The Association between TRP Channels Expression and Clinicopathological Characteristics of Patients with Pancreatic Adenocarcinoma
Abstract
:1. Introduction
2. Results
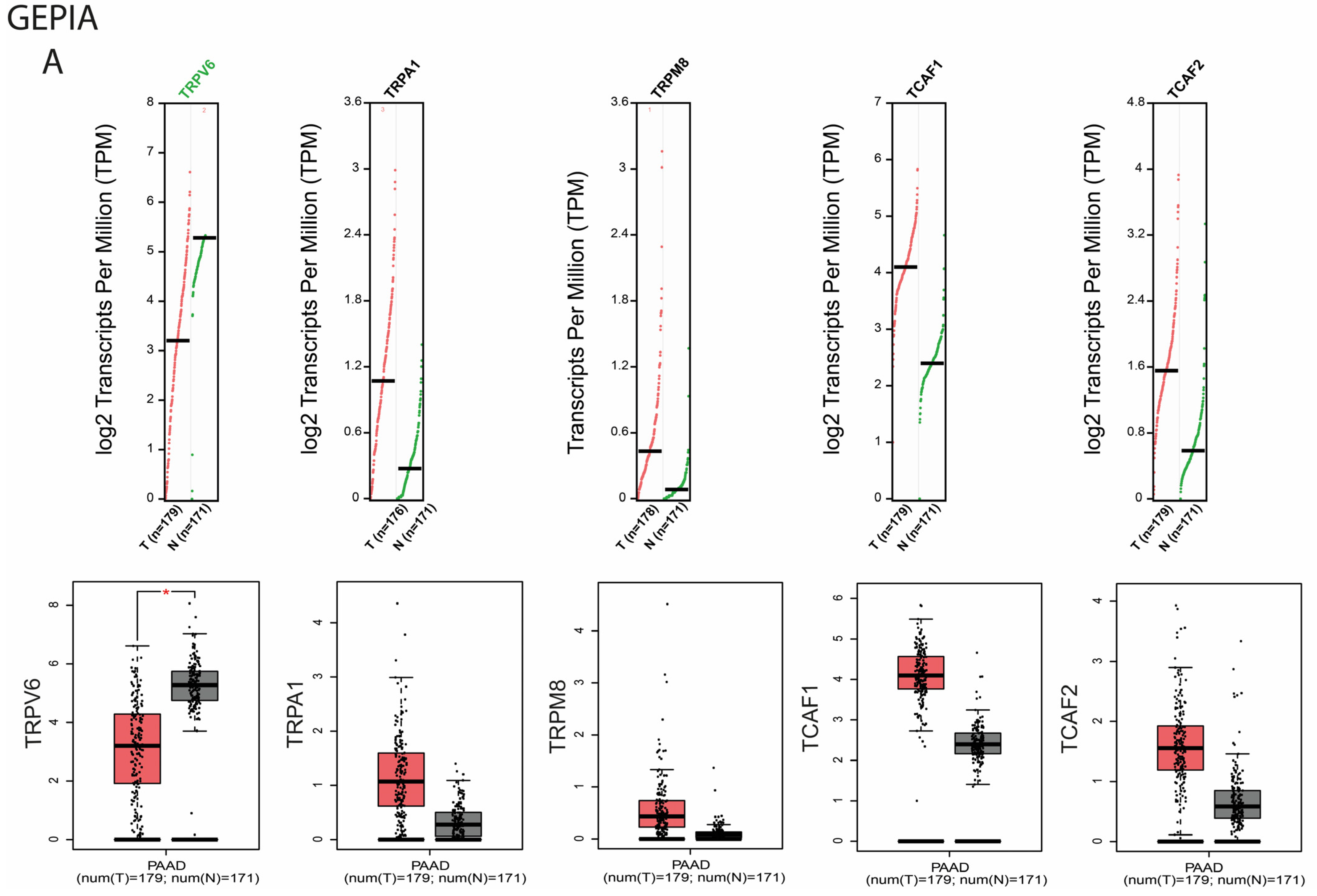
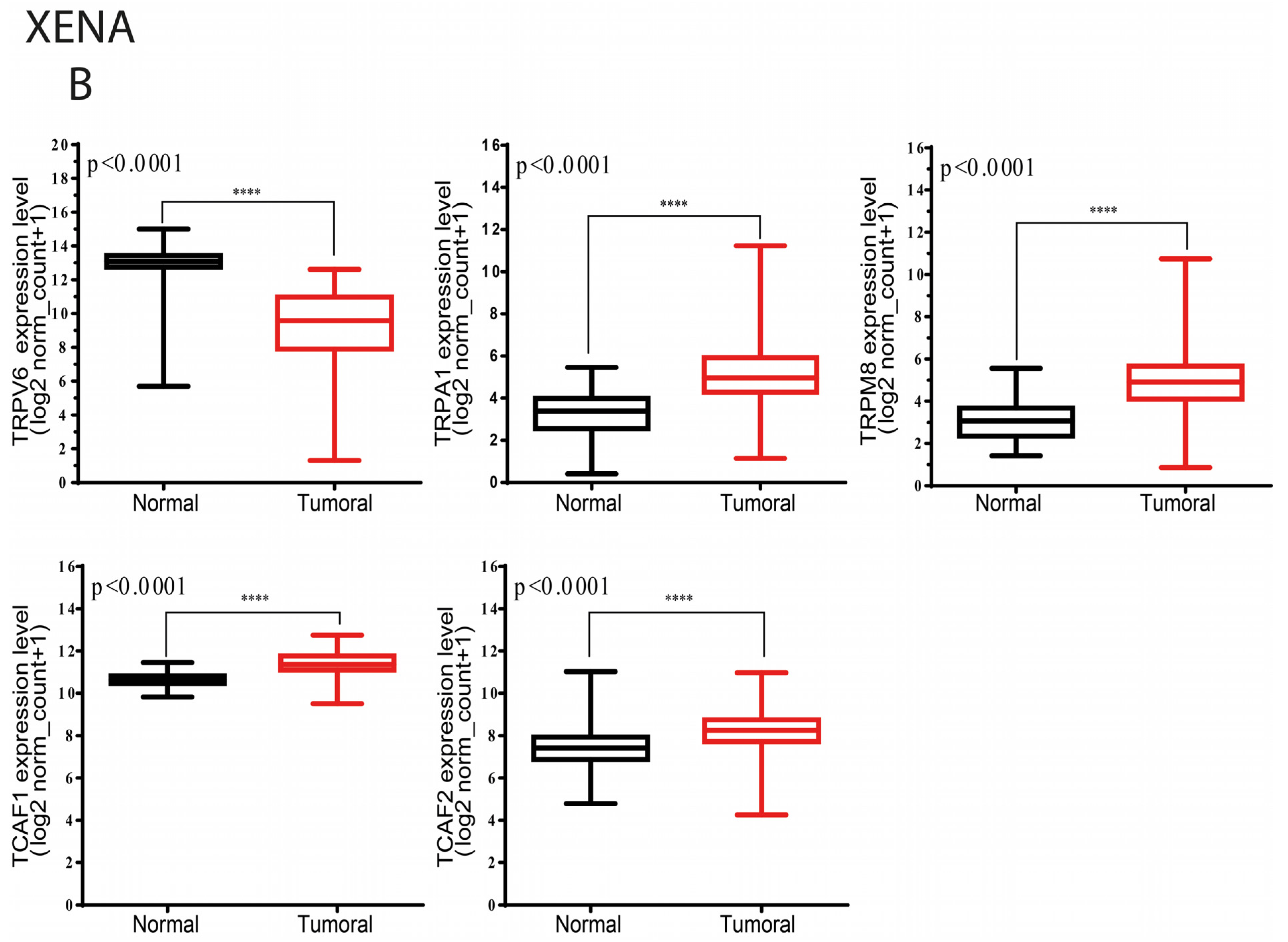
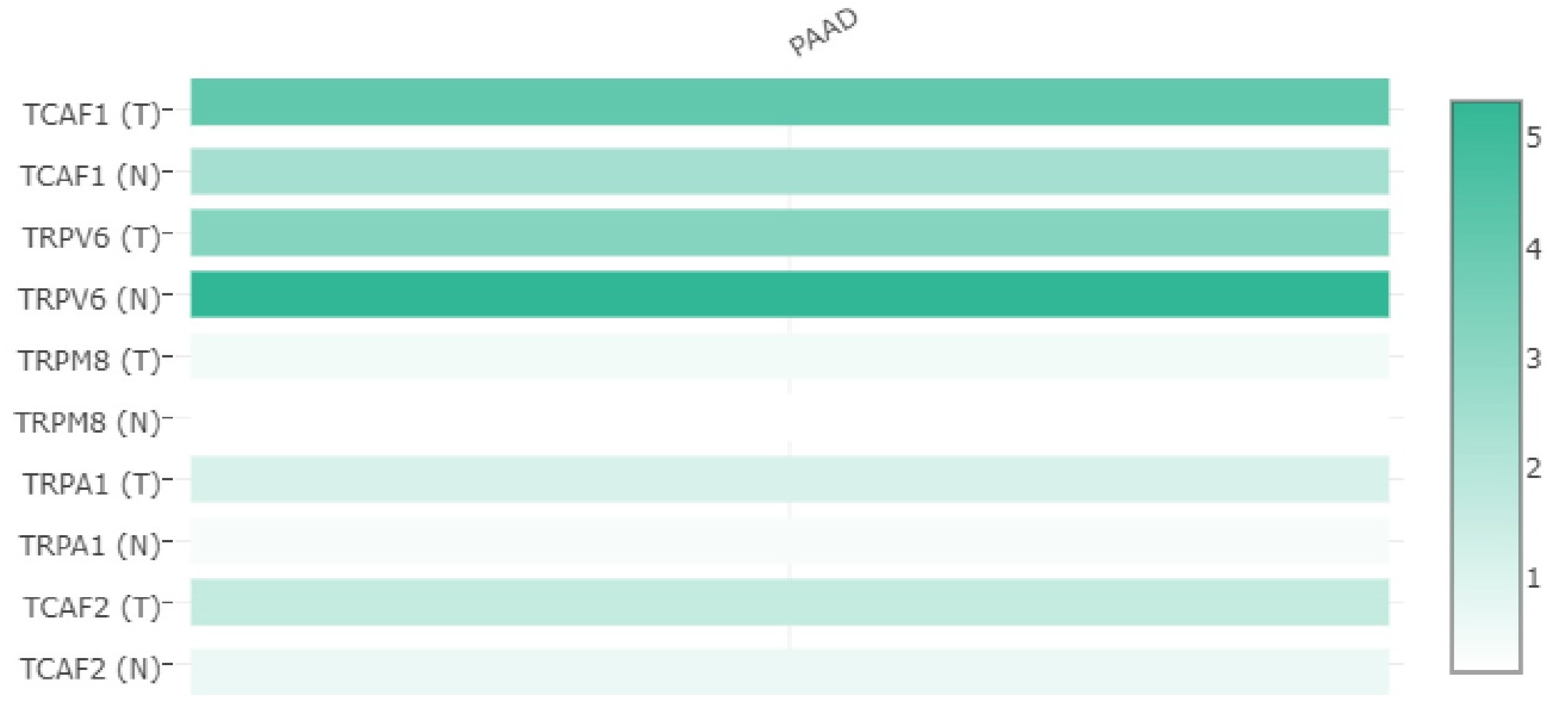
2.1. Studies of the Selected TRP Genes in Patients Included in the TCGA and GTEx Public Datasets
2.1.1. Differential Expression Analysis of Selected TRP Channels
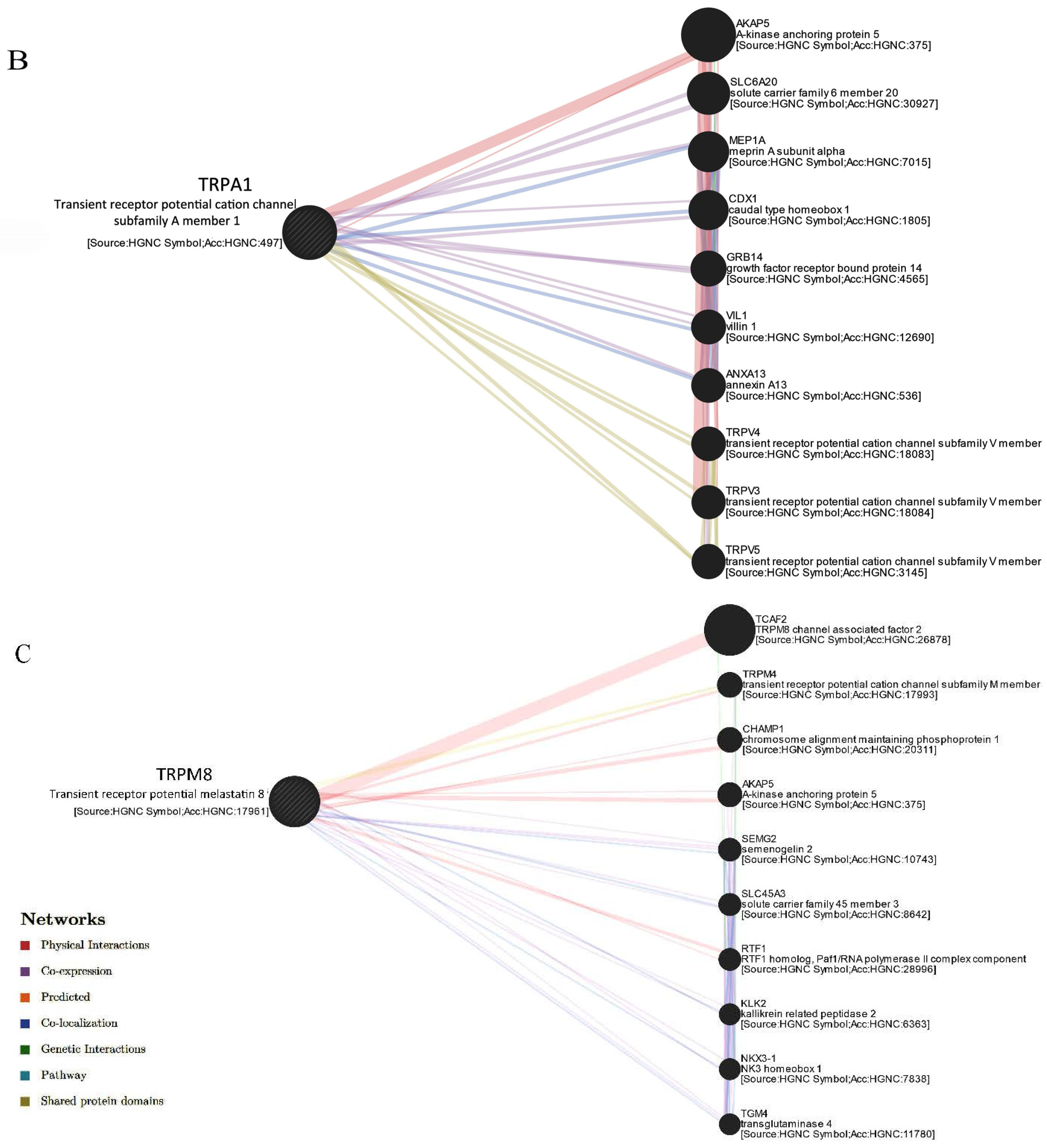
2.1.2. Selected TRP Channels Interact with Associated Factors and Calcium-Binding Proteins
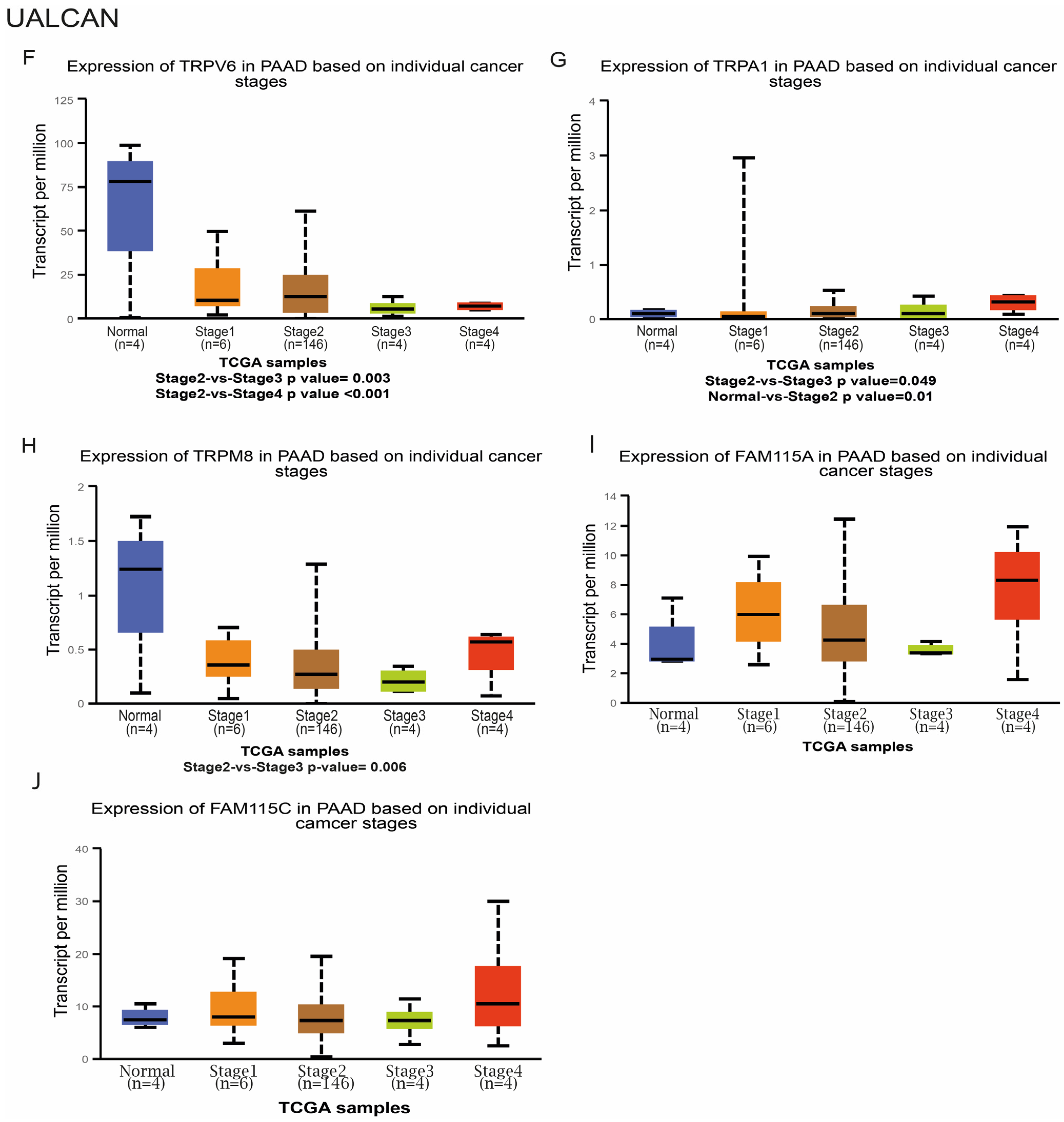
2.1.3. Selected TRP Genes Differently Correlates with Tumor Stages and Metastasis
2.2. Studies on the Validation Cohort of the Centre of Digestive Disease and Liver Transplantation in Fundeni Clinical Institute, Bucharest
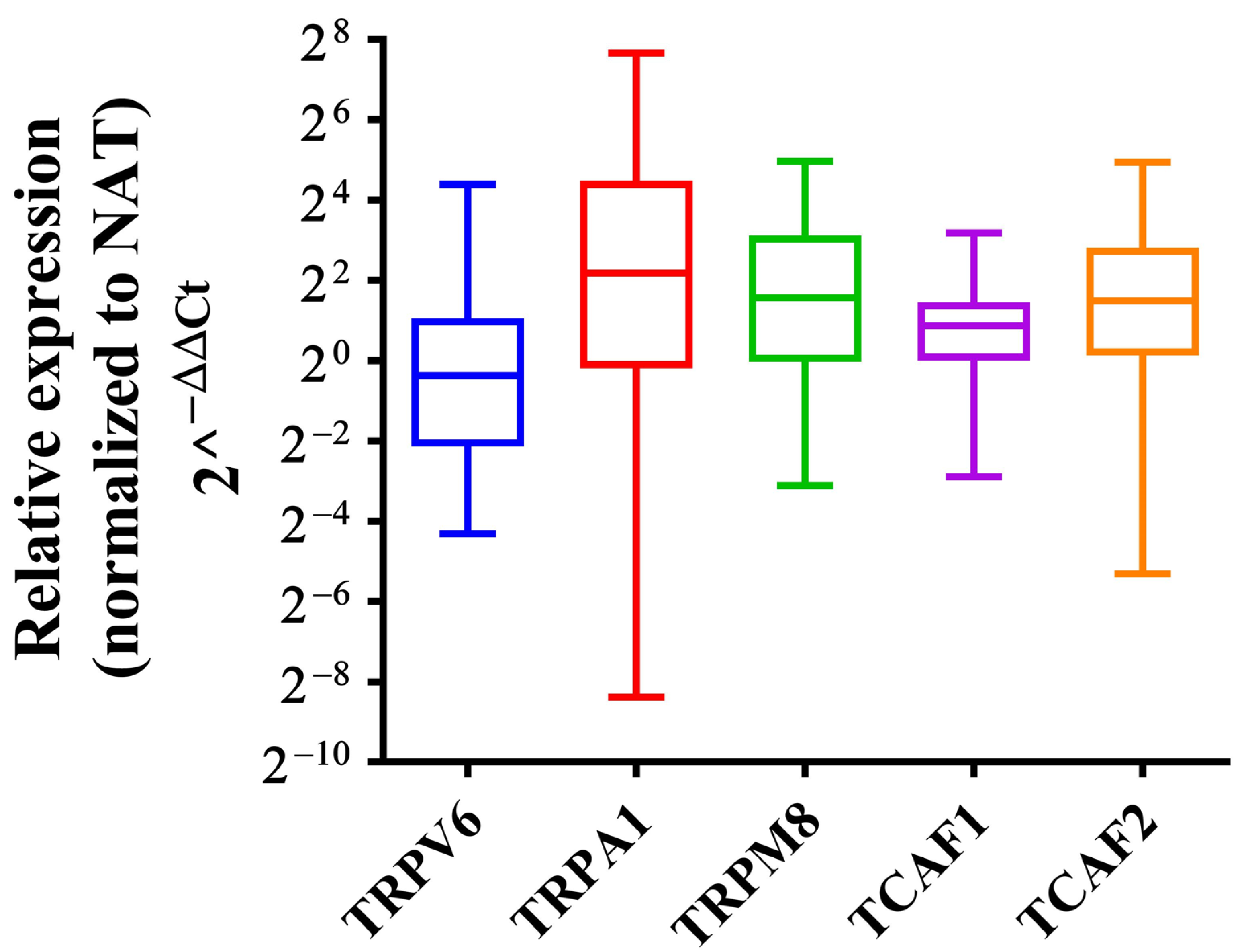
2.2.1. Independent Validation of the Expression Level of the Selected Genes by qPCR
2.2.2. Clinicopathologic Characteristics of the PDAC Patients in the Validation Cohort
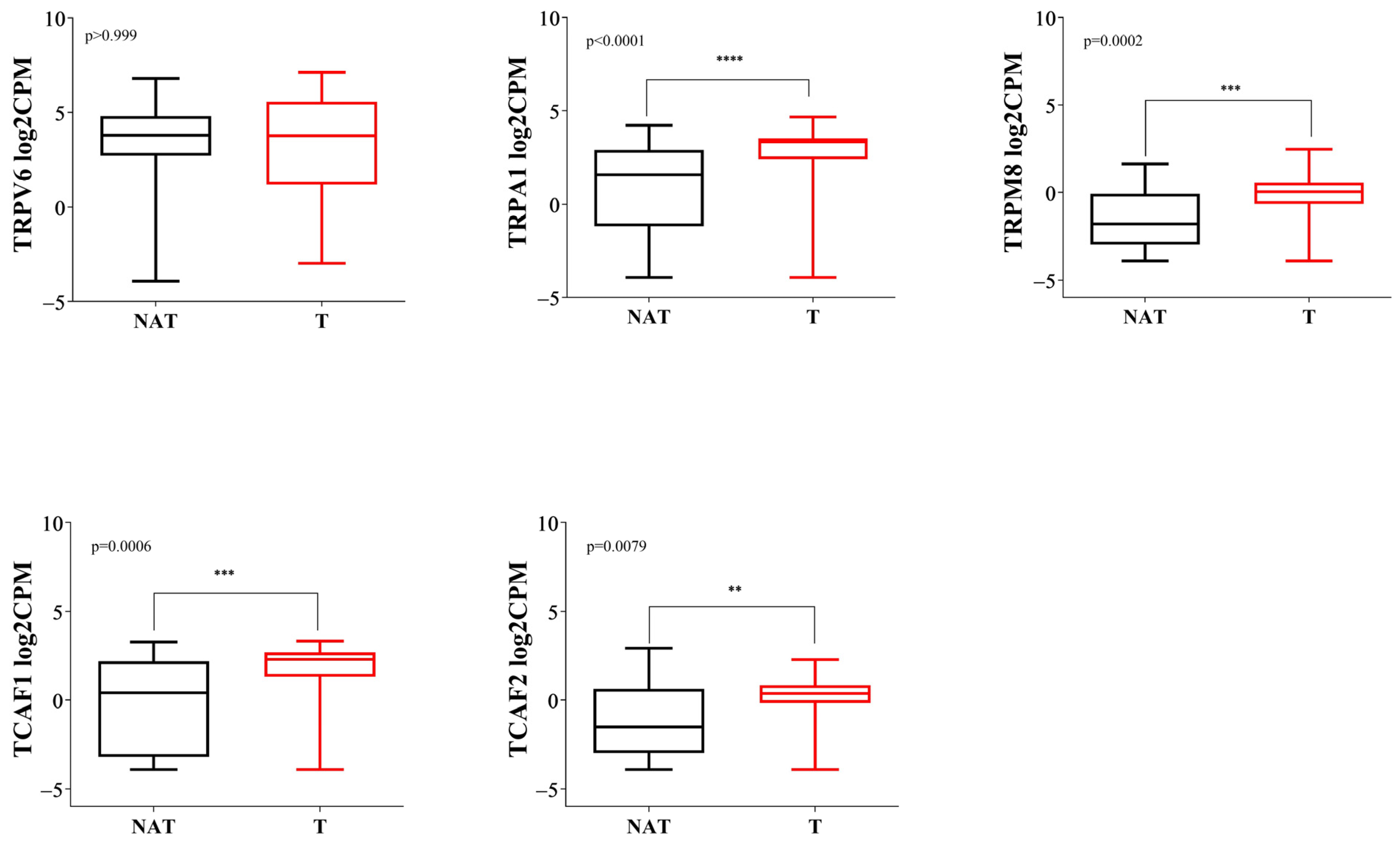
2.2.3. Gene Expression Analysis of the TRP Family Members by RNA-Seq Confirms Overexpression of TRPA1, TRPM8, TCAF1 and TCAF2 and Lower Expression of TRPV6 in PDAC Tissues
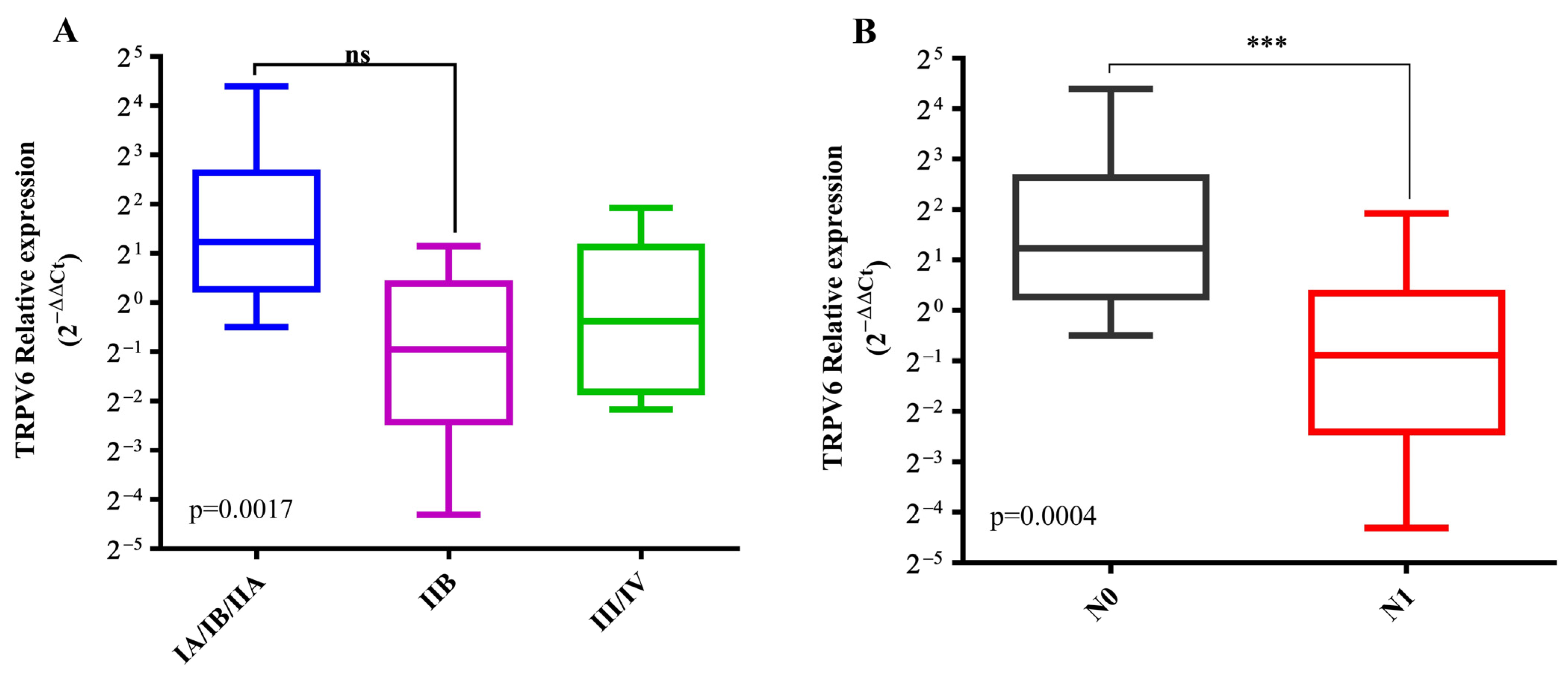
2.2.4. Correlation Analysis with Clinicopathological Characteristics of the PDAC Patients in the Validation Cohort
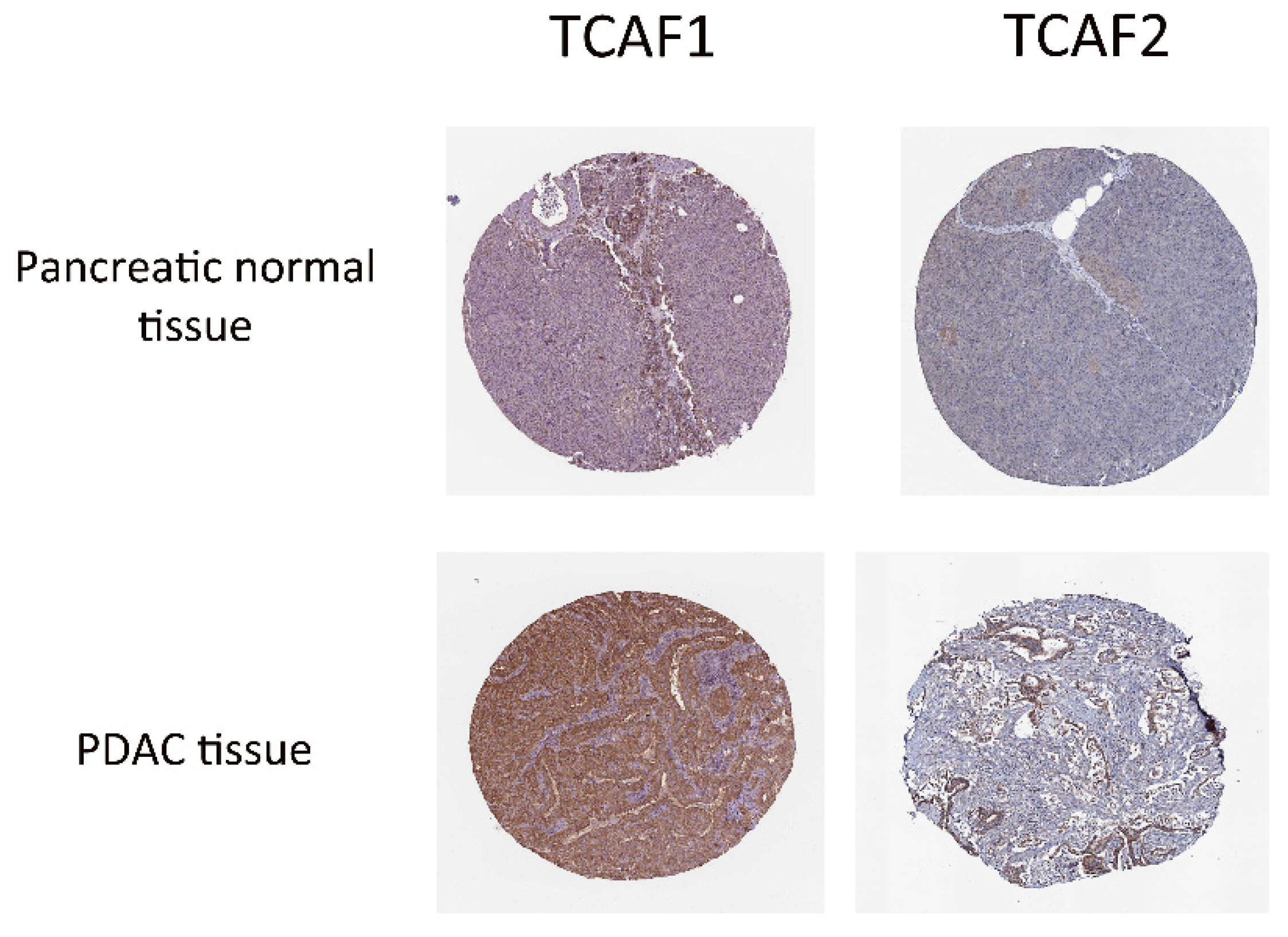
2.2.5. TRP Protein Expression and Function in PDAC Tissues
3. Discussion
3.1. Transient Receptor Potential Vanilloid 6 (TRPV6)
3.2. Transient Receptor Potential Ankyrin 1 (TRPA1)
3.3. Transient Receptor Potential Melastatin 8 (TRPM8)
3.4. Channels Functionality
4. Materials and Methods
4.1. Differential Expression Analysis by GEPIA
4.2. Differential Expression by UCSC Xena
4.3. Differential Expression by Ualcan
4.4. Differential Expression by TNMplot
4.5. Interaction Network by GeneMANIA
4.6. Patients and Sample Preparation
4.7. RNA Sequencing Analysis (RNA-Seq)
4.8. Quantitative Real-Time PCR Analysis
4.9. Statistics
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schizas, D.; Charalampakis, N.; Kole, C.; Economopoulou, P.; Koustas, E.; Gkotsis, E.; Ziogas, D.; Psyrri, A.; Karamouzis, M.V. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat. Rev. 2020, 86, 102016. [Google Scholar] [CrossRef]
- Bakasa, W.; Viriri, S. Pancreatic Cancer Survival Prediction: A Survey of the State-of-the-Art. Comput. Math. Methods Med. 2021, 2021, 1188414. [Google Scholar] [CrossRef]
- Ay, S.; Atci, M.M.; Arikan, R.; Dulgar, O.; Ozyukseler, D.T.; Paksoy, N.; Dogan, I.; Oztosun, B.; Tastekin, D.; Oven, B.B.; et al. FOLFIRINOX versus gemcitabine plus nab-paclitaxel as the first-line chemotherapy in metastatic pancreatic cancer. J. Chemother. 2022, 1–7. [Google Scholar] [CrossRef]
- Du, J.D.; Zheng, X.; Chen, Y.L.; Huang, Z.Q.; Cai, S.W.; Jiao, H.B.; Zhu, Z.M.; Hu, B. Elevated Transient Receptor Potential Melastatin 8 (TRPM8) Expression Is Correlated with Poor Prognosis in Pancreatic Cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 3720–3725. [Google Scholar] [CrossRef]
- Yee, N.S.; Brown, R.D.; Lee, M.S.; Zhou, W.; Jensen, C.; Gerke, H.; Yee, R.K. TRPM8 ion channel is aberrantly expressed and required for preventing replicative senescence in pancreatic adenocarcinoma: Potential role of TRPM8 as a biomarker and target. Cancer Biol. Ther. 2012, 13, 592–599. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, X.-Y.; Li, Q.; Cui, C.; Qiao, C.-M.; Shen, Y.-Q.; Zhao, W.-J. Comprehensive Pan-Cancer Analysis of TRPM8 in Tumor Metabolism and Immune Escape. Front. Oncol. 2022, 12, 914060. [Google Scholar] [CrossRef]
- Cucu, D.; Chiritoiu, G.; Petrescu, S.; Babes, A.; Stanica, L.; Duda, D.G.; Horii, A.; Dima, S.O.; Popescu, I. Characterization of functional transient receptor potential melastatin 8 channels in human pancreatic ductal adenocarcinoma cells. Pancreas 2014, 43, 795–800. [Google Scholar] [CrossRef]
- Song, H.; Dong, M.; Zhou, J.; Sheng, W.; Li, X.; Gao, W. Expression and prognostic significance of TRPV6 in the development and progression of pancreatic cancer. Oncol. Rep. 2018, 39, 1432–1440. [Google Scholar] [CrossRef]
- Takahashi, N.; Chen, H.Y.; Harris, I.S.; Stover, D.G.; Selfors, L.M.; Bronson, R.T.; Deraedt, T.; Cichowski, K.; Welm, A.L.; Mori, Y.; et al. Cancer Cells Co-opt the Neuronal Redox-Sensing Channel TRPA1 to Promote Oxidative-Stress Tolerance. Cancer Cell 2018, 33, 985–1003. [Google Scholar] [CrossRef]
- Cojocaru, F.; Selescu, T.; Domocos, D.; Marutescu, L.; Chiritoiu, G.; Chelaru, N.R.; Dima, S.; Mihailescu, D.; Babes, A.; Cucu, D. Functional expression of the transient receptor potential ankyrin type 1 channel in pancreatic adenocarcinoma cells. Sci. Rep. 2021, 11, 2018. [Google Scholar] [CrossRef]
- Mesquita, G.; Prevarskaya, N.; Schwab, A.; Lehen’kyi, V. Role of the TRP Channels in Pancreatic Ductal Adenocarcinoma Development and Progression. Cells 2021, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, C.; Wartmann, T.; Kahlert, C.; Du, R.; Perrakis, A.; Brunner, T.; Croner, R.S.; Kahlert, U.D. Sensory Ion Channel Candidates Inform on the Clinical Course of Pancreatic Cancer and Present Potential Targets for Repurposing of FDA-Approved Agents. J. Pers. Med. 2022, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Ulareanu, R.; Chiritoiu, G.; Cojocaru, F.; Deftu, A.; Ristoiu, V.; Stanica, L.; Mihailescu, D.F.; Cucu, D. N-glycosylation of the transient receptor potential melastatin 8 channel is altered in pancreatic cancer cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39, 1010428317720940. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.S. Roles of TRPM8 Ion Channels in Cancer: Proliferation, Survival, and Invasion. Cancers 2015, 7, 2134–2146. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, G.; Gong, Y.; Yu, Q.; He, B.; Li, W.; He, Z.; Hao, W.; Liu, Y. Silencing of TRPM8 inhibits aggressive tumor phenotypes and enhances gemcitabine sensitivity in pancreatic cancer. Pancreatology 2018, 18, 935–944. [Google Scholar] [CrossRef]
- Zaccagnino, A.; Pilarsky, C.; Tawfik, D.; Sebens, S.; Trauzold, A.; Novak, I.; Schwab, A.; Kalthoff, H. In silico analysis of the transportome in human pancreatic ductal adenocarcinoma. Eur. Biophys. J. EBJ 2016, 45, 749–763. [Google Scholar] [CrossRef]
- Fan, H.; Shen, Y.X.; Yuan, Y.F. Expression and prognostic roles of TRPV5 and TRPV6 in non-small cell lung cancer after curative resection. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 2559–2563. [Google Scholar] [CrossRef]
- Wu, Y.; Miyamoto, T.; Li, K.; Nakagomi, H.; Sawada, N.; Kira, S.; Kobayashi, H.; Zakohji, H.; Tsuchida, T.; Fukazawa, M.; et al. Decreased expression of the epithelial Ca2+ channel TRPV5 and TRPV6 in human renal cell carcinoma associated with vitamin D receptor. J. Urol. 2011, 186, 2419–2425. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xie, X.; Wen, J.; Luo, K.J.; Liu, Q.W.; Yang, H.; Hu, Y.; Fu, J.H. TRPV6 plays a new role in predicting survival of patients with esophageal squamous cell carcinoma. Diagn. Pathol. 2016, 11, 14. [Google Scholar] [CrossRef]
- Gkika, D.; Lemonnier, L.; Shapovalov, G.; Gordienko, D.; Poux, C.; Bernardini, M.; Bokhobza, A.; Bidaux, G.; Degerny, C.; Verreman, K.; et al. TRP channel-associated factors are a novel protein family that regulates TRPM8 trafficking and activity. J. Cell Biol. 2015, 208, 89–107. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, N.; Li, X.; Guo, C.; Li, C.; Jiang, B.; Wang, H.; Shi, D. Inhibition of PTP1B blocks pancreatic cancer progression by targeting the PKM2/AMPK/mTOC1 pathway. Cell Death Dis. 2019, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.Z.D.; Glicksberg, B.S.; Li, Y.; Chen, B. Selecting precise reference normal tissue samples for cancer research using a deep learning approach. BMC Med. Genom. 2019, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Camarda, R.; Odegaard, J.; Paik, H.; Oskotsky, B.; Krings, G.; Goga, A.; Sirota, M.; Butte, A.J. Comprehensive analysis of normal adjacent to tumor transcriptomes. Nat. Commun. 2017, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Dhennin-Duthille, I.; Gautier, M.; Faouzi, M.; Guilbert, A.; Brevet, M.; Vaudry, D.; Ahidouch, A.; Sevestre, H.; Ouadid-Ahidouch, H. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: Correlation with pathological parameters. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2011, 28, 813–822. [Google Scholar] [CrossRef]
- Zhao, D.; Han, D.F.; Wang, S.S.; Lv, B.; Wang, X.; Ma, C. Roles of tumor necrosis factor-alpha and interleukin-6 in regulating bone cancer pain via TRPA1 signal pathway and beneficial effects of inhibition of neuro-inflammation and TRPA1. Mol. Pain 2019, 15, 1744806919857981. [Google Scholar] [CrossRef]
- Zhong, Z.; Ye, Z.; He, G.; Zhang, W.; Wang, J.; Huang, S. Low expression of A-kinase anchor protein 5 predicts poor prognosis in non-mucin producing stomach adenocarcinoma based on TCGA data. Ann. Transl. Med. 2020, 8, 115. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef]
- Grolez, G.P.; Gkika, D. TRPM8 Puts the Chill on Prostate Cancer. Pharmaceuticals 2016, 9, 44. [Google Scholar] [CrossRef]
- Hemida, A.S.; Hammam, M.A.; Heriz, N.; Shehata, W.A. Expression of Transient Receptor Potential Channel of Melastatin number 8 (TRPM8) in Non- Melanoma Skin Cancer: A Clinical and Immunohistochemical study. J. Immunoass. Immunochem. 2021, 42, 620–632. [Google Scholar] [CrossRef]
- Huang, Y.; Li, S.; Jia, Z.; Zhao, W.; Zhou, C.; Zhang, R.; Ali, D.W.; Michalak, M.; Chen, X.Z.; Tang, J. Transient Receptor Potential Melastatin 8 (TRPM8) Channel Regulates Proliferation and Migration of Breast Cancer Cells by Activating the AMPK-ULK1 Pathway to Enhance Basal Autophagy. Front. Oncol. 2020, 10, 573127. [Google Scholar] [CrossRef]
- Liu, J.J.; Li, L.Z.; Xu, P. Upregulation of TRPM8 can promote the colon cancer liver metastasis through mediating Akt/GSK-3 signal pathway. Biotechnol. Appl. Biochem. 2022, 69, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Manolache, A.; Selescu, T.; Maier, G.L.; Mentel, M.; Ionescu, A.E.; Neacsu, C.; Babes, A.; Szedlacsek, S.E. Regulation of TRPM8 channel activity by Src-mediated tyrosine phosphorylation. J. Cell. Physiol. 2020, 235, 5192–5203. [Google Scholar] [CrossRef]
- Stewart, J.M. TRPV6 as A Target for Cancer Therapy. J. Cancer 2020, 11, 374–387. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; Yang, B.; Ding, L. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm. Sinica. B 2022, 12, 1761–1780. [Google Scholar] [CrossRef]
- Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repecka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. Theor. Den. Biowiss. 2012, 131, 281–285. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Bartha, A.; Gyorffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef] [PubMed]
- Montojo, J.; Zuberi, K.; Rodriguez, H.; Bader, G.D.; Morris, Q. GeneMANIA: Fast gene network construction and function prediction for Cytoscape. F1000Research 2014, 3, 153. [Google Scholar] [CrossRef]



| Clinicopathological Parameters | Percentage % (n) | |
|---|---|---|
| Age | ≥60 | 61.46% (n = 67) |
| <60 | 38.54% (n = 42) | |
| Gender | Male | 51.37% (n = 56) |
| Female | 48.63% (n = 53) | |
| Tumor size | ≥2 cm | 73.40% (n = 80) |
| <2 cm | 26.60% (n = 23) | |
| TNM | I | 31.2% (n = 34) |
| II | 49.55% (n = 54) | |
| III | 13.75% (n = 15) | |
| IV | 5.5% (n = 6) | |
| Tumor differentiation | G1 | 40.74% (n = 44) |
| G1-G2 | 20.36% (n = 22) | |
| G2 | 27.78% (n = 30) | |
| G2-G3 | 11.12% (n = 12) | |
| CA 19-9 (U/mL) | ≥36 | 77.9% (n = 74) |
| <36 | 22.1% (n = 21) | |
| Diabetes | Yes | 38.53% (n = 42) |
| No | 61.47% (n = 67) | |
| Vascular invasion | Yes | 42.45% (n = 45) |
| No | 57.55% (n = 61) | |
| Perineural invasion | Yes | 47.66% (n = 51) |
| No | 52.34% (n = 56) | |
| Pancreatitis | Yes | 20.44% (n = 19) |
| No | 79.56% (n = 74) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelaru, N.-R.; Chiosa, A.; Sorop, A.; Spiridon, A.; Cojocaru, F.; Domocos, D.; Cucu, D.; Popescu, I.; Dima, S.-O. The Association between TRP Channels Expression and Clinicopathological Characteristics of Patients with Pancreatic Adenocarcinoma. Int. J. Mol. Sci. 2022, 23, 9045. https://doi.org/10.3390/ijms23169045
Chelaru N-R, Chiosa A, Sorop A, Spiridon A, Cojocaru F, Domocos D, Cucu D, Popescu I, Dima S-O. The Association between TRP Channels Expression and Clinicopathological Characteristics of Patients with Pancreatic Adenocarcinoma. International Journal of Molecular Sciences. 2022; 23(16):9045. https://doi.org/10.3390/ijms23169045
Chicago/Turabian StyleChelaru, Nicoleta-Raluca, Andrei Chiosa, Andrei Sorop, Andreea Spiridon, Florentina Cojocaru, Dan Domocos, Dana Cucu, Irinel Popescu, and Simona-Olimpia Dima. 2022. "The Association between TRP Channels Expression and Clinicopathological Characteristics of Patients with Pancreatic Adenocarcinoma" International Journal of Molecular Sciences 23, no. 16: 9045. https://doi.org/10.3390/ijms23169045
APA StyleChelaru, N.-R., Chiosa, A., Sorop, A., Spiridon, A., Cojocaru, F., Domocos, D., Cucu, D., Popescu, I., & Dima, S.-O. (2022). The Association between TRP Channels Expression and Clinicopathological Characteristics of Patients with Pancreatic Adenocarcinoma. International Journal of Molecular Sciences, 23(16), 9045. https://doi.org/10.3390/ijms23169045






