Graphene Oxide (GO): A Promising Nanomaterial against Infectious Diseases Caused by Multidrug-Resistant Bacteria
Abstract
:1. Introduction
2. Graphene Oxide
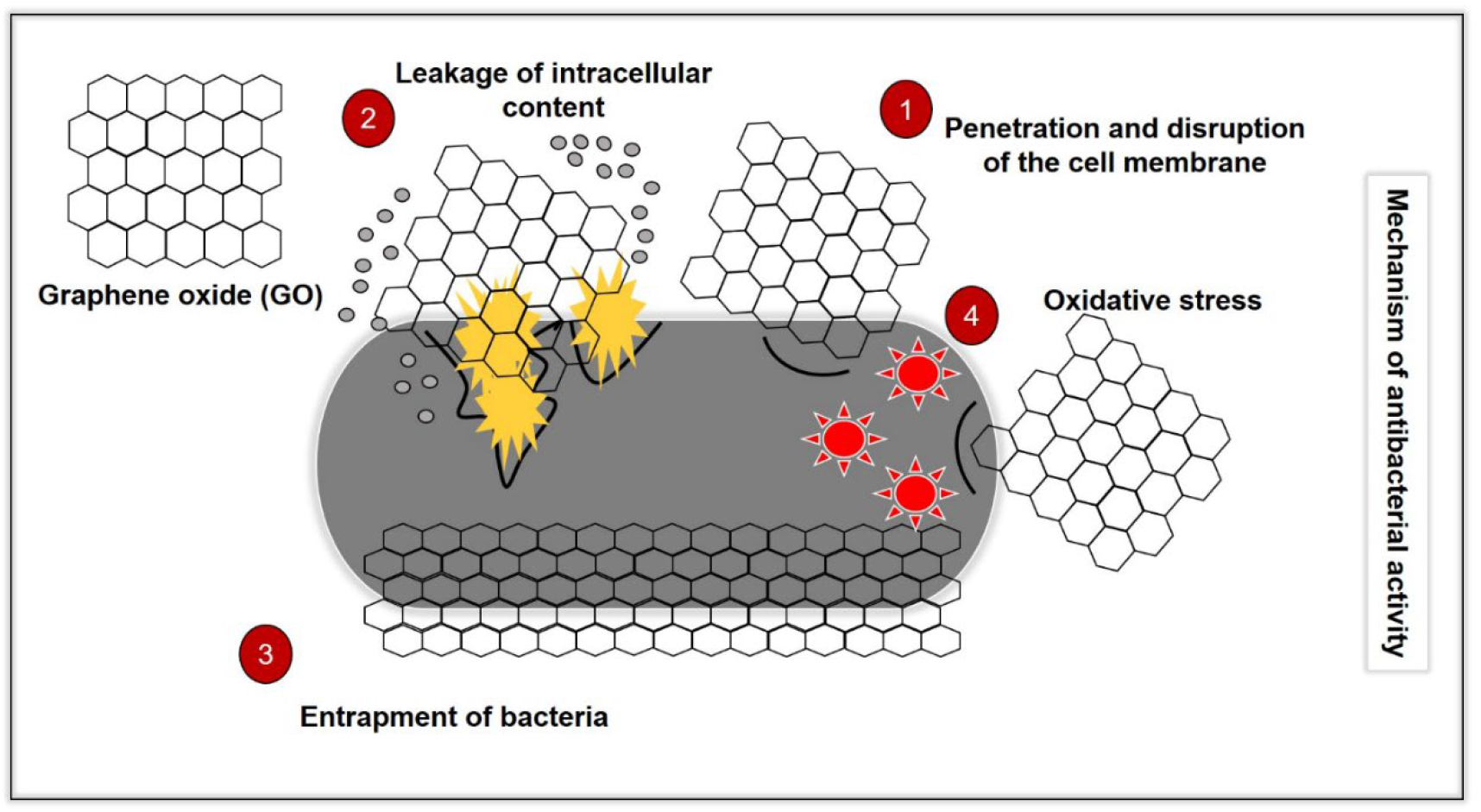
3. Mechanisms of Action
3.1. Disruption of Bacteria Cell Membrane
3.2. Bacteria Entrapping (Wrapping) Effect
- The method of size measurement used is different (area and diameter).
- The difference in ranges of the GO sizes. For example, one study may synthesise nanosize GO, while, in another study, microsized GO is used.
- The different types of bacteria with different sizes and concentrations used.
3.3. Oxidative Stress
4. Photoactivation of GO
5. Physicochemical Properties of GO Influence Its Antibacterial Effects
6. In Vitro Antibacterial Effects of GO
7. In Vivo Antibacterial Effects of GO
8. Biocompatibility of GO
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orecchioni, M.; Cabizza, R.; Bianco, A.; Delogu, L.G. Graphene as Cancer Theranostic Tool: Progress and Future Challenges. Theranostics 2015, 5, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Crocetta, V.; Scocchi, M.; Pomponio, S.; Di Vincenzo, V.; Mardirossian, M.; Gherardi, G.; Fiscarelli, E.; Dicuonzo, G.; Gennaro, R.; et al. Potential Novel Therapeutic Strategies in Cystic Fibrosis: Antimicrobial and Anti-Biofilm Activity of Natural and Designed -Helical Peptides against Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. BMC Microbiol. 2012, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Alsheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 23, 464–472. [Google Scholar]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid.-Based Complement. Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef]
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef]
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in Vivo Models. Front. Microbiol. 2021, 12, 677. [Google Scholar] [CrossRef]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, Facts, Opportunities, and Challenges in Nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef]
- Masoudipour, E.; Kashanian, S.; Maleki, N. A Targeted Drug Delivery System Based on Dopamine Functionalized Nano Graphene Oxide. Chem. Phys. Lett. 2017, 668, 56–63. [Google Scholar] [CrossRef]
- Wu, X.; Tan, S.; Xing, Y.; Pu, Q.; Wu, M.; Zhao, J.X. Graphene Oxide as an Efficient Antimicrobial Nanomaterial for Eradicating Multi-Drug Resistant Bacteria in Vitro and in Vivo. Colloids Surf. B Biointerfaces 2017, 157, 1–9. [Google Scholar] [CrossRef]
- Aunkor, M.T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial Activity of Graphene Oxide Nanosheet against Multidrug Resistant Superbugs Isolated from Infected Patients. R. Soc. Open Sci. 2020, 7, 200640. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, R.R.; Mohammad, M.R.; Hussien, A.M.A. Antibacterial Activity and Morphological Characterization of Synthesis Graphene Oxide Nanosheets by Simplified Hummer’s Method. Biosci. Biotechnol. Res. Asia 2018, 15, 627–633. [Google Scholar] [CrossRef]
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; Di Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and Antibiofilm Efficacy of Graphene Oxide against Chronic Wound Microorganisms. Antimicrob. Agents Chemother. 2018, 62, e00547-18. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of Antibacterial Mechanism of Graphene Oxide Using Raman Spectroscopy. Sci. Rep. 2016, 6, 28443. [Google Scholar] [CrossRef]
- Yu, C.H.; Chen, G.Y.; Xia, M.Y.; Xie, Y.; Chi, Y.Q.; He, Z.Y.; Zhang, C.L.; Zhang, T.; Chen, Q.M.; Peng, Q. Understanding the Sheet Size-Antibacterial Activity Relationship of Graphene Oxide and the Nano-Bio Interaction-Based Physical Mechanisms. Colloids Surf. B Biointerfaces 2020, 191, 111009. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Impact of Graphene Oxide on the Antibacterial Activity of Antibiotics against Bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]
- Zou, F.; Zhou, H.; Jeong, D.Y.; Kwon, J.; Eom, S.U.; Park, T.J.; Hong, S.W.; Lee, J. Wrinkled Surface-Mediated Antibacterial Activity of Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 1343–1351. [Google Scholar] [CrossRef]
- Barbolina, I.; Woods, C.R.; Lozano, N.; Kostarelos, K.; Novoselov, K.S.; Roberts, I.S. Purity of Graphene Oxide Determines Its Antibacterial Activity. 2D Mater. 2016, 3, 025025. [Google Scholar] [CrossRef]
- Karahan, H.E.; Wei, L.; Goh, K.; Liu, Z.; Birer, Ö.; Dehghani, F.; Xu, C.; Wei, J.; Chen, Y. Bacterial Physiology Is a Key Modulator of the Antibacterial Activity of Graphene Oxide. Nanoscale 2016, 8, 17181–17189. [Google Scholar] [CrossRef]
- Jayanthi, S.; Eswar, N.K.; Satyapaul, A.S.; Chatterjee, K.; Madras, G.; Sood, A.K. Macroporous Three-Dimensional Graphene Oxide Foams for Dye Adsorption and Antibacterial Applications. RSC Adv. 2015, 6, 1231–1242. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Umasuthan, N.; Mohan, R.; Lee, J.; Kim, S.J. Antibacterial Activity of Graphene Oxide Nanosheets. Sci. Adv. Mater. 2012, 4, 1111–1117. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Abdal Dayem, A.; Eppakayala, V.; Kim, J.H. Oxidative Stress-Mediated Antibacterial Activity of Graphene Oxide and Reduced Graphene Oxide in Pseudomonas Aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral Dimension-Dependent Antibacterial Activity of Graphene Oxide Sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Mokkapati, V.R.S.S.; Pandit, S.; Kim, J.; Martensson, A.; Lovmar, M.; Westerlund, F.; Mijakovic, I. Bacterial Response to Graphene Oxide and Reduced Graphene Oxide Integrated in Agar Plates. R. Soc. Open Sci. 2018, 5, 181083. [Google Scholar] [CrossRef] [PubMed]
- Seifi, T.; Kamali, A.R. Anti-Pathogenic Activity of Graphene Nanomaterials: A Review. Colloids Surf. B Biointerfaces 2021, 199, 111509. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Lu, X.; Feng, X.; Werber, J.R.; Chu, C.; Zucker, I.; Kim, J.-H.; Osuji, C.O.; Elimelech, M. Enhanced Antibacterial Activity through the Controlled Alignment of Graphene Oxide Nanosheets. Proc. Natl. Acad. Sci. USA 2017, 114, E9793–E9801. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; Von Dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene Microsheets Enter Cells through Spontaneous Membrane Penetration at Edge Asperities and Corner Sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Shi, X.; Gao, H. Cellular Entry of Graphene Nanosheets: The Role of Thickness, Oxidation and Surface Adsorption. RSC Adv. 2013, 3, 15776–15782. [Google Scholar] [CrossRef]
- Mao, J.; Guo, R.; Yan, L.T. Simulation and Analysis of Cellular Internalization Pathways and Membrane Perturbation for Graphene Nanosheets. Biomaterials 2014, 35, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive Extraction of Phospholipids from Escherichia Coli Membranes by Graphene Nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Bugli, F.; Lauriola, M.C.; Cacaci, M.; Torelli, R.; Ciasca, G.; Conti, C.; Sanguinetti, M.; Papi, M.; De Spirito, M. Bacteria Meet Graphene: Modulation of Graphene Oxide Nanosheet Interaction with Human Pathogens for Effective Antimicrobial Therapy. ACS Biomater. Sci. Eng. 2017, 3, 619–627. [Google Scholar] [CrossRef]
- Wu, L.; Liu, L.; Gao, B.; Muñoz-Carpena, R.; Zhang, M.; Chen, H.; Zhou, Z.; Wang, H. Aggregation Kinetics of Graphene Oxides in Aqueous Solutions: Experiments, Mechanisms, and Modeling. Langmuir 2013, 29, 15174–15181. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Rout, T.K.; Prusty, A.D.; Ajayan, P.M.; Nayak, S. Electron Transfer Directed Antibacterial Properties of Graphene Oxide on Metals. Adv. Mater. 2018, 30, 1702149. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene Oxide Exhibits Broad-Spectrum Antimicrobial Activity against Bacterial Phytopathogens and Fungal Conidia by Intertwining and Membrane Perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Deokar, A.R.; Lin, L.Y.; Chang, C.C.; Ling, Y.C. Single-Walled Carbon Nanotube Coated Antibacterial Paper: Preparation and Mechanistic Study. J. Mater. Chem. B 2013, 1, 2639–2646. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Sharma, A.; Varshney, M.; Nanda, S.S.; Shin, H.J.; Kim, N.; Yi, D.K.; Chae, K.H.; Ok Won, S. Structural, Electronic Structure and Antibacterial Properties of Graphene-Oxide Nano-Sheets. Chem. Phys. Lett. 2018, 698, 85–92. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, C.; Zhang, P.; Zhang, J.; Wang, G.; He, X.; Ma, Y.; Zhao, B.; Zhang, Z. Toxicity and Transformation of Graphene Oxide and Reduced Graphene Oxide in Bacteria Biofilm. Sci. Total Environ. 2017, 580, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Ricci, R.; Leite, N.C.S.; da-Silva, N.S.; Pacheco-Soares, C.; Canevari, R.A.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Graphene Oxide Nanoribbons as Nanomaterial for Bone Regeneration: Effects on Cytotoxicity, Gene Expression and Bactericidal Effect. Mater. Sci. Eng. C 2017, 78, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping Bacteria by Graphene Nanosheets for Isolation from Environment, Reactivation by Sonication, and Inactivation by near-Infrared Irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef]
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity of Cellular Uptake of Cellulose Nanocrystals. Nano Life 2012, 2, 1241006. [Google Scholar] [CrossRef]
- Male, K.B.; Leung, A.C.W.; Montes, J.; Kamen, A.; Luong, J.H.T. Probing Inhibitory Effects of Nanocrystalline Cellulose: Inhibition versus Surface Charge. Nanoscale 2012, 4, 1373–1379. [Google Scholar] [CrossRef]
- De Maio, F.; Palmieri, V.; Salustri, A.; Perini, G.; Sanguinetti, M.; De Spirito, M.; Delogu, G.; Papi, M. Graphene Oxide Prevents Mycobacteria Entry into Macrophages through Extracellular Entrapment. Nanoscale Adv. 2019, 1, 1421–1431. [Google Scholar] [CrossRef]
- Castrillón, S.R.-V.; Perreault, F.; De Faria, A.F.; Elimelech, M. Interaction of Graphene Oxide with Bacterial Cell Membranes: Insights from Force Spectroscopy. Environ. Sci. Technol. Lett. 2015, 2, 112–117. [Google Scholar] [CrossRef]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal Effect of Graphene Oxide and Reduced Graphene Oxide: Influence of Shape of Bacteria. Colloid Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Appaturi, J.N.; Lai, C.W.; Leo, B.F. Mechanistic Actions and Contributing Factors Affecting the Antibacterial Property and Cytotoxicity of Graphene Oxide. Chemosphere 2021, 281, 130739. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the Wall: Extracellular Vesicles in Gram-Positive Bacteria, Mycobacteria and Fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review. Chem. Res. Toxicol. 2011, 25, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wen, J.; Gao, Y.; Li, T.; Wang, H.; Yan, H.; Niu, B.; Guo, R. Antibacterial Graphene Oxide Coatings on Polymer Substrate. Appl. Surf. Sci. 2018, 436, 624–630. [Google Scholar] [CrossRef]
- Liu, X.; Sen, S.; Liu, J.; Kulaots, I.; Geohegan, D.; Kane, A.; Puretzky, A.A.; Rouleau, C.M.; More, K.L.; Palmore, G.T.R.; et al. Antioxidant Deactivation on Graphenic Nanocarbon Surfaces. Small 2011, 7, 2775–2785. [Google Scholar] [CrossRef]
- Koch, G.; Yepes, A.; Förstner, K.U.; Wermser, C.; Stengel, S.T.; Modamio, J.; Ohlsen, K.; Foster, K.R.; Lopez, D. Evolution of Resistance to a Last-Resort Antibiotic in Staphylococcus aureus via Bacterial Competition. Cell 2014, 158, 1060–1071. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; De Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the Antibacterial Mechanism of Graphene Oxide (GO) Langmuir–Blodgett Films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef]
- Hui, L.; Piao, J.G.; Auletta, J.; Hu, K.; Zhu, Y.; Meyer, T.; Liu, H.; Yang, L. Availability of the Basal Planes of Graphene Oxide Determines Whether It Is Antibacterial. ACS Appl. Mater. Interfaces 2014, 6, 13183–13190. [Google Scholar] [CrossRef]
- Sodhi, N. New Mechanism of Resistance in a Last-Resort Antibiotic. Aust. Vet. J. 2016, 94, N8–N9. [Google Scholar]
- Pumera, M. Graphene-Based Nanomaterials and Their Electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Sarkar, R.; Pakhira, B.; Ghosh, S.; Sarkar, R.; Barui, A.; Sarkar, S. ROS Generation by Reduced Graphene Oxide (RGO) Induced by Visible Light Showing Antibacterial Activity: Comparison with Graphene Oxide (GO). RSC Adv. 2015, 5, 80192–80195. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Escherichia Coli Bacteria Reduce Graphene Oxide to Bactericidal Graphene in a Self-Limiting Manner. Carbon 2012, 50, 1853–1860. [Google Scholar] [CrossRef]
- Nourmohammadi, A.; Rahighi, R.; Akhavan, O.; Moshfegh, A. Graphene Oxide Sheets Involved in Vertically Aligned Zinc Oxide Nanowires for Visible Light Photoinactivation of Bacteria. J. Alloys Compd. 2014, 612, 380–385. [Google Scholar] [CrossRef]
- Zhao, M.; Shan, T.; Wu, Q.; Gu, L. The Antibacterial Effect of Graphene Oxide on Streptococcus Mutans. J. Nanosci. Nanotechnol. 2019, 20, 2095–2103. [Google Scholar] [CrossRef]
- Pang, L.; Dai, C.; Bi, L.; Guo, Z.; Fan, J. Biosafety and Antibacterial Ability of Graphene and Graphene Oxide In Vitro and In Vivo. Nanoscale Res. Lett. 2017, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Antibacterial Action of Nanoparticle Loaded Nanocomposites Based on Graphene and Its Derivatives: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 3563. [Google Scholar] [CrossRef]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of Enhanced Stem Cell Growth and Differentiation on Graphene and Graphene Oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shahsavar, M. Graphene Nanogrids for Selective and Fast Osteogenic Differentiation of Human Mesenchymal Stem Cells. Carbon 2013, 59, 200–211. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.T.; Liu, J.H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In Vitro Toxicity Evaluation of Graphene Oxide on A549 Cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Wang, A.; Pu, K.; Dong, B.; Liu, Y.; Zhang, L.; Zhang, Z.; Duan, W.; Zhu, Y. Role of Surface Charge and Oxidative Stress in Cytotoxicity and Genotoxicity of Graphene Oxide towards Human Lung Fibroblast Cells. J. Appl. Toxicol. 2013, 33, 1156–1164. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef]
- Yue, H.; Wei, W.; Yue, Z.; Wang, B.; Luo, N.; Gao, Y.; Ma, D.; Ma, G.; Su, Z. The Role of the Lateral Dimension of Graphene Oxide in the Regulation of Cellular Responses. Biomaterials 2012, 33, 4013–4021. [Google Scholar] [CrossRef] [PubMed]
- Gies, V.; Zou, S. Systematic Toxicity Investigation of Graphene Oxide: Evaluation of Assay Selection, Cell Type, Exposure Period and Flake Size. Toxicol. Res. 2018, 7, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Hu, P.P.; Zhang, L.; Huang, S.Z.; Luo, L.F.; Huang, C.Z. Toxicity of Graphene Oxide and Multi-Walled Carbon Nanotubes against Human Cells and Zebrafish. Sci. China Chem. 2012, 55, 2209–2216. [Google Scholar] [CrossRef]
- Liu, X.T.; Mu, X.Y.; Wu, X.L.; Meng, L.X.; Guan, W.B.; Ma, Y.Q.; Sun, H.; Wang, C.J.; Li, X.F. Toxicity of Multi-Walled Carbon Nanotubes, Graphene Oxide, and Reduced Graphene Oxide to Zebrafish Embryos. Biomed. Environ. Sci. 2014, 27, 676–683. [Google Scholar]
- Chen, Y.; Hu, X.; Sun, J.; Zhou, Q. Specific Nanotoxicity of Graphene Oxide during Zebrafish Embryogenesis. Nanotoxicology 2015, 10, 42–52. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, M.-H.; Min, D.-H. Biocompatible Reduced Graphene Oxide Prepared by Using Dextran as a Multifunctional Reducing Agent. Chem. Commun. 2011, 47, 3195. [Google Scholar] [CrossRef]
- Mu, Q.; Su, G.; Li, L.; Gilbertson, B.O.; Yu, L.H.; Zhang, Q.; Sun, Y.P.; Yan, B. Size-Dependent Cell Uptake of Protein-Coated Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2012, 4, 2259–2266. [Google Scholar] [CrossRef]
- Cheng, C.; Nie, S.; Li, S.; Peng, H.; Yang, H.; Ma, L.; Sun, S.; Zhao, C. Biopolymer Functionalized Reduced Graphene Oxide with Enhanced Biocompatibility via Mussel Inspired Coatings/Anchors. J. Mater. Chem. B 2013, 1, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ghafor, A.A.H.A.; Elias, N.; Shamsi, S.; Yasin, F.M.; Sarchio, S.N.E. Toxicity Assessment of Gallic Acid Loaded Graphene Oxide (GAGO) Nano-Formulation in Zebrafish (Danio rerio) Embryos. Pertanika J. Sci. Technol. 2020, 28, 311–326. [Google Scholar]
- Shamsi, S.; Elias, N.; Narti Edayu Sarchio, S.; Md Yasin, F. Gallic Acid Loaded Graphene Oxide Based Nanoformulation (GAGO) as Potential Anti-Bacterial Agent against Staphylococcus aureus. Mater. Today Proc. 2018, 5, S160–S165. [Google Scholar] [CrossRef]
- Shamsi, S.; Alagan, A.A.; Sarchio, S.N.E.; Md Yasin, F. Synthesis, Characterization, and Toxicity Assessment of Pluronic F127-Functionalized Graphene Oxide on the Embryonic Development of Zebrafish (Danio rerio). Int. J. Nanomed. 2020, 15, 8311–8329. [Google Scholar] [CrossRef] [PubMed]

| Findings | Concentration | Time | Reference |
|---|---|---|---|
| Results showed concentration-dependent decrease in the survival rate of K. pneumoniae, E. coli and P. aeruginosa. Antibacterial effect was most effective on K. pneumoniae. This was evident with bioluminescence indicating live bacteria. | 0–500 µg/mL | 2 h | [11] |
| The MIC for E. coli, K. pneumoniae, P. mirabilis and S. aureus is 0.065 µg/mL. The MIC for P. aeruginosa and S. marcescens is 0.032 µg/µL. The MBC of P. aeruginosa and S. marcescens is 0.065 µg/mL. The MBC for E. coli, K. pneumoniae, P. mirabilis and S. aureus is 0.12 µg/mL. | 0.004–1 µg/mL | 24 h | [12] |
| Results showed concentration-dependent increase in the zone of inhibition for E. coli and S. aureus. Zone of inhibition was bigger on S. aureus. | 250–1000 µg/mL | 24 h | [13] |
| Results showed thesignificant growth inhibition of S. aureus at 2 and 24 h and for P. aeruginosa at 2 h. | 50 mg/L | 2 and 24 h | [14] |
| MIC for E. coli and E. faecalis was 1 µg/mL and 4 µg/mL, respectively. | - | 24 h | [15] |
| Results showed that the percentage loss in viability increases as the concentration and time increases. | 12–50 µg/mL | 30–180 min | [16] |
| Results showed concentration- and time-dependent decreased viability of bacteria. The significant reduction of viability of S. aureus was 12 h, while, for E. coli, it was 168 h. | 0–40 mg/mL | - | [17] |
| After 15 min, there was 99% loss in viability of Mycobacterium smegmatis, E. coli and S. aureus. | 1 mg/mL | 15 min in the dark | [18] |
| Results showed a decrease in the recovery of E. coli. | 1 mg/mL | 2 and 4 h | [19] |
| The survival rate of E. coli is 4% at 100 µg/mL. Results also showed that the average growth delay of exponential cells varies around 4 to 6 h. E. coli and P. aeruginosa also showed alow survival rate. On the other hand, a bacteriostatic effect was observed on S. aureus, as indicated by the high survival rate. | 0–100 µg/mL | 30 min | [20] |
| Results showed time-dependent decrease in the viability of E. coli. | 0.1 g/L | 0–5 h | [21] |
| The MIC and EC50 on E. coli are 100 µg/mL and 38 µg/mL, respectively. The MIC and EC50 on S. iniae are 125 µg/mL and 29 µg/mL, respectively. | 25–150 µg/mL | 3 h | [22] |
| Results showed a time-dependent loss in viability of P. aeruginosa, and the percentage loss at 4 h is 87%. There was a concentration-dependent loss in viability that reached a complete loss at 175 µg/mL. Bacterial growth was shown to increase and decrease after 3 h at 75 µg/mL. There was a 92% growth inhibition at after 15 h. | 0–200 µg/mL | 2 h | [23] |
| Results showed the concentration- and time-dependent decreases in viability of E. coli. | 0–80 µg/mL Time study: 40 µg/mL | 2 h | [24] |
| Findings | Nature of GO | Concentration, Time | Reference |
|---|---|---|---|
| The size of GO flakes affects the antibacterial activity on S. mutans. It was shown that GO-1 demonstrated better cutting activity, while GO-2–4 were better at entrapping bacteria as the size increases. GO-2 is most effective with the combination of cutting and entrapping activity. | Size: GO-1 (2 µm), 2 (4 µm), 3 (6 µm), 4 (8 µm) | 25 µg/mL, 10 s | [16] |
| GO with roughness of 505 nm decreased 20% viability of E. coli and S. aureus, while 845 nm GO decreased 30% viability of M. smegmatis. SEM showed wrapping and leakage of intracellular substances. The cell membranes were completely decomposed. There was a significant decrease in cell volume. If the surface roughness of GO nanosheets was consistent with the diameter of bacteria, this increases contact area and bacterial adhesion. As the GO nanosheet looks like a mountain range, the peaks improve charge transfer causing destruction of the bacterial cell membrane leading to intracellular leakage. Deep terrains trap bacteria of matching diameter, enhancing the strong interaction with the bacterial cells and promoting direct oxidation of cellular components. Fluorescent staining also indicated leakage of DNA due to a compromised membrane. | Roughness: 465, 505, 845, 1179 nm | 1 mg/mL, 15 min in the dark at room temperature | [18] |
| Results showed that increase in washes increased the recovery of E. coli. Interestingly, highly purified GO does not affect growth curve of E. coli and S. aureus and rather showed concentration-dependent increase in the growth rate (10–250 µg/mL). Size of highly purified GO does not affect growth rate (50 µg/mL) and morphology (100 µg/mL) of E. coli.Results suggested that increase in pH decrease the bactericidal effects. It may also be due to chemical contaminants present in the GO preparations as a consequence of the generation of the GO. Moreover, highly purified GO had no effects on growth or inhibition of bacteria. | GO washes: 2 (pH 3.5), 4 (pH 4), 6 (pH 5), 8 (pH 5.5) | 1 mg/mL, 2 and 4 h at 30 °C. | [19] |
| Survival rate of exponential E. coli is 4%. Average growth delay of exponential cells is longer than those in stationary and decline phases for E. coli, S. aureus and P. aeruginosa. In conclusion, bacteria is more susceptible to GO at the exponential phase. It was mentioned that as bacteria gradually matures through the growth phases, they generate phenotypically different subpopulations. | Cells in exponential, stationary, decline phases. | 100 µg/mL, 30 min | [20] |
| GO foam demonstrated more efficient antibacterial activity on E. coli than GO precipitate. | GO foam and GO precipitate | 0.1 g/L, 0–5 h | [21] |
| Increase size GO, decreased the viability of E. coli. Results showed that larger GO showed efficient antibacterial effects with lower concentrations of GO (10 µg/mL) and less treatment time (1 h). | Sizes of GO (µm2) GO-0: 0.753 GO-10: 0.127 GO-30: 0.065 GO-50: 035 GO-120: 0.013 GO-240: 0.010 | 40 µg/mL, 2 h | [24] |
| Results showed enhanced antibacterial activity on E. coli through the controlled alignment of GO nanosheets leading to cell membrane disruption and oxidative stress caused by electron transfer. Vertically aligned GO was the most effective. | Random, vertical, planar alignment | 200 µg/mL, 3 h | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, I.M.J.; Shamsi, S. Graphene Oxide (GO): A Promising Nanomaterial against Infectious Diseases Caused by Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2022, 23, 9096. https://doi.org/10.3390/ijms23169096
Ng IMJ, Shamsi S. Graphene Oxide (GO): A Promising Nanomaterial against Infectious Diseases Caused by Multidrug-Resistant Bacteria. International Journal of Molecular Sciences. 2022; 23(16):9096. https://doi.org/10.3390/ijms23169096
Chicago/Turabian StyleNg, Ida M. J., and Suhaili Shamsi. 2022. "Graphene Oxide (GO): A Promising Nanomaterial against Infectious Diseases Caused by Multidrug-Resistant Bacteria" International Journal of Molecular Sciences 23, no. 16: 9096. https://doi.org/10.3390/ijms23169096
APA StyleNg, I. M. J., & Shamsi, S. (2022). Graphene Oxide (GO): A Promising Nanomaterial against Infectious Diseases Caused by Multidrug-Resistant Bacteria. International Journal of Molecular Sciences, 23(16), 9096. https://doi.org/10.3390/ijms23169096






