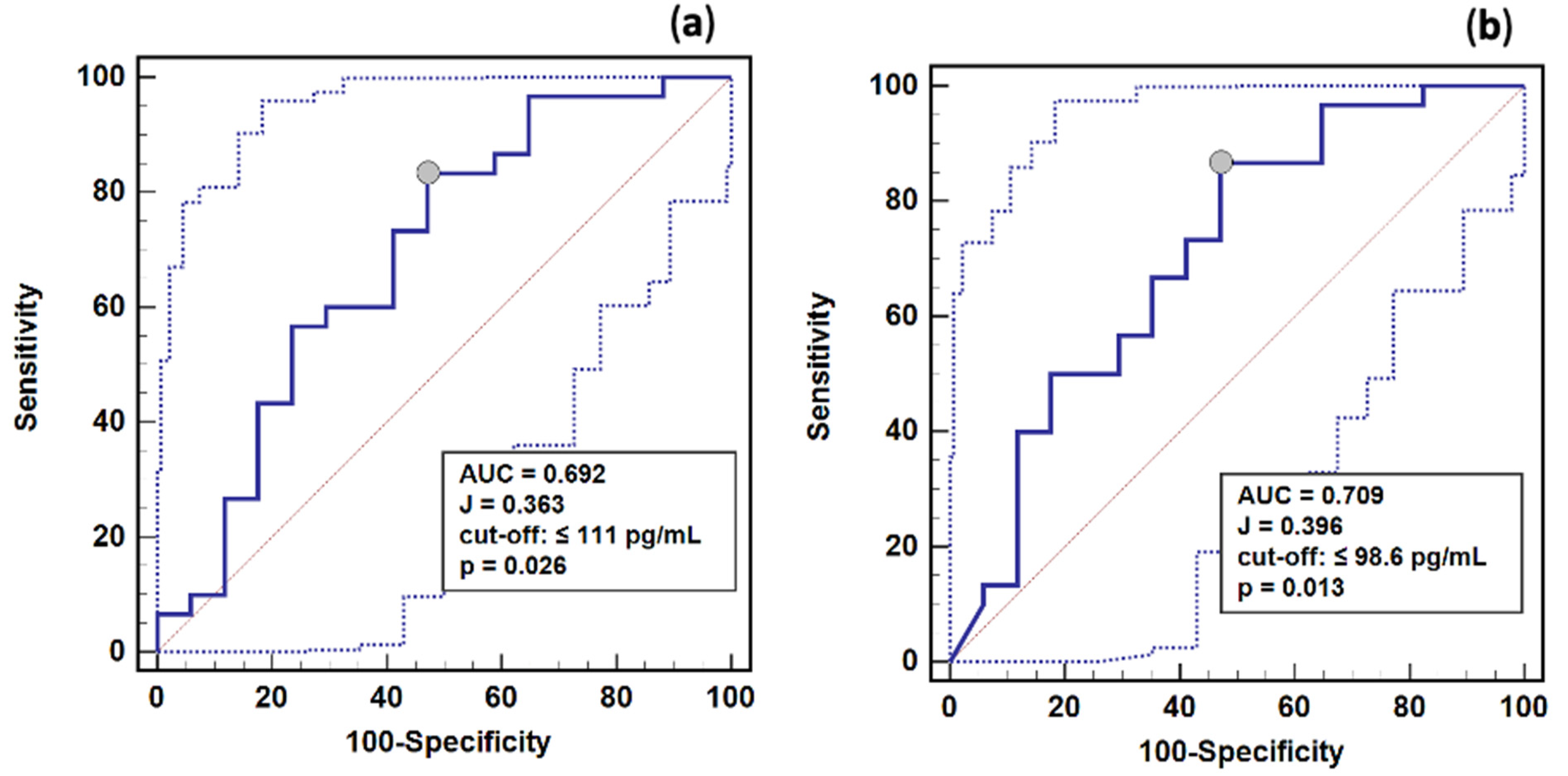
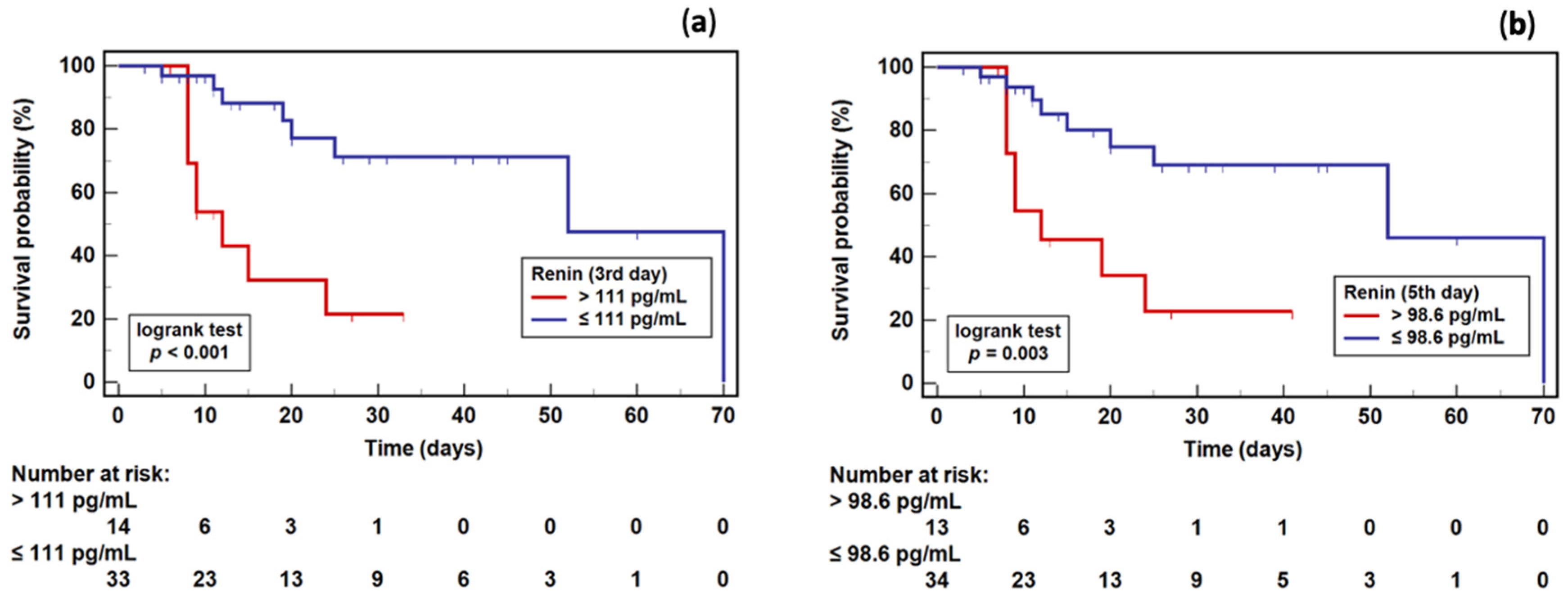
2.7. Renin as a Survival Marker
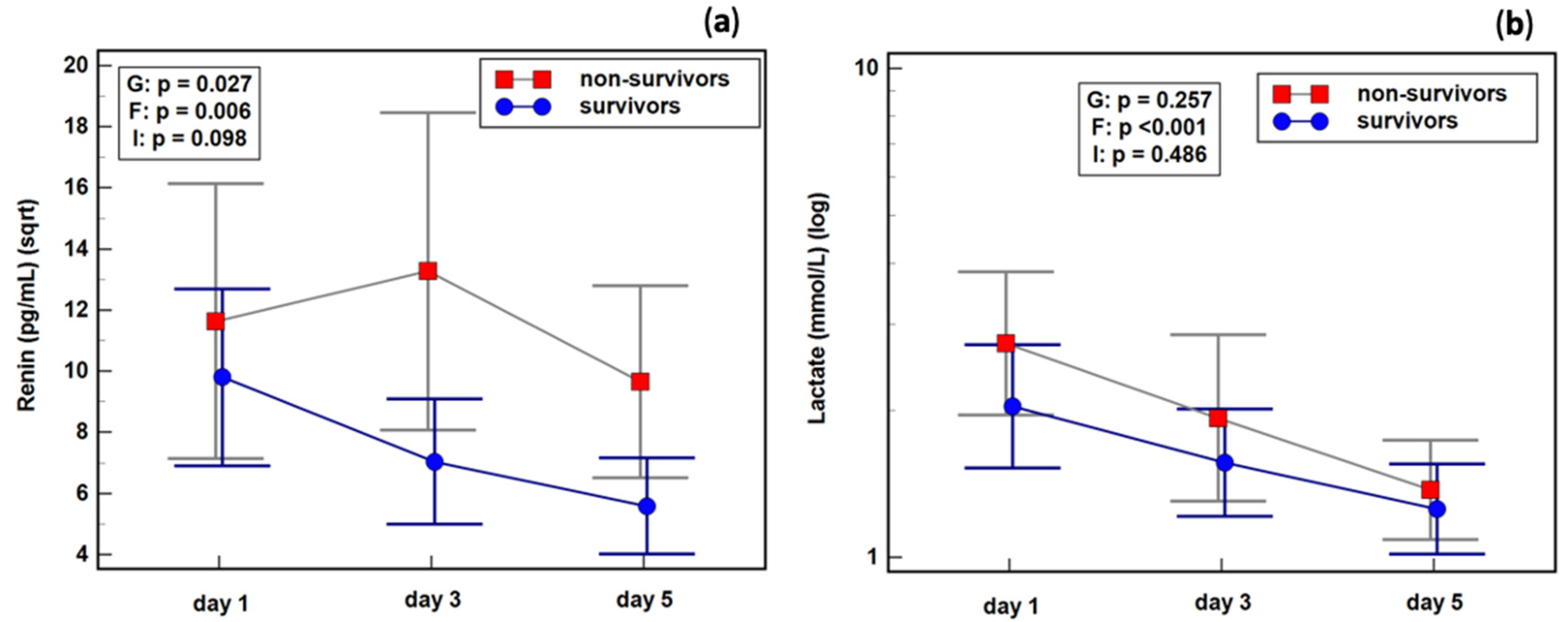
2.8. Renin Dynamics in Sepsis Survivors and Non-Survivors
3. Discussion
4. Materials and Methods
4.1. Study Patients
4.2. Study Design
4.3. Blood Sampling and Renin Determination
4.4. Ethical Considerations
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- World Health Organization. Sepsis. Available online: https://www.who.int/news-room/fact-sheets/detail/sepsis (accessed on 8 April 2022).
- Rhee, C.; Dantes, R.; Epstein, L.; Murphy, D.J.; Seymour, C.W.; Iwashyna, T.J.; Kadri, S.S.; Angus, D.C.; Danner, R.L.; Fiore, A.E.; et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009–2014. JAMA 2017, 318, 1241–1249. [Google Scholar] [CrossRef]
- Kaukonen, K.-M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality Related to Severe Sepsis and Septic Shock among Critically Ill Patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the Incidence and Mortality of Severe Sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Harrison, D.A.; Rubenfeld, G.D.; Rowan, K. Epidemiology of Sepsis and Septic Shock in Critical Care Units: Comparison between Sepsis-2 and Sepsis-3 Populations Using a National Critical Care Database. Br. J. Anaesth. 2017, 119, 626–636. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Hsu, W.-T.; Galm, B.P.; Schrank, G.; Hsu, T.-C.; Lee, S.-H.; Park, J.Y.; Lee, C.-C. Effect of Renin-Angiotensin-Aldosterone System Inhibitors on Short-Term Mortality After Sepsis: A Population-Based Cohort Study. Hypertension 2020, 75, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Nethathe, G.D.; Cohen, J.; Lipman, J.; Anderson, R.; Feldman, C. Mineralocorticoid Dysfunction during Critical Illness: A Review of the Evidence. Anesthesiology 2020, 133, 439–457. [Google Scholar] [CrossRef]
- Fyhrquist, F.; Saijonmaa, O. Renin-Angiotensin System Revisited. J. Intern. Med. 2008, 264, 224–236. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Du Cheyron, D.; Lesage, A.; Daubin, C.; Ramakers, M.; Charbonneau, P. Hyperreninemic Hypoaldosteronism: A Possible Etiological Factor of Septic Shock-Induced Acute Renal Failure. Intensive Care Med. 2003, 29, 1703–1709. [Google Scholar] [CrossRef]
- Gleeson, P.J.; Crippa, I.A.; Mongkolpun, W.; Cavicchi, F.Z.; Van Meerhaeghe, T.; Brimioulle, S.; Taccone, F.S.; Vincent, J.-L.; Creteur, J. Renin as a Marker of Tissue-Perfusion and Prognosis in Critically Ill Patients. Crit. Care Med. 2019, 47, 152–158. [Google Scholar] [CrossRef]
- Plataki, M.; Kashani, K.; Cabello-Garza, J.; Maldonado, F.; Kashyap, R.; Kor, D.J.; Gajic, O.; Cartin-Ceba, R. Predictors of Acute Kidney Injury in Septic Shock Patients: An Observational Cohort Study. Clin. J. Am. Soc. Nephrol. 2011, 6, 1744–1751. [Google Scholar] [CrossRef]
- Rizvi, M.S.; Kashani, K.B. Biomarkers for Early Detection of Acute Kidney Injury. J. Appl. Lab. Med. 2017, 2, 386–399. [Google Scholar] [CrossRef]
- Laesser, M.; Oi, Y.; Ewert, S.; Fändriks, L.; Aneman, A. The Angiotensin II Receptor Blocker Candesartan Improves Survival and Mesenteric Perfusion in an Acute Porcine Endotoxin Model. Acta Anaesthesiol. Scand. 2004, 48, 198–204. [Google Scholar] [CrossRef]
- Hirano, Y.; Takeuchi, H.; Suda, K.; Hagiwara, T.; Miyasho, T.; Kawamura, Y.; Yamada, S.; Oyama, T.; Takahashi, T.; Wada, N.; et al. (Pro)Renin Receptor Blocker Improves Survival of Rats with Sepsis. J. Surg. Res. 2014, 186, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.K. Tissue Perfusion and Prognosis in the Critically Ill-Is Renin the New Lactate? Crit. Care Med. 2019, 47, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Vieweg, R.; Järemo, M.; Steinvall, I.; Elmasry, M.; Abdelrahman, I.; Sjöberg, F. Renin Signals Renal Hypoperfusion during Parkland Fluid Resuscitation of Severe Burns—A Prospective Longitudinal Cohort Study. Int. J. Burns Trauma 2020, 10, 331–337. [Google Scholar] [PubMed]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and Therapeutic Approach to Elevated Lactate Levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef]
- Chung, K.S.; Song, J.H.; Jung, W.J.; Kim, Y.S.; Kim, S.K.; Chang, J.; Park, M.S. Implications of Plasma Renin Activity and Plasma Aldosterone Concentration in Critically Ill Patients with Septic Shock. Korean J. Crit. Care Med. 2017, 32, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, A. Control of Renin Synthesis and Secretion. Am. J. Hypertens. 2012, 25, 839–847. [Google Scholar] [CrossRef]
- Choi, S.J.; Ha, E.-J.; Jhang, W.K.; Park, S.J. Elevated Central Venous Pressure Is Associated with Increased Mortality in Pediatric Septic Shock Patients. BMC Pediatr. 2018, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Doerschug, K.C.; Delsing, A.S.; Schmidt, G.A.; Ashare, A. Renin-Angiotensin System Activation Correlates with Microvascular Dysfunction in a Prospective Cohort Study of Clinical Sepsis. Crit. Care 2010, 14, R24. [Google Scholar] [CrossRef]
- Wang, X.-T.; Yao, B.; Liu, D.-W.; Zhang, H.-M. Central Venous Pressure Dropped Early Is Associated with Organ Function and Prognosis in Septic Shock Patients: A Retrospective Observational Study. Shock 2015, 44, 426–430. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients | Condition/Subtype | Survival | ||
|---|---|---|---|---|---|
| Sepsis | Septic Shock | Survivors | Non-Survivors | ||
| N | 48 | 19 | 29 | 31 | 17 |
| Sex, F/M (%) | 19/29 | 5/14 | 14/15 1 | 14/17 | 5/12 2 |
| Age (years), Me (IQR) | 65 (54.3; 71.5) | 69 (51.3–73.5) | 63.5 (55–70) 1 | 63 (54.3–70) | 66 (54.5–73) 2 |
| APACHE II, Me (IQR) | 23.5 (16.5; 29) | 16 (13.3–21) | 26 (22–30) 3 | 19 (15–28.5) | 25 (22.8–29.3) 2 |
| SOFA, Me (IQR) | 10 (8; 13) | 8 (6.3–10.8) | 12 (9.8–14) 3 | 10 (7–12.8) | 11 (9–14) 2 |
| GCS, Me (IQR) | 14.5 (10.5; 15) | 15 (13.3–15) | 13 (8–15) 3 | 15 (13–15) | 12 (8–13.5) 4 |
| HGB (g/dL), Me (IQR) | 10.4 (9.7; 12.1) | 10.1 (8.3; 10.7) | 10.6 (9.9; 12.6) 3 | 10.4 (9.2; 12.3) | 10.2 (9.9; 11.9) 2 |
| HCT (%), Me (IQR) | 32 (28.5; 36.4) | 30.6 (25.4; 31.8) | 33 (30; 40) 3 | 31.3 (27.6; 38.4) | 32.2 (30.1; 35.3) 2 |
| WBC (×103/µL), M ± SD | 15.5 ± 10.4 | 14.97 ± 9.2 | 15.88 ± 11.3 1 | 14.66 ± 9.7 | 17.08 ± 11.8 2 |
| PLT (×103/µL), M ± SD | 228 ± 107 | 253 ±112 | 212 ± 103 1 | 243 ± 106 | 200 ± 107 2 |
| INR, Me (IQR) | 1.241 (1.11; 1.45) | 1.2 (1.07; 1.28) | 1.32 (1.12; 1.6) 1 | 1.211 (1.12; 1.47) | 1.286 (1.17; 1.41) 2 |
| D-dim. (µg/mL), Me (IQR) | 6.1 (4.3; 13.7) | 8.55 (4.6; 13.8) | 5.58 (4.1; 13.7) 1 | 7.68 (4; 14.7) | 5.55 (4.9; 13.1) 2 |
| SaO2 (%), Me (IQR) | 97.8 (95.3; 99) | 98.4 (97.5; 99.2) | 96.2 (92; 98.8) 3 | 97.6 (95.1; 99) | 98 (95.6; 99.1) 2 |
| PaO2 (mmHg), Me (IQR) | 111 (88; 133) | 126 (107; 175) | 84 (67; 135) 3 | 111 (71; 145) | 111 (81; 162) 2 |
| PaCO2 (mmHg), Me (IQR) | 38.7 (35.9; 45.4) | 38.4 (36.6; 42.4) | 38.8 (30.8; 45.8) 1 | 39 (36.7; 45.5) | 37.8 (29.9; 44) 2 |
| PaO2/FiO2, M ± SD | 245.8 ± 151.5 | 273.5 ± 155.3 | 224.2 ± 148.2 1 | 233.2 ± 155.8 | 270.2 ± 145.2 2 |
| BE (mM), M ± SD | −3.61 ± 5.9 | −3.28 ± 4.7 | −3.83 ± 6.6 1 | −3.16 ± 5.9 | −4.42 ± 5.9 2 |
| HCO3- (mM), Me (IQR) | 21.5 (17.6; 25.3) | 21.8 (19; 25.5) | 20.9 (17.3; 24) 1 | 21.8 (19; 25.2) | 20.8 (16.7; 26.5) 2 |
| Lactate, Me (IQR) | 1.95 (1.4; 4.0) | 1.3 (0.9; 1.6) | 2.7 (2.1; 5.1) 3 | 1.7 (1.2; 4.1) | 2.4 (1.9; 3.8) 2 |
| GLU (mg%), Me (IQR) | 145 (118; 194) | 130 (109; 149) | 150 (124; 207) 3 | 146 (115; 202) | 140 (118; 182) 2 |
| Urea (mg/dL), Me (IQR) | 77 (48; 120) | 57 (42; 87) | 88.5 (58.5; 146.5) 3 | 75 (52; 107) | 85 (45; 132) 2 |
| CREAT (mg/dL), Me (IQR) | 1.64 (0.85; 2.35) | 1.03 (0.74; 2.19) | 1.76 (1.17; 2.47) 1 | 1.72 (0.8; 2.55) | 1.39 (0.9; 2.3) 2 |
| DIUR (mL/day), Me (IQR) | 1140 (330; 230) | 1095 (913; 1910) | 1185 (100; 2375) 1 | 1095 (379; 1968) | 1710 (80; 2145) 2 |
| BIL (mg/dL), Me (IQR) | 0.9 (0.6; 1.5) | 0.9 (0.6; 1.3) | 0.9 (0.5; 1.6) 1 | 0.9 (0.6; 1.4) | 0.95 (0.6; 1.8) 2 |
| ASPAT (U/L), Me (IQR) | 64 (27.5; 218) | 27 (14.5; 74.8) | 128 (47.5; 354) 3 | 56 (39.5; 279) | 100 (24; 183) 2 |
| ALAT (U/L), Me (IQR) | 58 (20.8; 83.5) | 27 (12; 63) | 63 (37.5; 221) 3 | 59 (13.8; 99.5) | 58 (46; 73) 2 |
| CRP (mg/L), M ± SD | 238.4 ± 119.4 | 261 ± 116.4 | 221.9 ± 121.1 1 | 240.2 ± 110.3 | 234.4 ± 141.9 2 |
| PCT (ng/mL), Me (IQR) | 6.43 (2.44; 43.1) | 3 (1.3; 21.7) | 8.4 (4.4; 55.5) 1 | 5.3 (2.4; 18.3) | 22.8 (3; 55) 2 |
| MAP (mmHg), Me (IQR) | 95.3 (78.8; 126.9) | 93.3 (82.4; 107) | 109 (70; 197) 1 | 95 (77.2; 112) | 107.4 (78.8; 166) 2 |
| CVP (mmHg), M ± SD | 7 ± 3.1 | 7.7 ± 3.3 | 6.4 ± 2.9 1 | 7.3 ± 2.8 | 6.6 ± 3.7 2 |
| Source of sepsis, n (%): | |||||
| IAI | 19 (39.6) | 11 (57.9) | 8 (27.6) 1 | 13 (41.9) | 6 (35.3) 2 |
| CNS | 3 (6.3) | 1 (5.3) | 2 (6.9) | 0 | 3 (17.6) |
| PNEU | 19 (39.6) | 6 (31.6) | 13 (44.8) | 12 (38.7) | 7 (41.2) |
| UTI | 4 (8.3) | 1 (5.3) | 3 (10.3) | 4 (12.9) | 0 |
| SSTI | 2 (4.2) | 0 | 2 (6.9) | 1 (3.2) | 1 (5.9) |
| BSI | 1 (2.1) | 0 | 1 (3.5) | 1 (3.22) | 0 |
| CRRT, n (%): | |||||
| Day 1 | 17 (35.4) | 3 (15.8) | 14 (48.3) 3 | 10 (32.3) | 7 (41.2) 2 |
| Day 3 | 14 (29.2) | 2 (10.5) | 12 (41.4) 3 | 8 (25.8) | 6 (35.3) 2 |
| Day 5 | 15 (31.2) | 2 (10.5) | 13 (44.8) 3 | 29 (29) | 6 (35.3) 2 |
| Parameter | Renin | ||
|---|---|---|---|
| 1st Day | 3rd Day | 5th Day | |
| ASPAT | 0.36 2 | 0.13 1 | 0 1 |
| ALAT | 0.34 2 | 0.26 1 | 0.17 1 |
| Bilirubin | 0.10 1 | 0.28 1 | 0.35 2 |
| CRP | −0.08 1 | 0.13 1 | 0.33 2 |
| WBC | 0 1 | 0 1 | 0.40 3 |
| CVP | 0.19 1 | 0.34 2 | 0.22 1 |
| pH | 0.13 1 | −0.16 1 | −0.32 2 |
| Lactate | 0.33 2 | 0.28 1 | 0.34 2 |
| Urea | −0.03 1 | −0.10 1 | −0.29 2 |
| HCT | 0.36 2 | −0.18 1 | 0 1 |
| HGB | 0.32 2 | −0.15 1 | 0.10 1 |
| SOFA | 0.02 1 | 0.31 2 | 0.35 2 |
| Parameter | AUC (95% CI), p | Cut-Off | J | Sens. % | Spec. % | LR+ | LR− |
|---|---|---|---|---|---|---|---|
| Renin | 0.770 (0.62–0.88), p < 0.0001 | >87 pg/mL | 0.634 | 69 | 94.4 | 12.4 | 0.33 |
| SOFA | 0.787 (0.65–0.89), p < 0.0001 | >8 | 0.528 | 89.7 | 63.2 | 2.4 | 0.16 |
| APACHE II | 0.815 (0.68–0.91), p < 0.0001 | >21 | 0.583 | 79.3 | 79 | 3.8 | 0.26 |
| Lactate | 0.904 (0.78–0.97), p < 0.0001 | >1.8 mmol/L | 0.809 | 86.2 | 94.7 | 16.4 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leśnik, P.; Łysenko, L.; Krzystek-Korpacka, M.; Woźnica-Niesobska, E.; Mierzchała-Pasierb, M.; Janc, J. Renin as a Marker of Tissue Perfusion, Septic Shock and Mortality in Septic Patients: A Prospective Observational Study. Int. J. Mol. Sci. 2022, 23, 9133. https://doi.org/10.3390/ijms23169133
Leśnik P, Łysenko L, Krzystek-Korpacka M, Woźnica-Niesobska E, Mierzchała-Pasierb M, Janc J. Renin as a Marker of Tissue Perfusion, Septic Shock and Mortality in Septic Patients: A Prospective Observational Study. International Journal of Molecular Sciences. 2022; 23(16):9133. https://doi.org/10.3390/ijms23169133
Chicago/Turabian StyleLeśnik, Patrycja, Lidia Łysenko, Małgorzata Krzystek-Korpacka, Ewa Woźnica-Niesobska, Magdalena Mierzchała-Pasierb, and Jarosław Janc. 2022. "Renin as a Marker of Tissue Perfusion, Septic Shock and Mortality in Septic Patients: A Prospective Observational Study" International Journal of Molecular Sciences 23, no. 16: 9133. https://doi.org/10.3390/ijms23169133
APA StyleLeśnik, P., Łysenko, L., Krzystek-Korpacka, M., Woźnica-Niesobska, E., Mierzchała-Pasierb, M., & Janc, J. (2022). Renin as a Marker of Tissue Perfusion, Septic Shock and Mortality in Septic Patients: A Prospective Observational Study. International Journal of Molecular Sciences, 23(16), 9133. https://doi.org/10.3390/ijms23169133






