Ethylene Acts as a Local and Systemic Signal to Mediate UV-B-Induced Nitrate Reallocation to Arabidopsis Leaves and Roots via Regulating the ERFs-NRT1.8 Signaling Module
Abstract
:1. Introduction
2. Results
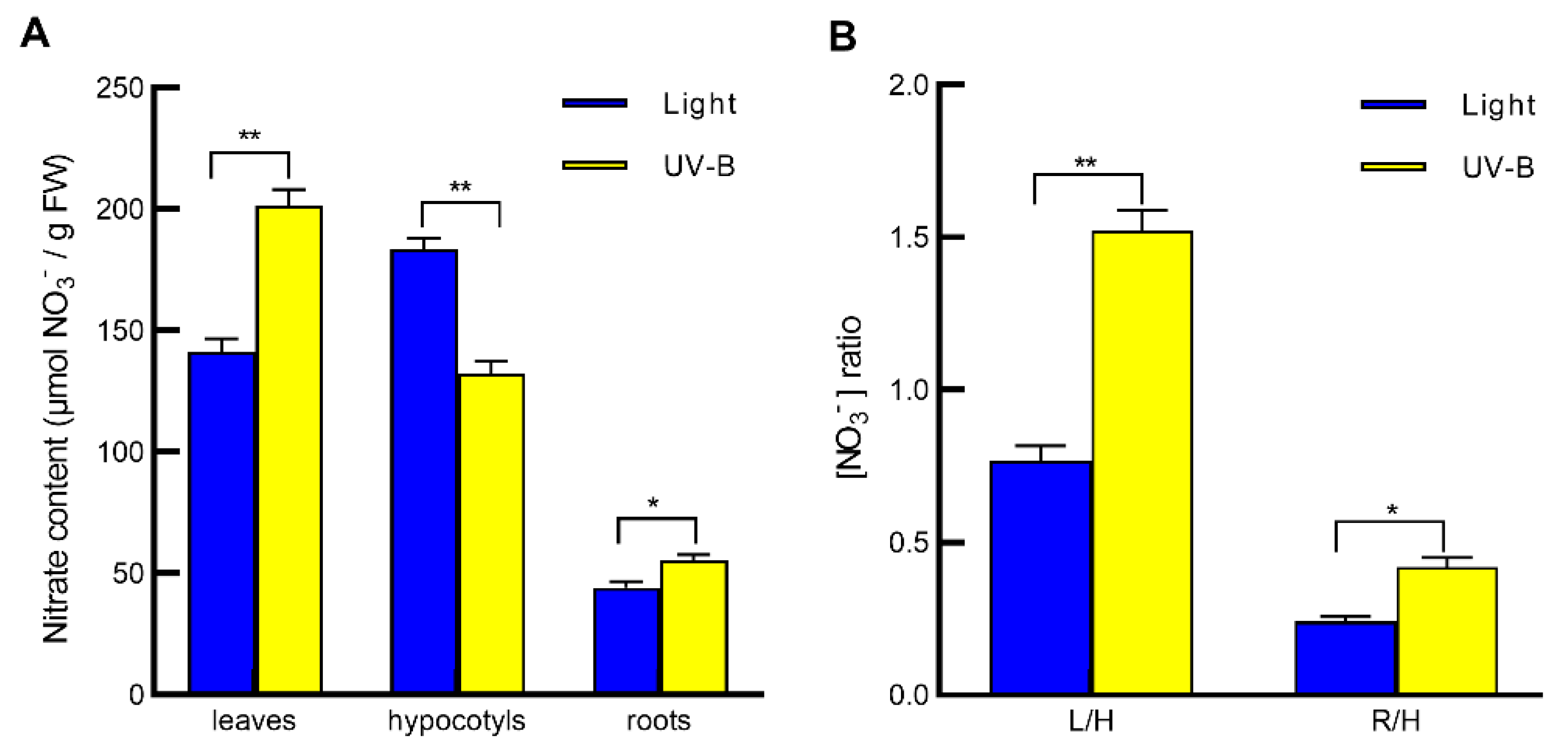
2.1. UV-B Induces Nitrate Reallocation from Hypocotyls to Leaves and Roots
2.2. UV-B Induces Nitrate Reallocation by Promoting NRT1.8 Expression in Shoots and Roots
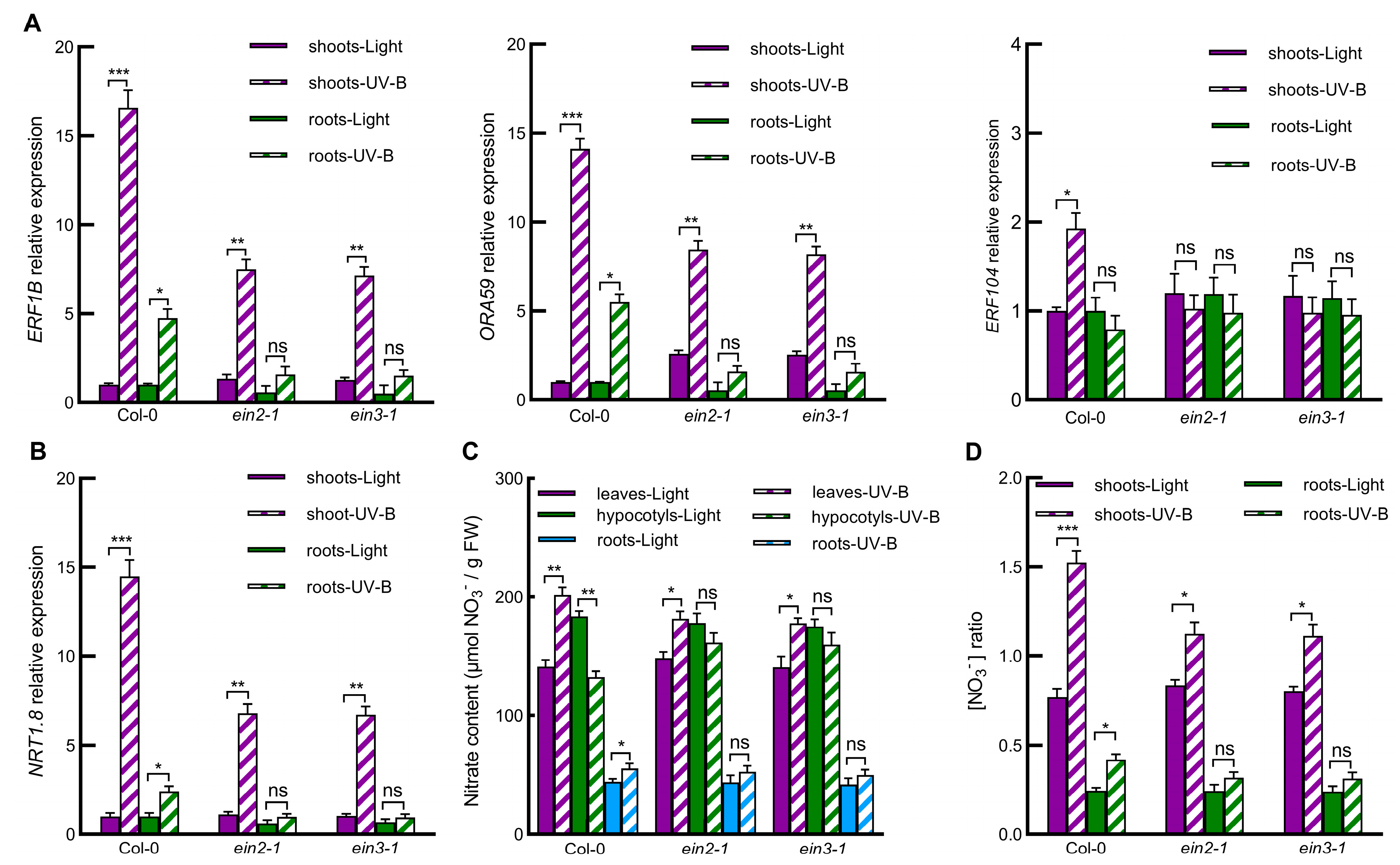
2.3. UV-B Induces Expression of ERF Genes, and ERFs Mediates UV-B-Induced NRT1.8 Expression and Subsequent Nitrate Reallocation to Leaves and Roots
2.4. Ethylene Signaling Pathway Is Involved in UV-B-Induced Nitrate Reallocation through Regulating Gene Expression of ERFs-NRT1.8 Signaling Module
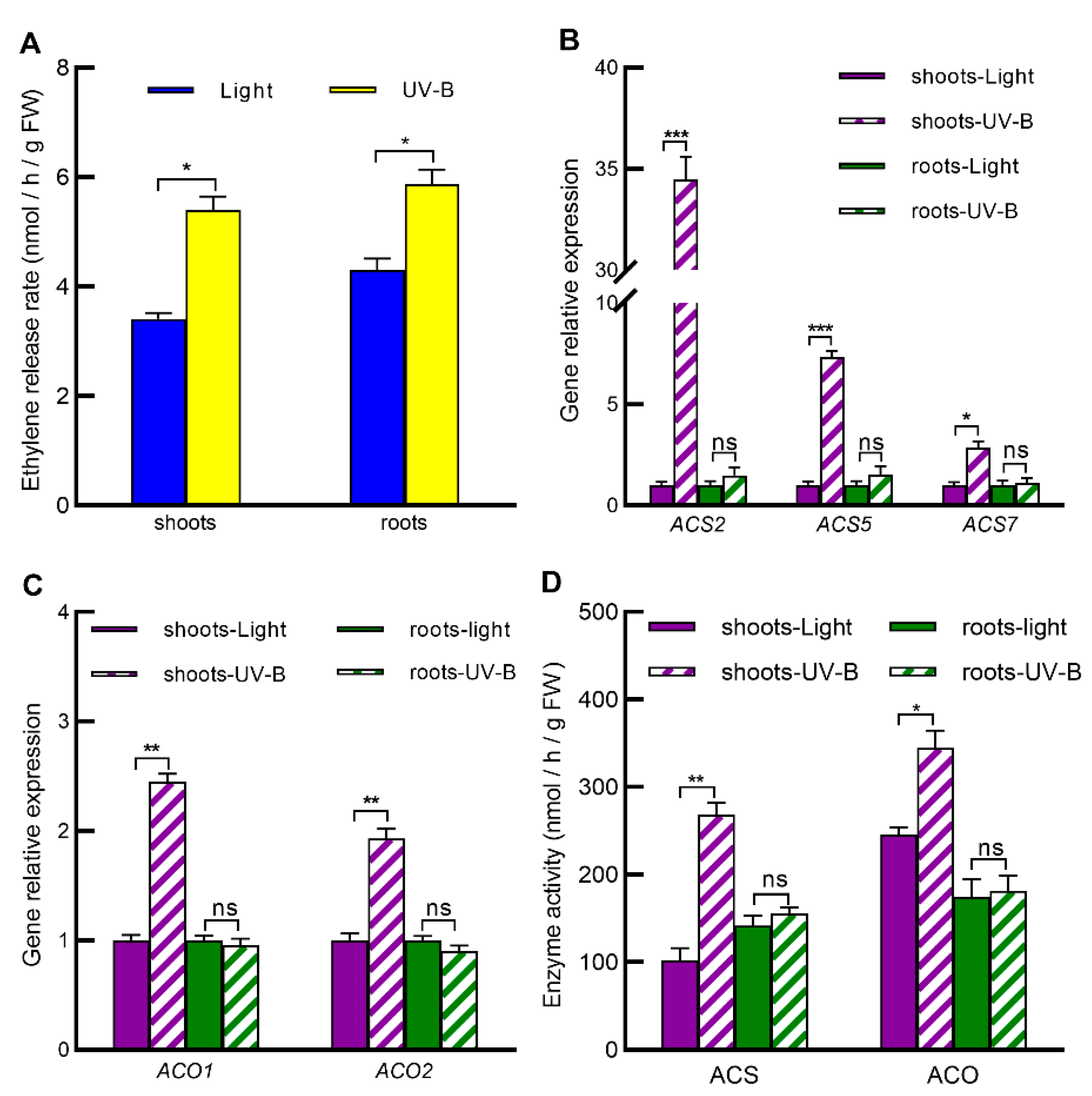
2.5. UV-B Increases Ethylene Levels in Both Shoots and Roots by Inducing Ethylene Biosynthesis Only in Shoots
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. UV-B Treatment
4.3. RNA Extraction and qPCR Analysis
4.4. Determination of Nitrate Content
4.5. Detection of Ethylene Release Rate
4.6. Assays for ACS Activity
4.7. Assays for ACO Activity
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.H.; Hu, B.; Chu, C.C. Towards understanding the hierarchical nitrogen signalling network in plants. Curr. Opin. Plant Biol. 2020, 55, 60–65. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Miller, A. Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 659–688. [Google Scholar] [CrossRef] [PubMed]
- Kant, S. Understanding nitrate uptake, signaling and remobilisation for improving plant nitrogen use efficiency. Semin. Cell Dev. Biol. 2018, 74, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Kuo, H.F.; Canivenc, G.; Lin, C.S.; Lepetit, M.; Hsu, P.K.; Tillard, P.; Lin, H.L.; Wang, Y.Y.; Tsai, C.B.; et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 2008, 20, 2514–2528. [Google Scholar] [CrossRef]
- Li, J.Y.; Fu, Y.L.; Pike, S.M.; Bao, J.; Tian, W.; Zhang, Y.; Chen, C.Z.; Zhang, Y.; Li, H.M.; Huang, J.; et al. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 2010, 22, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.C.; Lin, C.S.; Hsu, P.K.; Lin, S.H.; Tsay, Y.F. The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell 2009, 21, 2750–2761. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Tsay, Y.F. Arabidopsis nitrate transporter NRT1.9 is important in phloem nitrate transport. Plant Cell 2011, 23, 1945–1957. [Google Scholar] [CrossRef]
- Chen, C.Z.; Lv, X.F.; Li, J.Y.; Yi, H.Y.; Gong, J.M. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 2012, 159, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.B.; Yi, H.Y.; Gong, J.M. The Arabidopsis ethylene/jasmonic acid-NRT signaling module coordinates nitrate reallocation and the trade-off between growth and environmental adaptation. Plant Cell 2014, 26, 3984–3998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, C.; Zhang, S.; Shi, C.; Cheng, H.; Liu, H.; Zhong, B. Origin and adaptive evolution of UV RESISTANCE LOCUS 8-mediated signaling during plant terrestrialization. Plant Physiol. 2022, 188, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Chen, Z.; Liu, Q.; Mao, W.; Chen, Y.; Tian, W.; Liu, Y.; Han, J.; Ouyang, X.; Huang, X. Coordinated transcriptional regulation by the UV-B photoreceptor and multiple transcription factors for plant UV-B responses. Mol. Plant 2020, 13, 777–792. [Google Scholar] [CrossRef]
- Brown, B.A.; Cloix, C.; Jiang, G.H.; Kaiserli, E.; Herzyk, P.; Kliebenstein, D.J.; Jenkins, G.I. A UV-B-specific signaling component orchestrates plant UV protection. Proc. Natl. Acad. Sci. USA 2005, 102, 18225–18230. [Google Scholar] [CrossRef]
- Brown, B.A.; Jenkins, G.I. UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol. 2008, 146, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.M. Solar UV-B irradiation and the growth and development of higher plant. In Photophysiology, 2nd ed.; Giese, A.C., Ed.; Academic Press: New York, NY, USA, 1971; Volume 6, pp. 131–137. [Google Scholar]
- He, J.M.; Ma, X.G.; Zhang, Y.; Sun, T.F.; Xu, F.F.; Chen, Y.P.; Liu, X.; Yue, M. Role and interrelationship of Gα protein, hydrogen peroxide, and nitric oxide in ultraviolet B-induced stomatal closure in Arabidopsis leaves. Plant Physiol. 2013, 161, 1570–1583. [Google Scholar] [CrossRef]
- Li, F.C.; Wang, J.; Wu, M.M.; Fan, C.M.; Li, X.; He, J.M. Mitogen-activated protein kinase phosphatases affect UV-B-induced stomatal closure via controlling NO in guard cells. Plant Physiol. 2017, 173, 760–770. [Google Scholar] [CrossRef]
- An, L.; Xu, X.; Tang, H.; Zhang, M.; Hou, Z.; Liu, Y.; Zhan, Z.; Feng, H.; Xu, S.; Wang, X. Ethylene production and 1-Aminocyclopropane-1-Carboxylate (ACC) synthase gene expression in tomato (Lycopsicon esculentum Mill.) leaves under enhanced UV-B radiation. J. Integr. Plant Biol. 2006, 48, 1190–1196. [Google Scholar] [CrossRef]
- Katerova, Z.; Ivanov, S.; Prinsen, E.; Onckelen, H.; Alexieva, V.; Azmi, A. Low doses of ultraviolet-B or ultraviolet-C radiation affect phytohormones in young pea plants. Biol. Plantarum 2009, 53, 365–368. [Google Scholar] [CrossRef]
- Li, H.F.; Testerink, C.; Zhang, Y.X. How roots and shoots communicate through stressful times. Trends Plant Sci. 2021, 26, 940–952. [Google Scholar] [CrossRef]
- Rossel, J.B.; Wilson, P.B.; Hussain, D.; Woo, N.S.; Gordon, M.J.; Mewett, O.P.; Howell, K.A.; Whelan, J.; Kazan, K.; Pogson, B.J. Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell 2007, 19, 4091–4110. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Devireddy, A.R.; Azad, R.K.; Shulaev, V.; Mittler, R. Local and systemic metabolic responses during light-induced rapid systemic signaling. Plant Physiol. 2018, 178, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van, B.F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Christmann, A.; Grill, E.; Huang, J. Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 2013, 16, 293–300. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.L.; Luo, X.; Shah, J.; Schlauch, K.; et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 2013, 25, 3553–3569. [Google Scholar] [CrossRef]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, F.; Xiang, X.; Ahammed, G.J.; Wang, M.; Onac, E.; Zhou, J.; Xia, X.; Shi, K.; Yin, X.; et al. Systemic induction of photosynthesis via illumination of the shoot apex is mediated sequentially by phytochrome B, auxin and hydrogen peroxide in tomato. Plant Physiol. 2016, 172, 1259–1272. [Google Scholar]
- Choi, W.G.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 2017, 90, 698–707. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Gómez-Cadenas, A.; Blumwald, E.; Mittler, R. Coordinating the overall stomatal response of plants: Rapid leaf-to-leaf communication during light stress. Sci. Signal. 2018, 11, 1126. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yao, Q.; Gao, X.; Jiang, C.; Harberd, N.P.; Fu, X. Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr. Biol. 2016, 26, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Sengupta, S.; Burks, D.; Azad, R.K.; Mittler, R. Identification and characterization of a core set of ROS wave-associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J. 2019, 98, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Bethke, G.; Unthan, T.; Uhrig, J.F.; Pöschl, Y.; Gust, A.A.; Scheel, D.; Lee, J. Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc. Natl. Acad. Sci. USA. 2009, 106, 8067–8072. [Google Scholar] [CrossRef] [PubMed]
- Oñate-Sánchez, L.; Singh, K.B. Identification of Arabidopsis ethylene-responsive element binding factors with distinct induction kinetics after pathogen infection. Plant Physiol. 2002, 128, 1313–1322. [Google Scholar] [CrossRef]
- McManus, M.T. The plant hormone ethylene. In Annual Plant Reviews, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 44, pp. 1–7. [Google Scholar]
- Poel, B.; Straeten, D. 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: More than just the precursor of ethylene! Front. Plant Sci. 2014, 5, 640. [Google Scholar]
- Lisa, V.; Dominique, V. Accumulation and transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: Current status, considerations for future research and agronomic applications. Front. Plant Sci. 2017, 8, 38. [Google Scholar]
- Zhang, T.Y.; Li, Z.Q.; Zhao, Y.D.; Shen, W.J.; Chen, M.S.; Gao, H.Q.; Ge, X.M.; Wang, H.Q.; Li, X.; He, J.M. Ethylene-induced stomatal closure is mediated via MKK1/3-MPK3/6 cascade to EIN2 and EIN3. J. Integr. Plant Biol. 2021, 63, 1324–1340. [Google Scholar] [CrossRef]
- Ge, X.M.; Hu, X.; Zhang, J.; Huang, Q.M.; Gao, Y.; Li, Z.Q.; Li, S.; He, J.M. UV RESISTANCE LOCUS8 mediates ultraviolet-B-induced stomatal closure in an ethylene-dependent manner. Plant Sci. 2020, 301, 110679. [Google Scholar] [CrossRef]
- Houben, M.; Poel, B. 1-Aminocyclopropane-1-carboxylic acid oxidase (ACO): The enzyme that makes the plant hormone ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 5, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Lin, C.S.; Hsia, A.P.; Su, R.C.; Lin, H.L.; Tsay, Y.F. Mutation of a nitrate transporter, AtNRT1:4, results in a reduced petiole nitrate content and altered leaf development. Plant Cell Physiol. 2004, 45, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Woeste, K.E.; Ye, C.; Kieber, J.J. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 1999, 119, 5215–5230. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Tian, Q.Y.; Zhao, M.G.; Dai, X.Y.; Huang, J.H.; Li, L.H.; Zhang, W.H. Aluminum-induced ethylene production is associated with inhibition of root elongation in Lotus japonicus L. Plant Cell Physiol. 2007, 48, 1229–1235. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-T.; Xiao, J.-H.; Li, L.; Guo, J.-F.; Zhang, M.-X.; An, Y.-Y.; He, J.-M. Ethylene Acts as a Local and Systemic Signal to Mediate UV-B-Induced Nitrate Reallocation to Arabidopsis Leaves and Roots via Regulating the ERFs-NRT1.8 Signaling Module. Int. J. Mol. Sci. 2022, 23, 9068. https://doi.org/10.3390/ijms23169068
Wang X-T, Xiao J-H, Li L, Guo J-F, Zhang M-X, An Y-Y, He J-M. Ethylene Acts as a Local and Systemic Signal to Mediate UV-B-Induced Nitrate Reallocation to Arabidopsis Leaves and Roots via Regulating the ERFs-NRT1.8 Signaling Module. International Journal of Molecular Sciences. 2022; 23(16):9068. https://doi.org/10.3390/ijms23169068
Chicago/Turabian StyleWang, Xiao-Ting, Jun-Hua Xiao, Li Li, Jiang-Fan Guo, Mei-Xiang Zhang, Yu-Yan An, and Jun-Min He. 2022. "Ethylene Acts as a Local and Systemic Signal to Mediate UV-B-Induced Nitrate Reallocation to Arabidopsis Leaves and Roots via Regulating the ERFs-NRT1.8 Signaling Module" International Journal of Molecular Sciences 23, no. 16: 9068. https://doi.org/10.3390/ijms23169068
APA StyleWang, X.-T., Xiao, J.-H., Li, L., Guo, J.-F., Zhang, M.-X., An, Y.-Y., & He, J.-M. (2022). Ethylene Acts as a Local and Systemic Signal to Mediate UV-B-Induced Nitrate Reallocation to Arabidopsis Leaves and Roots via Regulating the ERFs-NRT1.8 Signaling Module. International Journal of Molecular Sciences, 23(16), 9068. https://doi.org/10.3390/ijms23169068




