Improvement of Drought Tolerance by Exogenous Spermidine in Germinating Wheat (Triticum aestivum L.) Plants Is Accompanied with Changes in Metabolite Composition
Abstract
1. Introduction
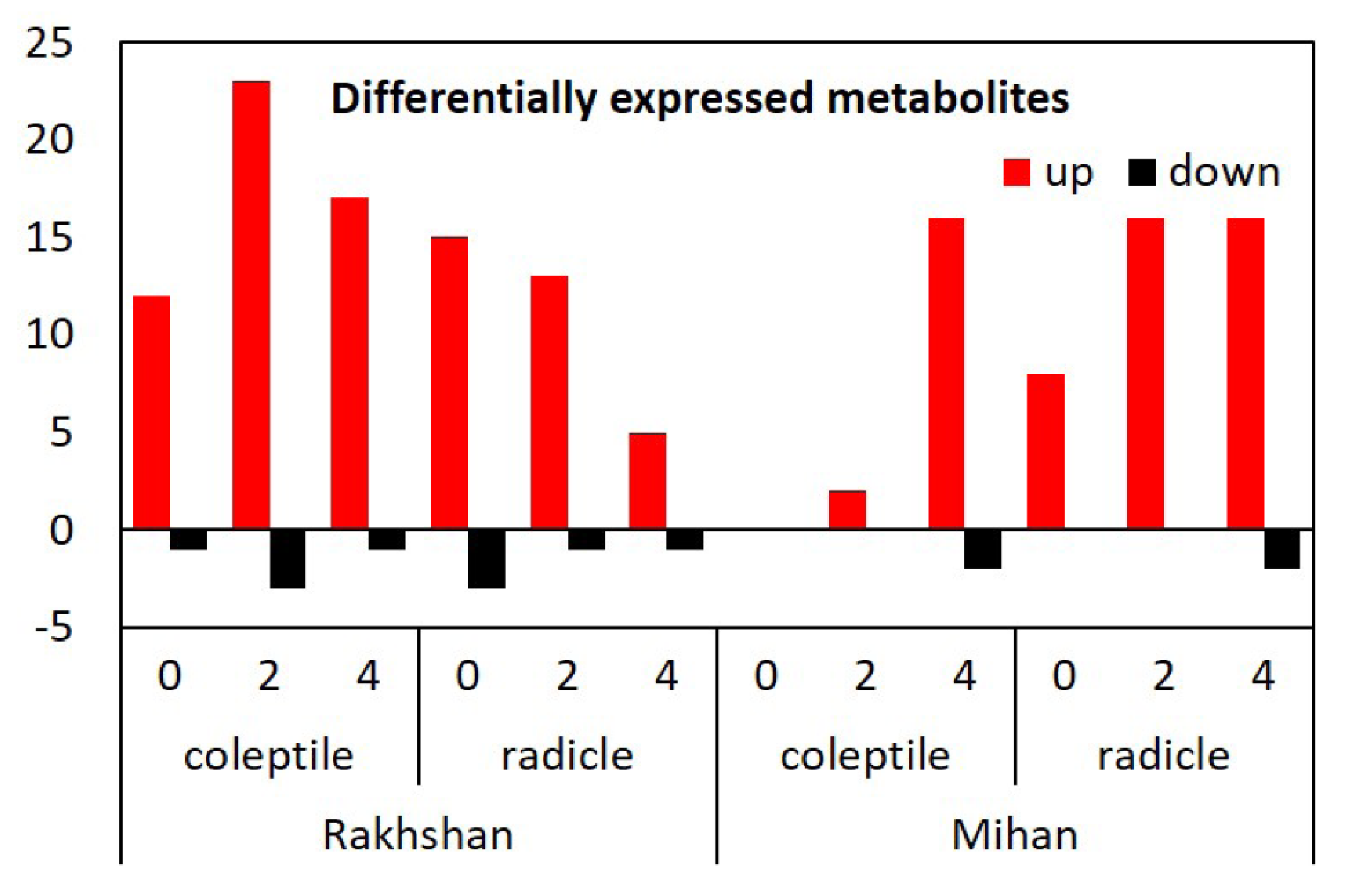
2. Results and Discussion
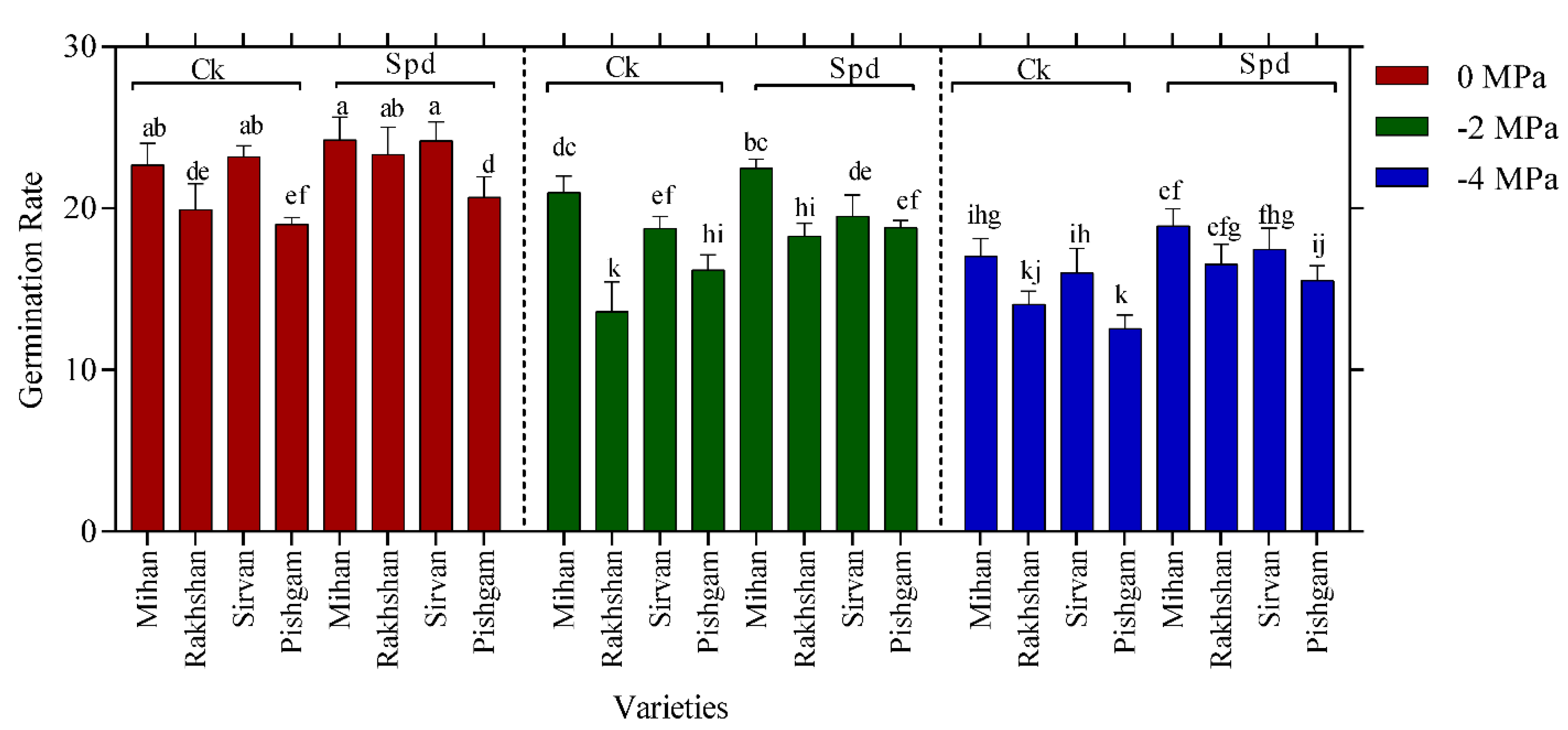
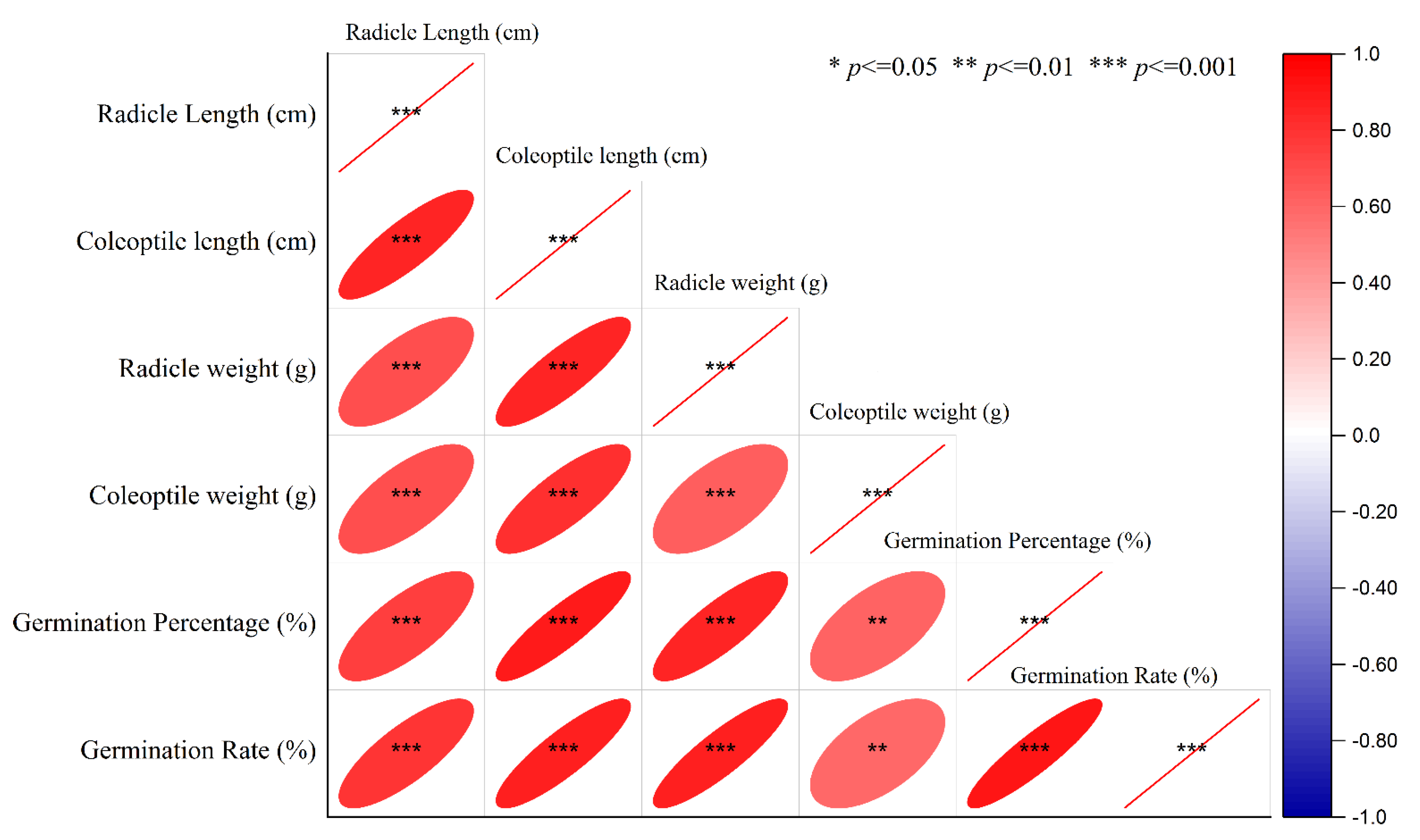
2.1. Germination Experiments
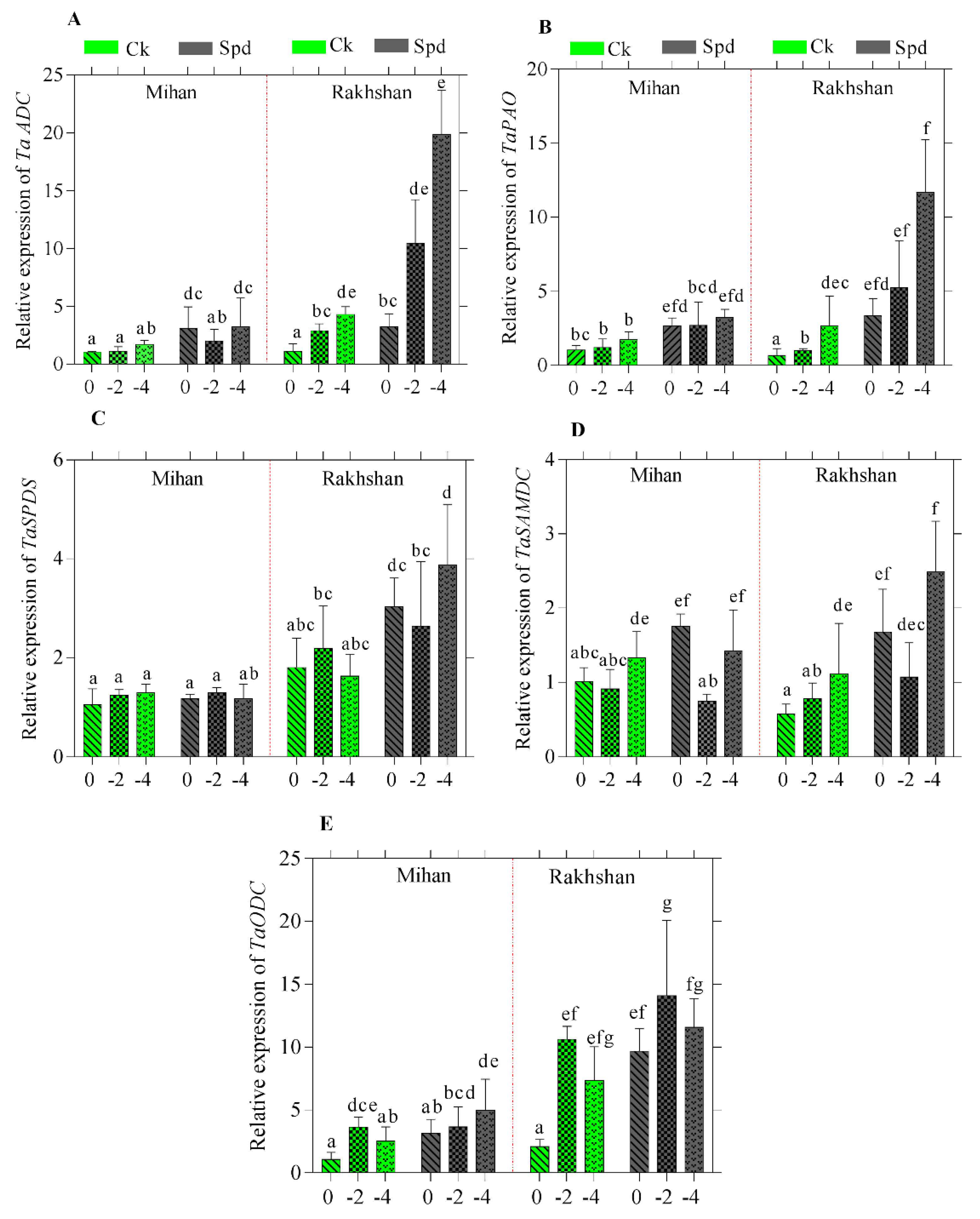
2.2. Gene Expression in Response to Drought Stress
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Gene Expression Analysis
3.3. GC-MS Analyses
3.4. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghassemi, S.; Farhangi-Abriz, S.; Faegi-Analou, R.; Ghorbanpour, M.; Lajayer, B.A. Monitoring Cell Energy, Physiological Functions and Grain Yield in Field-Grown Mung Bean Exposed to Exogenously Applied Polyamines under Drought Stress. Soil Sci. Plant Nutr. 2018, 18, 1108–1125. [Google Scholar] [CrossRef]
- Peng, J.H.; Sun, D.; Nevo, E.J.M.B. Domestication Evolution, Genetics and Genomics in Wheat. Mol. Breed. 2011, 28, 281–301. [Google Scholar] [CrossRef]
- Hassan, N.; Ebeed, H.; Aljaarany, A. Exogenous Application of Spermine and Putrescine Mitigate Adversities of Drought Stress in Wheat by Protecting Membranes and Chloroplast Ultra-Structure. Physiol. Mol. Biol. Plants 2020, 26, 233–245. [Google Scholar] [CrossRef]
- Singh, G.; Chaudhary, H. Selection Parameters and Yield Enhancement of Wheat (Triticum aestivum L.) under Different Moisture Stress Conditions. Asian J. Plant Sci. 2006, 5, 894–898. [Google Scholar] [CrossRef][Green Version]
- Farooq, M.; Wahid, A.; Lee, D.J. Exogenously Applied Polyamines Increase Drought Tolerance of Rice by Improving Leaf Water Status, Photosynthesis and Membrane Properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Zheng, B. Photosynthetic Response of Plants under Different Abiotic Stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Hubbard, M.; Germida, J.; Vujanovic, V.J.B. Fungal Endophytes Improve Wheat Seed Germination under Heat and Drought Stress. Botany 2012, 90, 137–149. [Google Scholar] [CrossRef]
- Zapata, P.J.; Serrano, M.A.; Pretel, M.T.; Amorós, A.; Botella, M.Á. Polyamines and Ethylene Changes during Germination of Different Plant Species under Salinity. Plant Sci. 2004, 167, 781–788. [Google Scholar] [CrossRef]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Janda, T.J.P.S. Speculation: Polyamines Are Important in Abiotic Stress Signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Szalai, G.; Gondor, O.K.; Janda, T.J.P.S. Unfinished Story of Polyamines: Role of Conjugation, Transport and Light-Related Regulation in The Polyamine Metabolism in Plants. Plant Sci. 2021, 308, 110923. [Google Scholar] [CrossRef]
- Groppa, M.D.; Benavides, M.P. Polyamines and Abiotic Stress: Recent Advances. Amino Acids 2008, 34, 35. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with Regulatory Functions in Plant Abiotic Stress Tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Yan, Y.H.J.M. Exogenous Spermidine Improves Seed Germination of White Clover under Water Stress via Involvement in Starch Metabolism, Antioxidant Defenses and Relevant Gene Expression. Molecules 2014, 19, 18003–18024. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene Networks Involved in Drought Stress Response and Tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Miyazaki, A.; Takahashi, T.; Michael, A.; Kusano, T.J.F.l. The Polyamine Spermine Protects against High Salt Stress in Arabidopsis thaliana. FEBS Let. 2006, 580, 6783–6788. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ziaf, K.; Amjad, M.; Iqbal, Q. Exogenous Application of Polyamines Improves Germination and Early Seedling Growth of Hot Pepper. Chil. J. Agric. Res. 2012, 72, 429–433. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, X.; Wen, X.X.; Liao, Y.C. Effect of Polyamine on Seed Germination of Wheat under Drought Stress Is Related to Changes in Hormones and Carbohydrates. J. Integr. Agric. 2016, 15, 2759–2774. [Google Scholar] [CrossRef]
- Capell, T.; Bassie, L.; Christou, P. Modulation of the Polyamine Biosynthetic Pathway in Transgenic Rice Confers Tolerance to Drought Stress. Proc. Natl. Acad. Sci. USA 2004, 101, 9909–9914. [Google Scholar] [CrossRef] [PubMed]
- Yiu, J.C.; Liu, C.W.; Fang, D.Y.T.; Lai, Y.S. Waterlogging Tolerance of Welsh Onion (Allium Fistulosum L.) Enhanced by Exogenous Spermidine and Spermine. Plant Physiol. Biochem. 2009, 47, 710–716. [Google Scholar] [CrossRef]
- Almaghrabi, O.A. Impact of Drought Stress on Germination and Seedling Growth Parameters of Some Wheat Cultivars. Life Sci. 2012, 9, 590–598. [Google Scholar]
- Dhanda, S.S.; Sethi, G.S.; Behl, R.K. Indices of Drought Tolerance in Wheat Genotypes at Early Stages of Plant Growth. J. Agron. Crop Sci. 2004, 190, 6–12. [Google Scholar] [CrossRef]
- Duman, I. Effects of Seed Priming with PEG or K3PO4 on Germination and Seedling Growth in Lettuce. Pak. J. Biol. Sci. 2006, 9, 923–928. [Google Scholar] [CrossRef]
- Kasukabe, Y.; He, L.; Nada, K.; Misawa, S.; Ihara, I.; Tachibana, S. Overexpression of Spermidine Synthase Enhances Tolerance to Multiple Environmental Stresses and Up-Regulates the Expression of Various Stress-Regulated Genes in Transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 712–722. [Google Scholar] [CrossRef]
- Huang, H.; Villanueva, V.R. Amino Acids, Polyamines and Proteins during Seed Germination of Two Species of Dipterocarpaceae. Trees 1993, 7, 189–193. [Google Scholar] [CrossRef]
- Aydin, M.; Hossein Pour, A.; Haliloğlu, K.; Tosun, M. Effect of Putrescine Application and Drought Stress on Germination of Wheat (Triticum aestivum L.). Atatürk Univ. J. Agric. Fac. 2015, 46, 43–55. [Google Scholar]
- Liu, Y.; Liang, H.; Lv, X.; Liu, D.; Wen, X.; Liao, Y. Effect of Polyamines on the Grain Filling of Wheat under Drought Stress. Plant Physiol. Biochem. 2016, 100, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Almansouri, M.; Kinet, J.M.; Lutts, S. Effect of Salt and Osmotic Stresses on Germination in Durum Wheat (Triticum durum Desf.). Plant Soil. 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Jie, S.; Kentian, Z. Organic Substance Metabolism during Seed Germination of Pinus Bungeana. J. N. Univ. 1993, 4, 70–75. [Google Scholar] [CrossRef]
- Gholizadeh, F.; Navabpour, S. Effect of Salinity on Morphological and Physiological Characteristics in Correlation to Selection of Salt Tolerance in Rice (Oryza sativa L.). Int. J. Agric. Res. 2011, 6, 780–788. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Liu, J.H. Change in Free Polyamine Contents and Expression Profiles of Two Polyamine Biosynthetic Genes in Citrus Embryogenic Callus under Abiotic Stresses. Biotechnol. Biotechnol. Equip. 2009, 23, 1289–1293. [Google Scholar] [CrossRef]
- Quinet, M.; Ndayiragije, A.; Lefevre, I.; Lambillotte, B.; Dupont-Gillain, C.C.; Lutts, S. Putrescine Differently Influences the Effect of Salt Stress on Polyamine Metabolism and Ethylene Synthesis in Rice Cultivars Differing in Salt Resistance. J. Exp. Bot. 2010, 61, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.J.; Kim, W.T.; Park, K.Y. Overexpression of Carnation S-Adenosylmethionine Decarboxylase Gene Generates a Broad-Spectrum Tolerance to Abiotic Stresses in Transgenic Tobacco Plants. Plant Cell Rep. 2006, 25, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Doneva, D.; Pál, M.; Brankova, L.; Szalai, G.; Tajti, J.; Khalil, R.; Ivanovska, B.; Velikova, V.; Misheva, S.; Janda, T. The Effects of Putrescine Pre-Treatment on Osmotic Stress Responses in Drought-Tolerant and Drought-Sensitive Wheat Seedlings. Physiol. Plant. 2021, 171, 200–216. [Google Scholar] [CrossRef]
- Liu, J.H.; Kitashiba, H.; Wang, J.; Ban, Y.; Moriguchi, T. Polyamines and Their Ability to Provide Environmental Stress Tolerance to Plants. Plant Biotechnol. 2007, 24, 117–126. [Google Scholar] [CrossRef]
- Gao, C.; Hu, J.; Zhang, S.; Zheng, Y.; Knapp, A. Association of Polyamines in Governing the Chilling Sensitivity of Maize Genotypes. Plant Growth Regul. 2009, 57, 31–38. [Google Scholar] [CrossRef]
- Li, J.; Hu, L.; Zhang, L.; Pan, X.; Hu, X. Exogenous Spermidine Is Enhancing Tomato Tolerance to Salinity–Alkalinity Stress by Regulating Chloroplast Antioxidant System and Chlorophyll Metabolism. BMC Plant Biol. 2015, 15, 303. [Google Scholar] [CrossRef]
- Li, Z.; Jing, W.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Yan, Y.H. Spermine Alleviates Drought Stress in White Clover with Different Resistance by Influencing Carbohydrate Metabolism and Dehydrins Synthesis. PLoS ONE 2015, 10, e0120708. [Google Scholar] [CrossRef]
- Kovács, H.; Aleksza, D.; Baba, A.I.; Hajdu, A.; Király, A.M.; Zsigmond, L.; Tóth, S.Z.; Kozma-Bognár, L.; Szabados, L. Light Control of Salt-Induced Proline Accumulation Is Mediated by Elongated Hypocotyl 5 in Arabidopsis. Front. Plant Sci. 2019, 10, 1584. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline Metabolism as Regulatory Hub. Trends Plant Sci. 2021, 27, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Gillaspy, G.E. The Cellular Language of Myo-Inositol Signaling. New Phytol. 2011, 192, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A.; Murthy, P.P. Myo-Inositol Metabolism in Plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The Osmotic Potential of Polyethylene Glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Sadaf, S.; Ullah, S.; Alshaya, H.; Okla, M.; Alwasel, Y.; Tariq, A. Using Halothermal Time Model to Describe Barley (Hordeumvulgare L.) Seed Germination Response to Water Potential and Temperature. Life 2022, 12, 209. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and Validation of Reference Genes for Quantitative RT-PCR Normalization in Wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef]
- Pál, M.; Tajti, J.; Szalai, G.; Peeva, V.; Végh, B.; Janda, T. Interaction of Polyamines, Abscisic Acid and Proline under Osmotic Stress in The Leaves of Wheat Plants. Sci. Rep. 2018, 8, 12839. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Li, J.; Liu, L. RNAseq Analysis Reveals Pathways and Candidate Genes Associated with Salinity Tolerance in a Spaceflight-induced Wheat Mutant. Sci. Rep. 2017, 7, 2731. [Google Scholar] [CrossRef]
- Gondor, O.K.; Tajti, J.; Hamow, K.Á.; Majláth, I.; Szalai, G.; Janda, T.; Pál, M. Polyamine Metabolism under Different Light Regimes in Wheat. Int. J. Mol. Sci. 2021, 22, 11717. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Seifert, E. OriginPro 9.1: Scientific data analysis and graphing software-software review. J. Chem. Inf. Model. 2014, 54, 1552. [Google Scholar] [CrossRef] [PubMed]
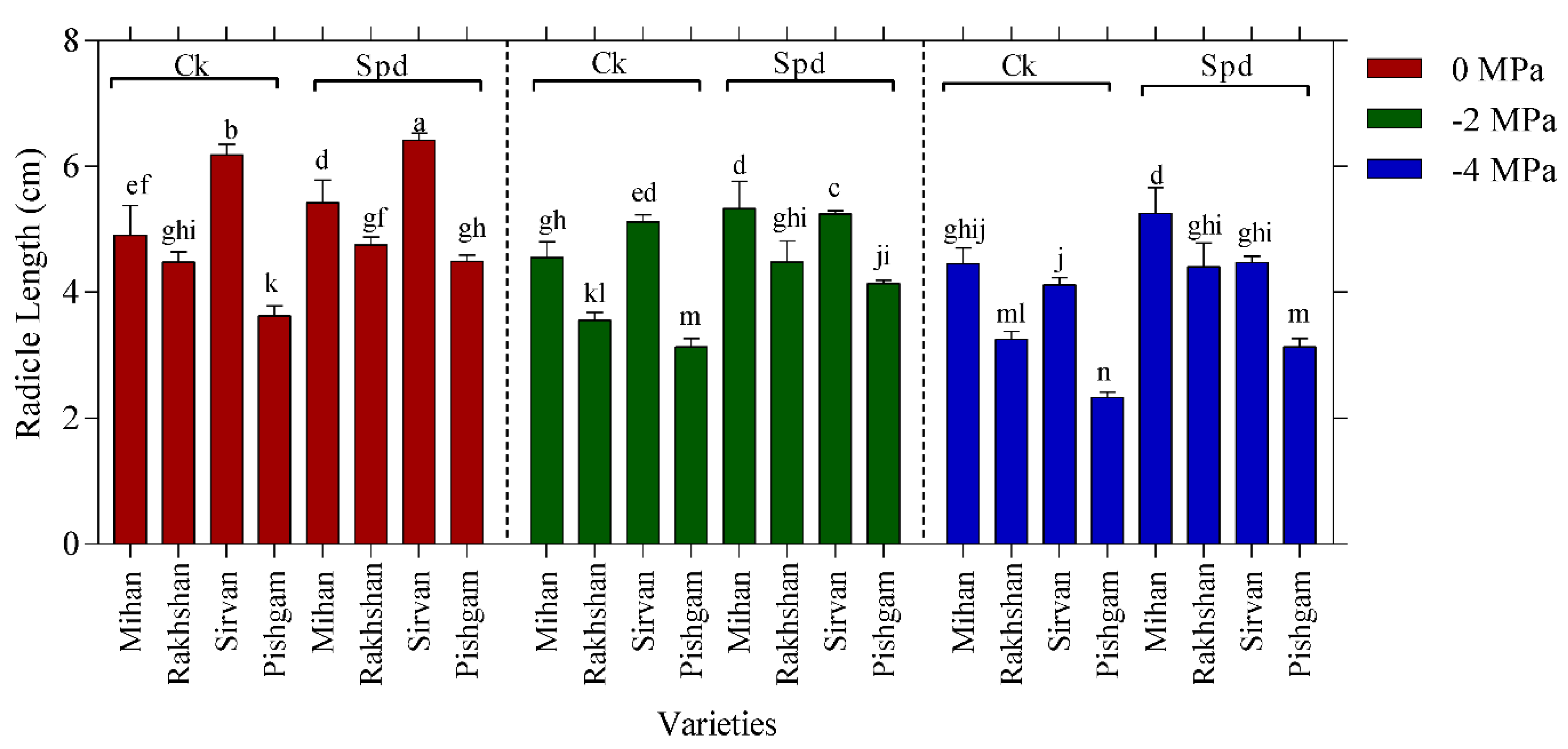

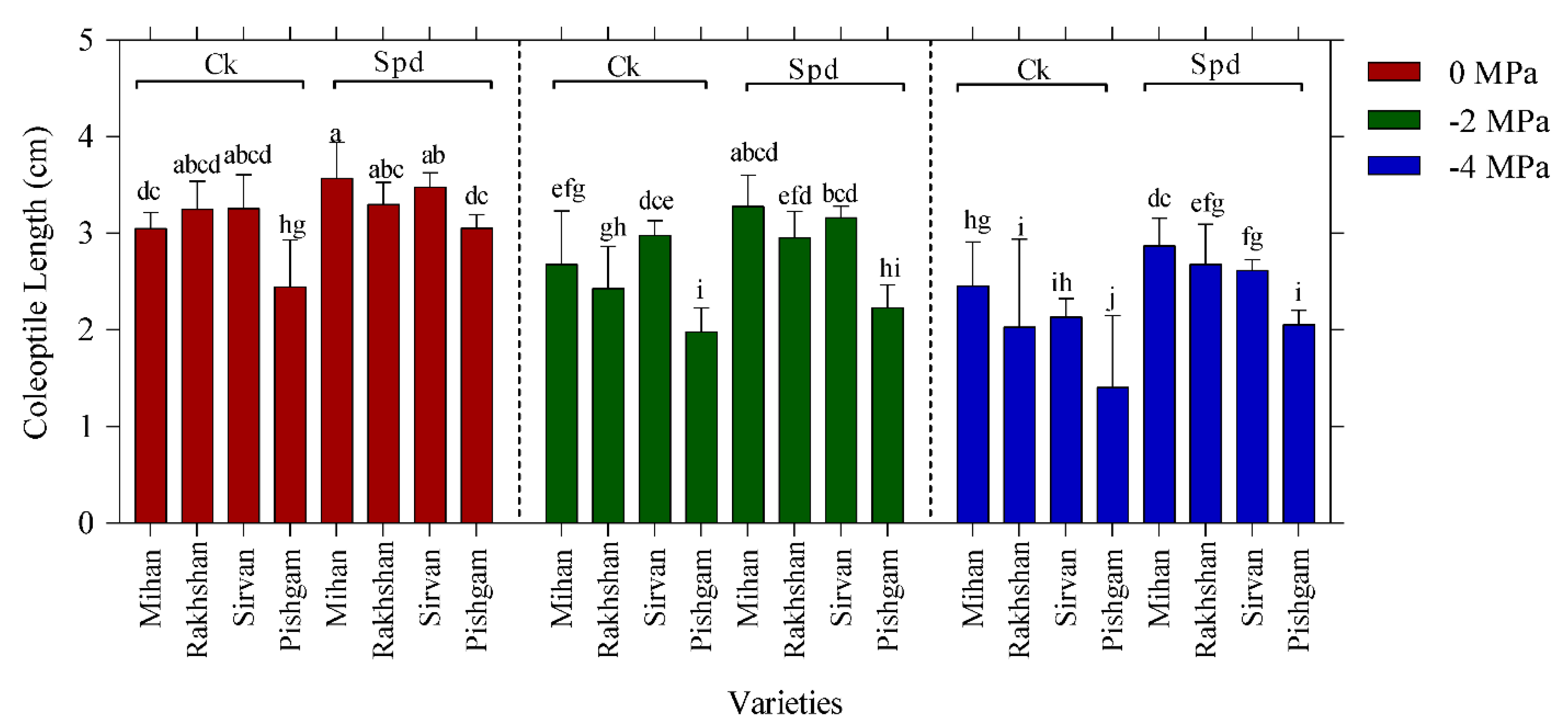
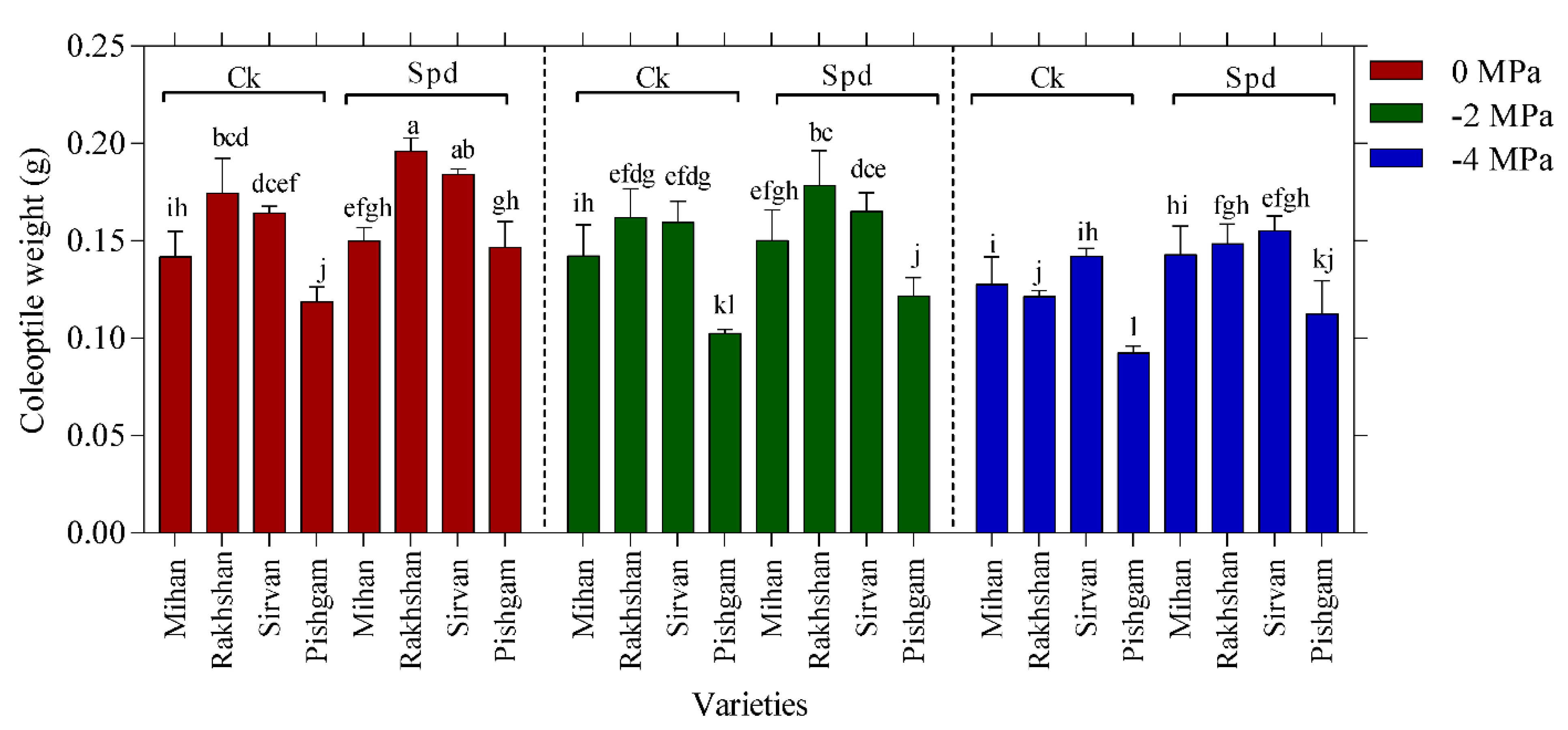
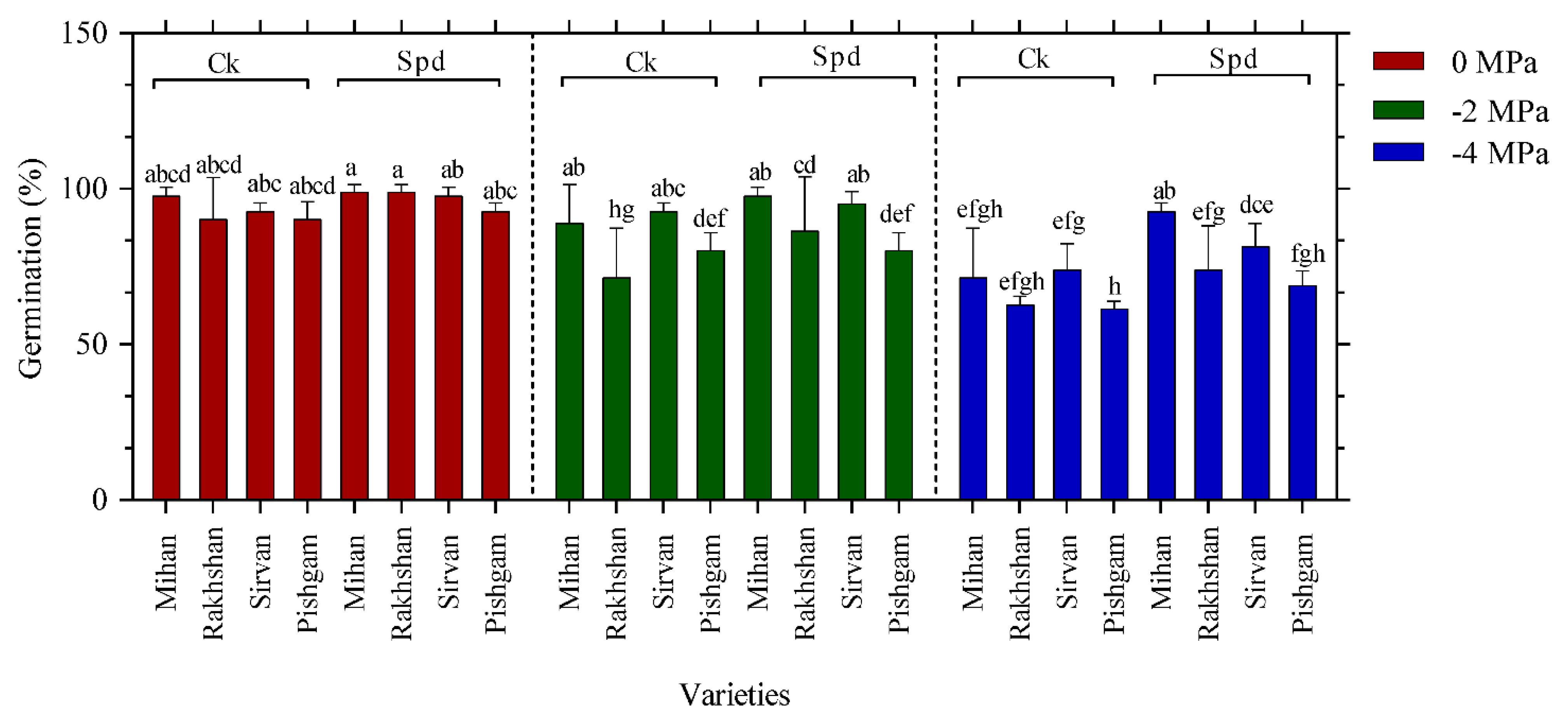


| Changes Sources | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| Radicle Length | Coleoptile Length | Radicle Weight | Coleoptile Weight | Germination Percentage | Germination Rate | ||
| Block | 3 | 0.009 ns | 0.01 ns | 0.0003 ns | 0.0001 ns | 12.76 ns | 3.36 ns |
| Drought stress (Ds) | 2 | 13.31 ** | 6.14 ** | 0.002 ** | 0.002 ** | 2863.3 ** | 288.3 ** |
| Spermidine (Spd) | 1 | 15.01 ** | 4.77 ** | 0.0009 ** | 0.006 ** | 1544.01 ** | 111.9 ** |
| Varieties (V) | 3 | 21.39 ** | 3.30 ** | 0.0007 ** | 0.011 ** | 971.09 ** | 82.42 ** |
| Spd × Ds | 2 | 0.03 ns | 0.15 ** | 0.0001 ns | 0.0005 ns | 51.82 ns | 1.26 ns |
| Ds × V | 6 | 2.29 ** | 0.16 ** | 0.0002 ** | 0.001 * | 276.05 ** | 13.78 ** |
| V × Spd | 3 | 0.03 ns | 0.09 ns | 0.0003 * | 0.0002 ns | 121.78 ns | 6.81 ** |
| V × Ds × Spd | 6 | 0.20 ** | 0.06 ns | 0.0001 ns | 0.0001 ns | 193.85 * | 0.267 ns |
| Error | 69 | 0.06 | 0.05 | 0.0009 | 0.0001 | 74.17 | 1.26 |
| CV (%) | 5.37 | 8.92 | 9.73 | 7.74 | 10.13 | 5.94 | |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Reference |
|---|---|---|---|
| Ta30797 (PGD) | 5′-GCCGTGTCCATGCCAGTG-3′ | 5′-TTAGCCTGAACCACCTGTGC-3′ | [47] |
| TaADC | 5′-TCTACCCCGTCAAGTGCAAC-3′ | 5′-GACGAGGCAGCTCATGGT-3′ | [48] |
| TaODC | 5′-CGTGCGTGGAGGTGATAGG-3′ | 5′-AGCTGAGGGTGCCGTAGA-3′ | [48] |
| TaSAMDC | 5′-ACAGCCTTCTCCACACAAGA-3′ | 5′-TCCAGACCAGTCATGCACA-3′ | [48] |
| TaSPDS | 5′-AGGTATTCAAGGGTGGCGTG-3′ | 5′-TGGGTTCACAGGAGTCAGGA-3′ | [48] |
| TaPAO | 5′-GCTCATAAATCAGCCCAATTCCA-3′ | 5′-TTCGCCATTTGTTGAGCTCT-3′ | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholizadeh, F.; Janda, T.; Gondor, O.K.; Pál, M.; Szalai, G.; Sadeghi, A.; Turkoglu, A. Improvement of Drought Tolerance by Exogenous Spermidine in Germinating Wheat (Triticum aestivum L.) Plants Is Accompanied with Changes in Metabolite Composition. Int. J. Mol. Sci. 2022, 23, 9047. https://doi.org/10.3390/ijms23169047
Gholizadeh F, Janda T, Gondor OK, Pál M, Szalai G, Sadeghi A, Turkoglu A. Improvement of Drought Tolerance by Exogenous Spermidine in Germinating Wheat (Triticum aestivum L.) Plants Is Accompanied with Changes in Metabolite Composition. International Journal of Molecular Sciences. 2022; 23(16):9047. https://doi.org/10.3390/ijms23169047
Chicago/Turabian StyleGholizadeh, Fatemeh, Tibor Janda, Orsolya Kinga Gondor, Magda Pál, Gabriella Szalai, Amirali Sadeghi, and Aras Turkoglu. 2022. "Improvement of Drought Tolerance by Exogenous Spermidine in Germinating Wheat (Triticum aestivum L.) Plants Is Accompanied with Changes in Metabolite Composition" International Journal of Molecular Sciences 23, no. 16: 9047. https://doi.org/10.3390/ijms23169047
APA StyleGholizadeh, F., Janda, T., Gondor, O. K., Pál, M., Szalai, G., Sadeghi, A., & Turkoglu, A. (2022). Improvement of Drought Tolerance by Exogenous Spermidine in Germinating Wheat (Triticum aestivum L.) Plants Is Accompanied with Changes in Metabolite Composition. International Journal of Molecular Sciences, 23(16), 9047. https://doi.org/10.3390/ijms23169047









