Disrupted Decision-Making: EcoHIV Inoculation in Cocaine Dependent Rats
Abstract
1. Introduction
2. Results
2.1. Phase 1.3: Escalation of Cocaine-Maintained Responding Supports the Development of a Drug Dependent Phenotype
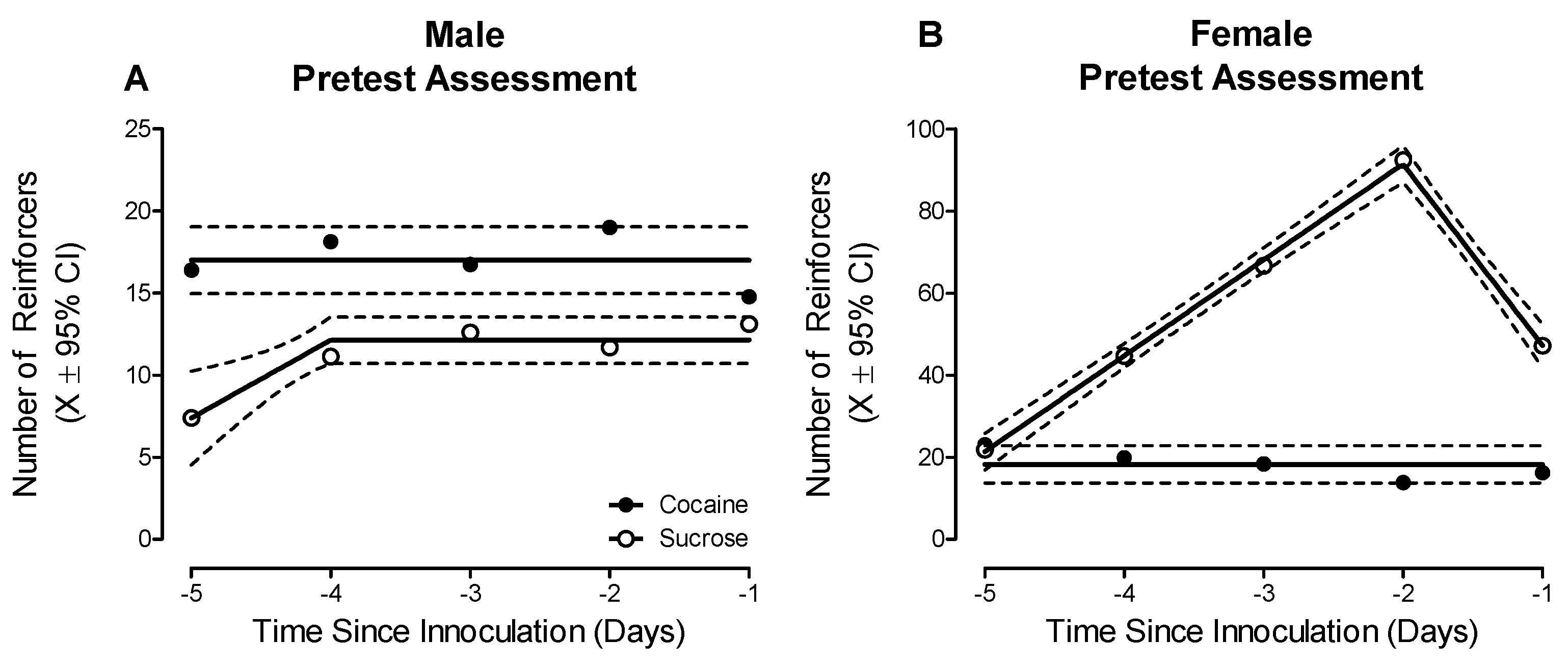
2.2. Phase 2.1: Prominent Sex Differences in Preference Judgment Were Observed during the Pretest Evaluation
2.3. Phase 3.1.: Bilateral Retro-Orbital Inoculation of EcoHIV Induced Significant HIV Infection in the Medial Prefrontal Cortex; Infection Which Is Primarily Harbored in Microglia
2.4. Phases 3.2 to 3.5: EcoHIV Inoculation Disrupted Preference Judgment, but Not Reinforcing Efficacy, during Posttest Evaluations
2.5. EcoHIV Animals Exhibited Blunted Extinction Learning following Removal of the Preferred Reinforcer
2.6. Phase 4: Inoculation with EcoHIV Induced Prominent Synaptodendritic Alterations in Pyramidal Neurons, and Their Associated Dendritic Spines, in the Medial Prefrontal Cortex
3. Discussion
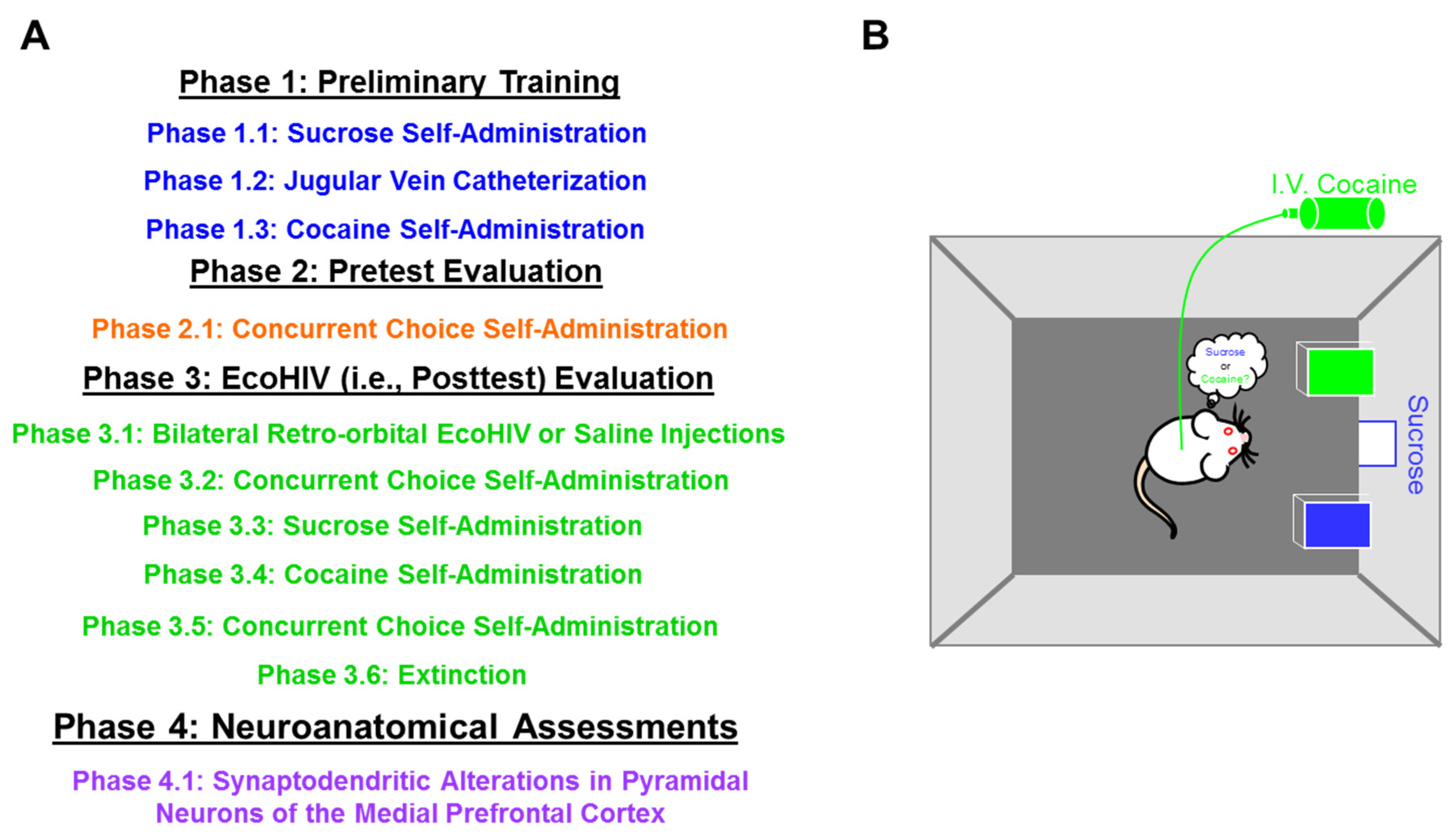
4. Materials and Methods
4.1. Animals
4.2. Apparatus
4.3. Drugs
4.4. Phase 1: Preliminary Training
4.4.1. Phase 1.1: Sucrose Self-Administration
4.4.2. Phase 1.2: Jugular Vein Catheterization
4.4.3. Phase 1.3: Cocaine Self-Administration
4.5. Phase 2: Pretest Evaluation
Phase 2.1: Concurrent Choice Self-Administration
4.6. Phase 3: EcoHIV (i.e., Posttest) Evaluation
4.6.1. Phase 3.1: Bilateral Retro-Orbital EcoHIV or Saline Injections
4.6.2. Phase 3.2: Concurrent Choice Self-Administration (3 to 9 Days Post Inoculation)
4.6.3. Phase 3.3: Sucrose Self-Administration (8 to 22 Days Post Inoculation)
4.6.4. Phase 3.4: Cocaine Self-Administration (21 to 39 Days Post Inoculation)
4.6.5. Phase 3.5: Concurrent Choice Self-Administration (37 to 45 Days Post Inoculation)
4.6.6. Phase 3.6: Extinction (42 to 52 Days Post Inoculation)
4.7. Phase 4: Neuroanatomical Assessments
Phase 4.1: Synaptodendritic Alterations in Pyramidal Neurons in the Medial Prefrontal Cortex
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. Available online: https://www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf (accessed on 14 April 2022).
- Novak, S.P.; Kral, A.H. Comparing injection and non-injection routes of administration for heroin, methamphetamine, and cocaine uses in the United States. J. Addict. Dis. 2011, 30, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, R.; McCusker, J. Cocaine use and risky injection and sexual behaviors. Drug Alcohol Depend. 1995, 37, 7–14. [Google Scholar] [CrossRef]
- Chaisson, R.E.; Bacchetti, P. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA 1989, 261, 561–565. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.B.; Lai, S. Injection drug use and crack cocaine smoking: Independent and dual risk behaviors for HIV infection. Ann. Epidemiol. 2004, 14, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Global HIV & Aids Statistics-Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 14 April 2022).
- Illenberger, J.M.; Harrod, S.B. HIV infection and neurocognitive disorders in the context of chronic drug abuse: Evidence for divergent findings dependent upon prior drug history. J. Neuroimmune Pharmacol. 2020, 15, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E.; DeLong, M.R. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef]
- Alexander, G.E.; Crutcher, M.D. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci. 1990, 13, 266–271. [Google Scholar] [CrossRef]
- Alexander, G.E. Basal ganglia-thalamocortical circuits: Their role in control of movements. J. Clin. Neurophysiol. 1994, 11, 420–431. [Google Scholar] [CrossRef]
- Haber, S.N.; Kunishio, K. The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci. 1995, 15, 4851–4867. [Google Scholar] [CrossRef]
- Sesack, S.R.; Deutch, A.Y. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 1989, 290, 213–242. [Google Scholar] [CrossRef]
- Sesack, S.R.; Pickel, V.M. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J. Comp. Neurol. 1992, 320, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Oades, R.D.; Halliday, G.M. Ventral tegmental (A10) system: Neurobiology. 1. Anatomy and connectivity. Brain Res. 1987, 434, 117–165. [Google Scholar] [CrossRef]
- Swanson, L.W. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 1982, 9, 321–353. [Google Scholar] [CrossRef]
- Ray, J.P.; Price, J.L. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1993, 337, 1–31. [Google Scholar] [CrossRef]
- Watabe-Uchida, M.; Zhu, L. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 2012, 74, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, C.W.; Nobiletti, J.B. Cocaine and cocaethylene: Microdialysis comparison of brain drug levels and effects on dopamine and serotonin. J. Neurochem. 1993, 60, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Ponteieri, F.E.; Tanda, G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc. Natl. Acad. Sci. USA 1995, 92, 12304–12308. [Google Scholar] [CrossRef]
- Yuen, J.; Goyal, A. Cocaine-induced changes in tonic dopamine concentrations measured using multiple-cyclic square wave voltammetry in vivo. Front. Pharmacol. 2021, 12, 705254. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am. J. Psychiatry 1990, 147, 719–724. [Google Scholar]
- Volkow, N.D.; Wang, G.J. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 1997, 386, 830–833. [Google Scholar] [CrossRef]
- Ferris, M.J.; Calipari, E.S. Cocaine self-administration produces pharmacodynamics tolerance: Differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology 2012, 37, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Willuhn, I.; Burgeno, L.M. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat. Neurosci. 2014, 17, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Briand, L.A.; Flagel, S.B. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology 2008, 33, 2969–2980. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Robinson, T.E.; Gorny, G. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 2001, 39, 257–266. [Google Scholar] [CrossRef]
- McLaurin, K.A.; Harris, M. HIV-associated apathy/depression and neurocognitive impairments reflect persistent dopamine deficits. Cells 2021, 10, 2158. [Google Scholar] [CrossRef]
- Kumar, A.M.; Fernandez, J.B. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J. Neurovirol. 2009, 15, 257–274. [Google Scholar] [CrossRef]
- Denton, A.R.; Samaranayake, S.A. Selective monoaminergic and histaminergic circuit dysregulation following long-term HIV-1 protein exposure. J. Neurovirol. 2019, 25, 540–550. [Google Scholar] [CrossRef]
- Vázquez-Santiago, F.J.; Noel, R.J. Glutamate metabolism and HIV-1 associated neurocognitive disorders. J. Neurovirol. 2014, 20, 315–331. [Google Scholar] [CrossRef]
- Moore, D.J.; Masliah, E. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 2006, 20, 879–887. [Google Scholar] [CrossRef]
- Roscoe, R.F.; Mactutus, C.F. HIV-1 transgenic female rat: Synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J. Neuroimmune Pharmacol. 2014, 9, 642–653. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.A.; Li, H. Disruption of timing: NeuroHIV progression in the post-cART era. Sci. Rep. 2019, 9, 827. [Google Scholar] [CrossRef]
- Festa, L.K.; Irollo, E. CXCL12-induced rescue of cortical dendritic spines and cognitive flexibility. Elife 2020, 9, e49717. [Google Scholar] [CrossRef]
- Speidell, A.; Asuni, G.P. Up-regulation of the p75 neurotrophin receptor is an essential mechanism for HIV-gp120 mediated synaptic loss in the striatum. Brain Behav. Immun. 2020, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wang, G.J. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage 2008, 42, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.M.; Ownby, R.L. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: Relationship with neuropsychological performance. J. Neurovirol. 2011, 17, 26–40. [Google Scholar] [CrossRef]
- Paul, R.H.; Brickman, A.M. Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 167–171. [Google Scholar] [CrossRef]
- Kamat, R.; Brown, G.G. Apathy is associated with white matter abnormalities in anterior, medial brain regions in persons with HIV infection. J. Clin. Exp. Neuropsychol. 2014, 36, 854–866. [Google Scholar] [CrossRef][Green Version]
- Potash, M.J.; Chao, W. A mouse model for study o systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc. Natl. Acad. Sci. USA 2005, 102, 3760–3765. [Google Scholar] [CrossRef]
- Kim, J.W.; Closs, E.I. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature 1991, 352, 725–728. [Google Scholar] [CrossRef]
- Wang, H.; Kavanaugh, M.P. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature 1991, 352, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, P.; Hadas, E. HIV infection model of chronic obstructive pulmonary disease in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L500–L509. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.J.; Borjabad, A. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog. 2018, 14, e1007061. [Google Scholar] [CrossRef] [PubMed]
- Kelschenbach, J.; He, H. Efficient expression of HIV in immunocompetent mouse brain reveals a novel nonneurotoxic viral function in hippocampal synaptodendritic injury and memory impairment. mBio 2019, 10, e00591-19. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; McLaurin, K.A. Microglial HIV-1 expression: Role in HIV-1 associated neurocognitive disorders. Viruses 2021, 13, 924. [Google Scholar] [CrossRef]
- Li, H.; McLaurin, K.A. A rat model of EcoHIV brain infection. J. Vis. Exp. 2021, 167, e62137. [Google Scholar] [CrossRef]
- Fellows, L.K. The role of orbitofrontal cortex in decision making: A component process account. Ann. N. Y. Acad. Sci. 2007, 1121, 421–430. [Google Scholar] [CrossRef]
- Edwards, S.; Koob, G.F. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav. Pharmacol. 2013, 24, 356–362. [Google Scholar] [CrossRef]
- Ko, A.; Kang, G. Macrophages but not astrocytes harbor HIV DNA in the brains of HIV-1-infected aviremic individuals on suppressive antiretroviral therapy. J. Neuroimmune Pharmacol. 2019, 14, 110–119. [Google Scholar] [CrossRef]
- Richardson, N.R.; Roberts, D.C. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J. Neurosci. Methods 1996, 66, 1–11. [Google Scholar] [CrossRef]
- Li, H.; McLaurin, K.A. Ballistic labeling of pyramidal neurons in brain slices and in primary cell culture. J. Vis. Exp. 2020, 158, e60989. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Koob, G.F. Transition from moderate to excessive drug intake: Change in hedonic set point. Science 1998, 282, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Koob, G.F. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999, 146, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.; Liu, Y. Rapid and persistent sensitization to the reinforcing effects of cocaine. Neuropsychopharmacology 2006, 31, 121–128. [Google Scholar] [CrossRef][Green Version]
- McLaurin, K.A.; Bertrand, S.J. S-Equol mitigates motivational deficits and dysregulation associated with HIV-1. Sci. Rep. 2021, 11, 11870. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Koob, G.F. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology (Berl) 2003, 172, 450–454. [Google Scholar] [CrossRef]
- Bertrand, S.J.; Mactutus, C.F. HIV-1 proteins dysregulate motivational processes and dopamine circuitry. Sci. Rep. 2018, 8, 7869. [Google Scholar] [CrossRef]
- Hao, Y.; Martin-Fardon, R. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: Factor in the transition to dependence. Biol. Psychiatry 2010, 68, 240–248. [Google Scholar] [CrossRef]
- Ben-Shahar, O.; Obara, I. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse 2009, 63, 598–609. [Google Scholar] [CrossRef]
- Ploense, K.L.; Vieira, P. Contributions of prolonged contingent and non-contingent cocaine exposure to escalation of cocaine intake and glutamatergic gene expression. Psychopharmacology (Berl) 2017, 235, 1347–1359. [Google Scholar] [CrossRef]
- Martin, E.M.; Pitrak, D.L. Cognitive impulsivity and HIV serostatus in substance dependent males. J. Int. Neuropsychol. Soc. 2004, 10, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.J.; Hinkin, C.H. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology 2006, 20, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Iudicello, J.E.; Woods, S.P. Risky decision-making in HIV-associated neurocognitive disorders (HAND). Clin. Neuropsychol. 2013, 27, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Damasio, H. Dissociation of working memory from decision making within the human prefrontal cortex. J. Neurosci. 1998, 18, 428–437. [Google Scholar] [CrossRef]
- Walton, M.E.; Bannerman, D.M. The role of rat medial frontal cortex in effort-based decision making. J. Neurosci. 2002, 22, 10996–11003. [Google Scholar] [CrossRef]
- Cardinal, R.N.; Pennicott, D.R. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 2001, 292, 2499–2501. [Google Scholar] [CrossRef] [PubMed]
- Hauber, W.; Sommer, S. Prefrontostriatal circuitry regulates effort-related decision making. Cereb. Cortex 2009, 19, 2240–2247. [Google Scholar] [CrossRef]
- Floresco, S.B.; Montes, D.R. Differential contributions of nucleus accumbens subregions to cue-guided risk/reward decision making and implementation of conditional rules. J. Neurosci. 2018, 38, 1901–1914. [Google Scholar] [CrossRef]
- Bernosky-Smith, K.A.; Qiu, Y.Y. Ventral tegmental area D2 receptor knockdown enhances choice impulsivity in a delay-discounting task in rats. Behav. Brain Res. 2018, 341, 129–134. [Google Scholar] [CrossRef]
- Baylis, L.L.; Gaffan, D. Amygdalectomy and ventromedial prefrontal ablation produce similar deficits in food choice and in simple object discrimination learning for an unseen reward. Exp. Brain Res. 1991, 86, 617–622. [Google Scholar] [CrossRef]
- Fellows, L.K.; Farah, M.J. The role of ventromedial prefrontal cortex in decision making: Judgment under uncertainty or judgment per se? Cereb. Cortex 2007, 17, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Henri-Bhargava, A.; Simioni, A. Ventromedial frontal lobe damage disrupts the accuracy, but not the speed, of value-based preference judgments. Neuropsychologia 2012, 50, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Hori, Y. Chemogenetic dissection of the primary prefronto-subcortical pathways for working memory and decision-making. Sci. Adv. 2021, 7, eabg4246. [Google Scholar] [CrossRef] [PubMed]
- Spruston, N. Pyramidal neurons: Dendritic structure and synaptic integration. Nat. Rev. Neurosci. 2008, 9, 206–221. [Google Scholar] [CrossRef]
- Hersch, S.M.; White, E.L. Quantification of synapses formed with apical dendrites of Golgi-impregnated pyramidal cells: Variability in thalamocortical inputs, but consistency in the ratios of asymmetrical to symmetrical synapses. Neuroscience 1981, 6, 1043–1051. [Google Scholar] [CrossRef]
- Santana, N.; Artigas, F. Laminar and cellular distribution of monoamine receptors in rat medial prefrontal cortex. Front. Neuroanat. 2017, 11, 87. [Google Scholar] [CrossRef]
- Peters, A.; Kaiserman-Abramof, I.R. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am. J. Anat. 1970, 127, 321–355. [Google Scholar] [CrossRef]
- Freund, T.F.; Powell, J.F. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience 1984, 13, 1189–1215. [Google Scholar] [CrossRef]
- Cirino, T.J.; Harden, S.W. Region-specific effects of HIV-1 Tat on intrinsic electophysiological properties of pyramidal neurons in mouse prefrontal cortex and hippocampus. J. Neurophysiol. 2020, 123, 1332–1341. [Google Scholar] [CrossRef]
- Cosenza, M.A.; Zhao, M.L. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002, 12, 442–455. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.E.; Lowery, R.L. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010, 8, e1000527. [Google Scholar] [CrossRef] [PubMed]
- Garvey, L.J.; Pavese, N. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS 2014, 28, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Cornell, J.; Salinas, S. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen. Res. 2022, 17, 705–716. [Google Scholar] [PubMed]
- Domercq, M.; Vazquez-Villoldo, N. Neurotransmitter signaling in the pathophysiology of microglia. Front. Cell. Neurosci. 2013, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Leak, R.K. Neurotrasmitter receptors on microglia. Stroke Vasc. Neurol. 2016, 1, 52–58. [Google Scholar] [CrossRef]
- Mishra, N.; Mohata, M. Expression of complement receptor 3 (CR3) and regulatory protein CD46 on dendritic cells of antiretroviral naïve and treated HIV-1 infected individuals: Correlation with immune activation status. Mol. Immunol. 2018, 96, 83–87. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Tremblay, M.E.; Marker, D.F. Ultrastructure of microglia-synapse interactions in the HIV-1 Tat-injected muring central nervous system. Commun. Integr. Biol. 2013, 6, e27670. [Google Scholar] [CrossRef]
- Gupta, V.D. Stability of cocaine hydrochloride solutions at various pH values as determined by high-pressure liquid chromatography. Int. J. Pharm. 1982, 10, 249–257. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Elsevier Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Radley, J.J.; Rocher, A.B. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol. 2008, 507, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Arellano, J.I.; Benavides-Piccione, R. Ultrastructure of dendritic spines: Correlation between synaptic and spine morphologies. Front. Neurosci. 2007, 1, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Ruszczycki, B.; Szepesi, Z. Sampling issues in quantitative analysis of dendritic spines morphology. BMC Bioinform. 2012, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Konur, S.; Rabinowitz, D. Systematic regulation of spine sizes and densities in pyramidal neurons. J. Neurobiol. 2003, 56, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Greenhouse, S.W.; Geisser, S. On methods in the analysis of profile data. Psychometrica 1959, 24, 95–112. [Google Scholar] [CrossRef]
- Denenberg, V.H. Some statistical and experimental considerations in the use of the analysis-of-variance procedure. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1984, 246, R403–R408. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLaurin, K.A.; Li, H.; Mactutus, C.F.; Harrod, S.B.; Booze, R.M. Disrupted Decision-Making: EcoHIV Inoculation in Cocaine Dependent Rats. Int. J. Mol. Sci. 2022, 23, 9100. https://doi.org/10.3390/ijms23169100
McLaurin KA, Li H, Mactutus CF, Harrod SB, Booze RM. Disrupted Decision-Making: EcoHIV Inoculation in Cocaine Dependent Rats. International Journal of Molecular Sciences. 2022; 23(16):9100. https://doi.org/10.3390/ijms23169100
Chicago/Turabian StyleMcLaurin, Kristen A., Hailong Li, Charles F. Mactutus, Steven B. Harrod, and Rosemarie M. Booze. 2022. "Disrupted Decision-Making: EcoHIV Inoculation in Cocaine Dependent Rats" International Journal of Molecular Sciences 23, no. 16: 9100. https://doi.org/10.3390/ijms23169100
APA StyleMcLaurin, K. A., Li, H., Mactutus, C. F., Harrod, S. B., & Booze, R. M. (2022). Disrupted Decision-Making: EcoHIV Inoculation in Cocaine Dependent Rats. International Journal of Molecular Sciences, 23(16), 9100. https://doi.org/10.3390/ijms23169100






