Small-Molecules as Chemiluminescent Probes to Detect Lipase Activity
Abstract
:1. Introduction
2. Results and Discussion
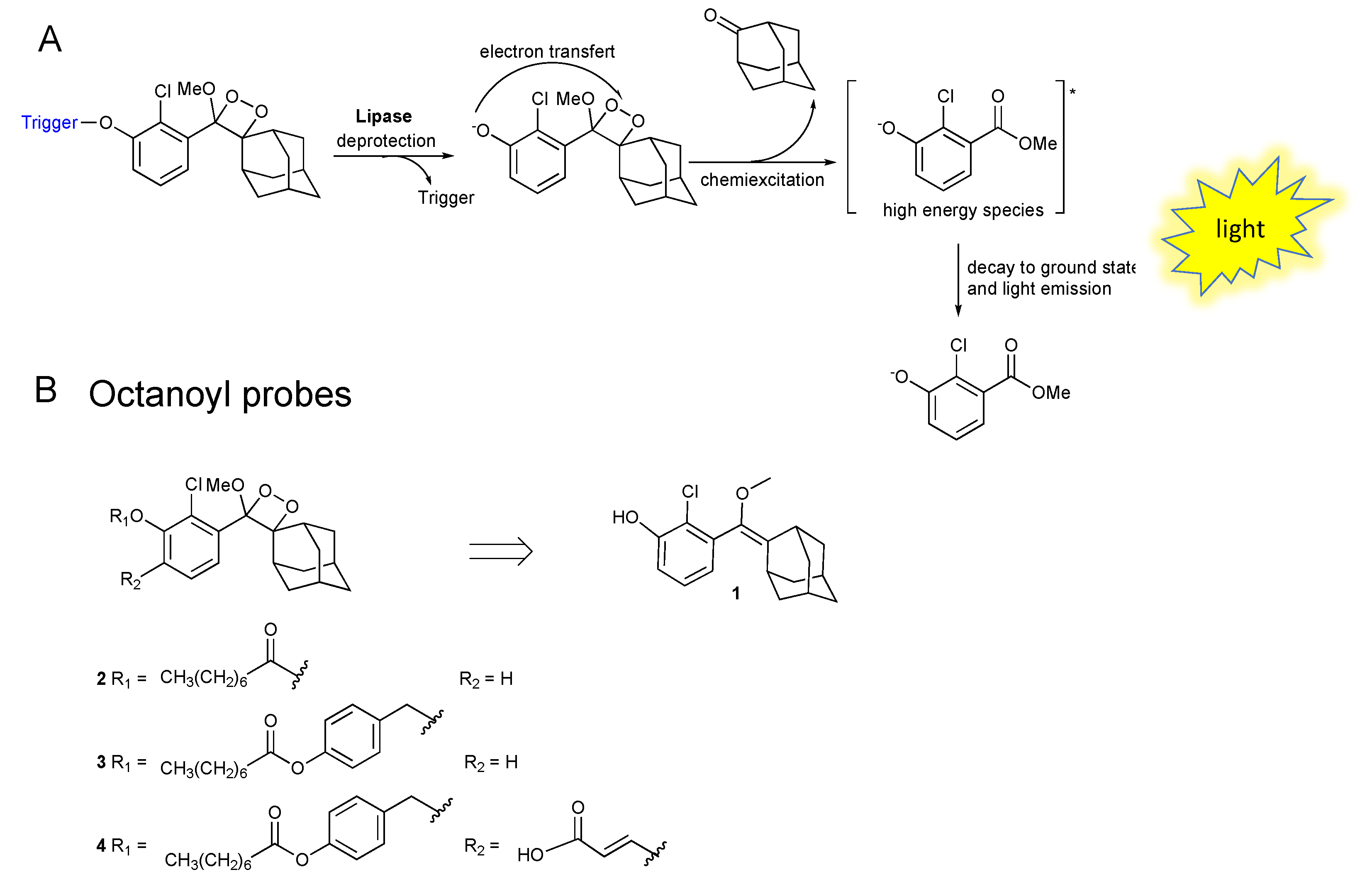
2.1. Synthesis of Phenoxy-1,2-Dioxetane Luminophores 2, 3, and 4
2.2. Chemiluminescence Emission Profile of 2, 3 and 4 over Time
3. Materials and Methods
3.1. Chemistry
- General Information
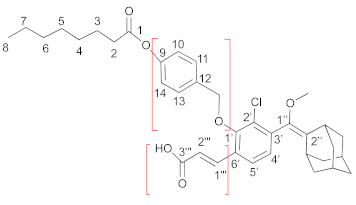
- Compound 5
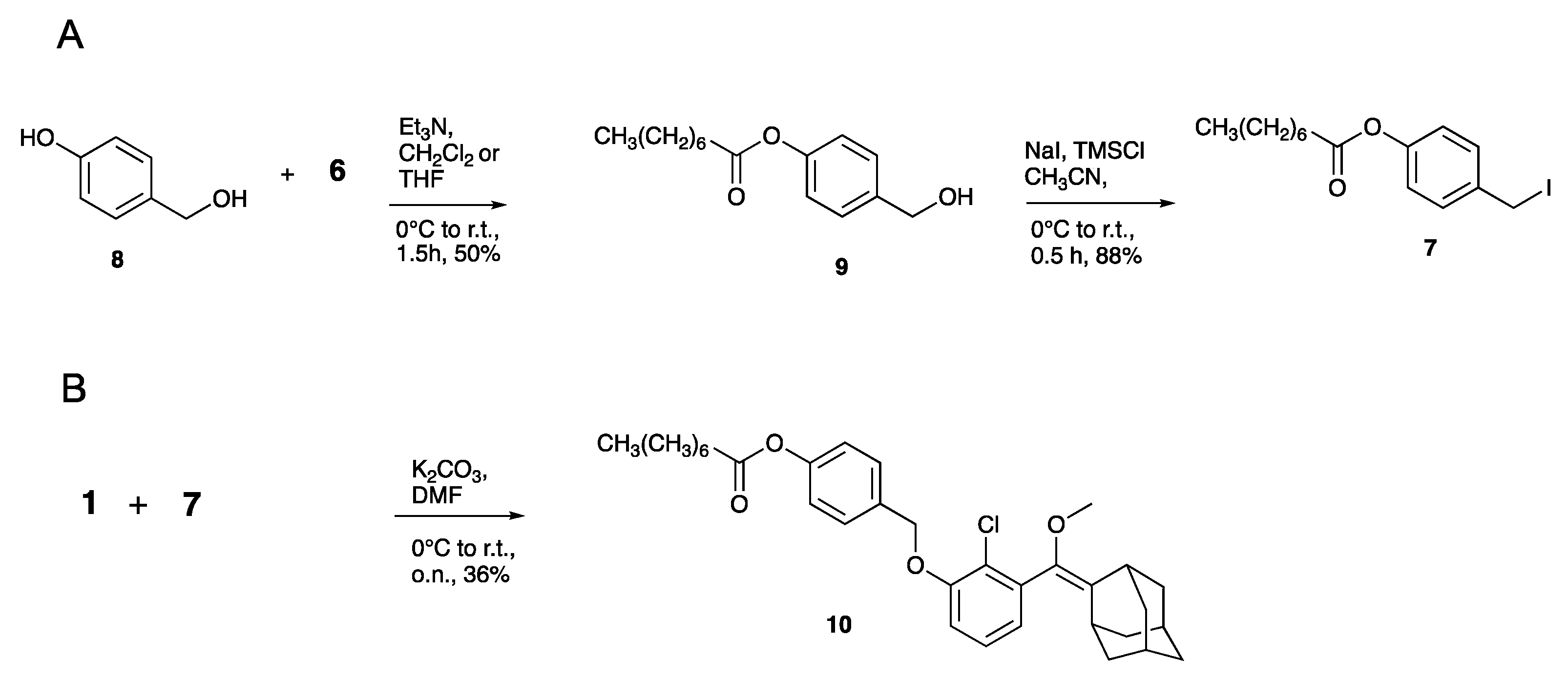
- Compound 9
- Compound 7
- Compound 10
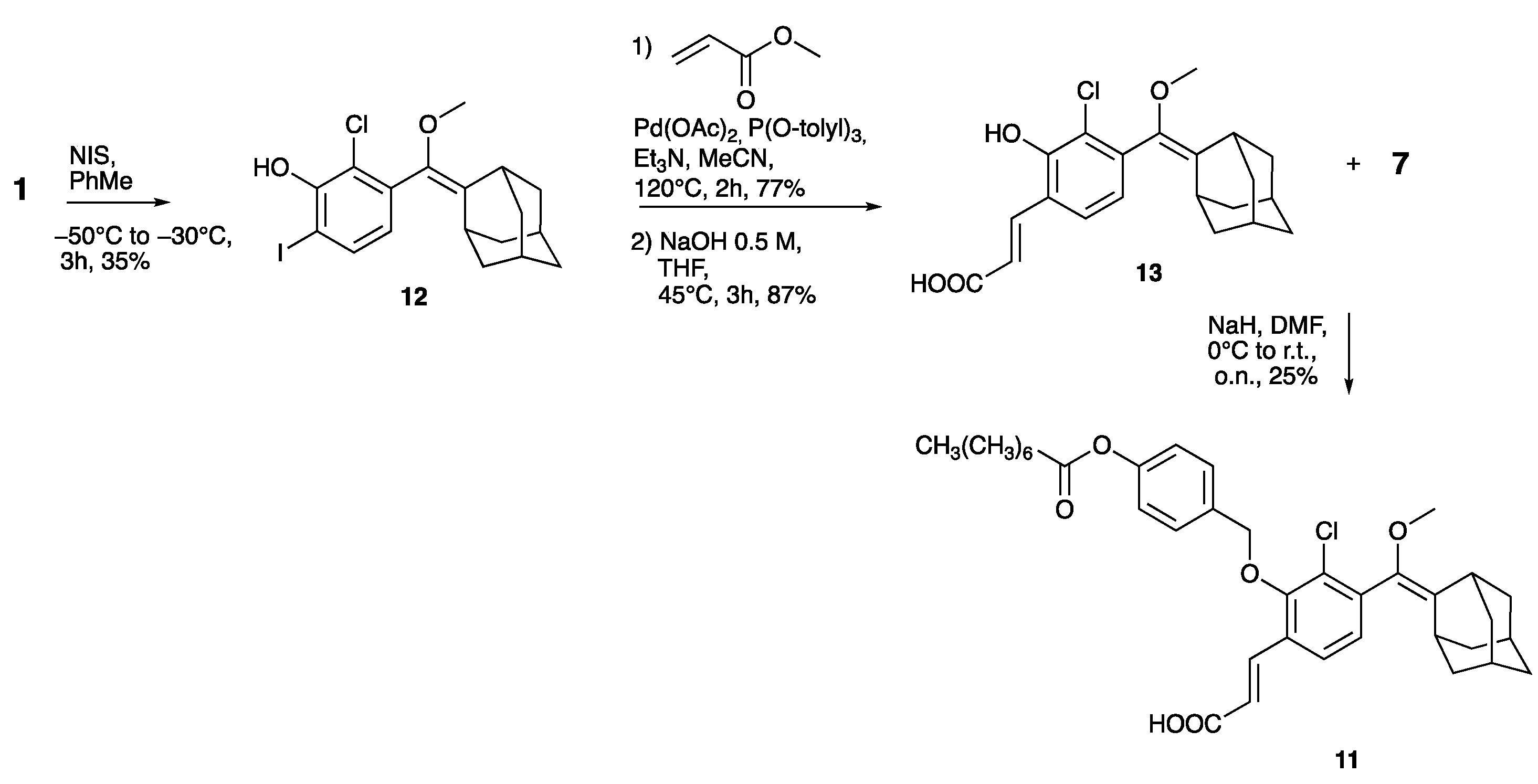
- Compound 12
- Compound 13
- Compound 11
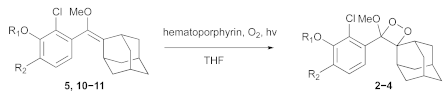
- General Procedure for the Radical Oxidation of Compounds 5, 10, and 11 to Obtain Final Compound 2–4.
- Compound 2
- Compound 3
- Compound 4
3.2. Biochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, S.; Ihssen, J.; Wick, L.; Spitz, U.; Shabat, D. Chemiluminescent Carbapenem-Based Molecular Probe for Detection of Carbapenemase Activity in Live Bacteria. Chem.—A Eur. J. 2020, 26, 3647–3652. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Mao, Y.; Wang, H.; Cao, Z. An activatable chemiluminescence probe based on phenoxy-dioxetane scaffold for biothiol imaging in living systems. J. Pharm. Biomed. Anal. 2021, 204, 114266. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.; Li, Q.; Yue, L.; Liu, Y.-J. Mechanistic Investigation on Chemiluminescent Formaldehyde Probes. Chem.—A Eur. J. 2021, 27, 5712–5720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, C.; Wang, C.; Guo, Z.; Liu, X.; Zhu, W.-H. A Sequential Dual-Lock Strategy for Photoactivatable Chemiluminescent Probes Enabling Bright Duplex Optical Imaging. Angew. Chem. Int. Ed. 2020, 59, 9059–9066. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, Z.; Yuan, Q. Recent advances in autofluorescence-free biosensing and bioimaging based on persistent luminescence nanoparticles. Chin. Chem. Lett. 2019, 30, 1547–1556. [Google Scholar] [CrossRef]
- Yan, Y.; Shi, P.; Song, W.; Bi, S. Chemiluminescence and Bioluminescence Imaging for Biosensing and Therapy: In Vitro and In Vivo Perspectives. Theranostics 2019, 9, 4047–4065. [Google Scholar] [CrossRef]
- Hananya, N.; Shabat, D. A Glowing Trajectory between Bio- and Chemiluminescence: From Luciferin-Based Probes to Triggerable Dioxetanes. Angew. Chem. Int. Ed. 2017, 56, 16454–16463. [Google Scholar] [CrossRef]
- Hananya, N.; Shabat, D. Recent Advances and Challenges in Luminescent Imaging: Bright Outlook for Chemiluminescence of Dioxetanes in Water. ACS Cent. Sci. 2019, 5, 949–959. [Google Scholar] [CrossRef]
- Kim, J.S.; Son, S.B.; Won, M.A.; Sharma, A.; Sessler, J.; Shabat, D. Luminescent Probe Compound for Cancer Cell Detection, and Luminescent Sensor for Cancer Cell Detection Comprising. Same. Patent WO2021125831 A2, 24 June 2021. [Google Scholar]
- Ye, D.; An, R.; Wei, S. Preparation GGT Activated Chemiluminescence Probe and Its. Application. Patent CN112409322 A, 26 February 2021. [Google Scholar]
- An, W.; Ryan, L.S.; Reeves, A.G.; Bruemmer, K.J.; Mouhaffel, L.; Gerberich, J.L.; Winters, A.; Mason, R.P.; Lippert, A.R. A Chemiluminescent Probe for HNO Quantification and Real-Time Monitoring in Living Cells. Angew. Chem. Int. Ed. Engl. 2019, 58, 1361–1365. [Google Scholar] [CrossRef]
- Ryan, L.S.; Lippert, A.R. Ultrasensitive Chemiluminescent Detection of Cathepsin B: Insights into the New Frontier of Chemiluminescent Imaging. Angew. Chem. Int. Ed. 2018, 57, 622–624. [Google Scholar] [CrossRef]
- Ryan, L.S.; Gerberich, J.; Cao, J.; An, W.; Jenkins, B.A.; Mason, R.P.; Lippert, A.R. Kinetics-Based Measurement of Hypoxia in Living Cells and Animals Using an Acetoxymethyl Ester Chemiluminescent Probe. ACS Sens. 2019, 4, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, Z.; Wang, R.; Zhang, S.; Zhang, X. A Highly Sensitive Chemiluminescent Probe for Detecting Nitroreductase and Imaging in Living Animals. Anal. Chem. 2019, 91, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Won, M.; Green, O.; Hananya, N.; Sharma, A.; Jeon, Y.; Kwak, J.H.; Sessler, J.L.; Shabat, D.; Kim, J.S. Chemiluminescent Probe for the In Vitro and In Vivo Imaging of Cancers Over-Expressing NQO1. Angew. Chem. Int. Ed. 2019, 58, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wei, S.; Huang, Z.; Liu, F.; Ye, D. An Activatable Chemiluminescent Probe for Sensitive Detection of γ-Glutamyl Transpeptidase Activity In Vivo. Anal. Chem. 2019, 91, 13639–13646. [Google Scholar] [CrossRef]
- Gutkin, S.; Green, O.; Raviv, G.; Shabat, D.; Portnoy, O. Powerful Chemiluminescence Probe for Rapid Detection of Prostate Specific Antigen Proteolytic Activity: Forensic Identification of Human Semen. Bioconjugate Chem. 2020, 31, 2488–2493. [Google Scholar] [CrossRef]
- Roth-Konforti, M.; Green, O.; Hupfeld, M.; Fieseler, L.; Heinrich, N.; Ihssen, J.; Vorberg, R.; Wick, L.; Spitz, U.; Shabat, D. Ultrasensitive Detection of Salmonella and Listeria monocytogenes by Small-Molecule Chemiluminescence Probes. Angew. Chem. Int. Ed. 2019, 58, 10361–10367. [Google Scholar] [CrossRef]
- Shabat, D.; Roth-Konforti; Eli, M.; Hananya, N.; Green, O.; Spitz, U.; Wick, L.; Ihssen, J.; Vorberg, R.; Cribiu, R.; et al. Dioxetane Compounds and Their Use for the Detection of. Microorganisms. Patent WO2019224338 A1, 28 November 2019. [Google Scholar]
- Shabat, D.; Green, O.; Bogyo, M. Preparation of Chemiluminescence Probes for Detection of Live Mycobacterium. Tuberculosis. Patent WO2021165959 A1, 26 August 2021. [Google Scholar]
- Yang, M.; Huang, J.; Fan, J.; Du, J.; Pu, K.; Peng, X. Chemiluminescence for bioimaging and therapeutics: Recent advances and challenges. Chem. Soc. Rev. 2020, 49, 6800–6815. [Google Scholar] [CrossRef]
- Vanleeuw, E.; Winderickx, S.; Thevissen, K.; Lagrain, B.; Dusselier, M.; Cammue, B.P.A.; Sels, B.F. Substrate-Specificity of Candida rugosa Lipase and Its Industrial Application. ACS Sustainable Chem. Eng. 2019, 7, 15828–15844. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Lipase immobilization on hyroxypropyl methyl cellulose support and its applications for chemo-selective synthesis of β-amino ester compounds. Process Biochem. 2016, 51, 1420–1433. [Google Scholar] [CrossRef]
- Sadaf, A.; Grewal, J.; Jain, I.; Kumari, A.; Khare, S.K. Stability and structure of Penicillium chrysogenum lipase in the presence of organic solvents. Prep. Biochem. Biotechnol. 2018, 48, 977–983. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Schaap, A.P.; Chen, T.-S.; Handley, R.S.; DeSilva, R.; Giri, B.P. Chemical and enzymatic triggering of 1,2-dioxetanes. 2: Fluoride-induced chemiluminescence from tert-butyldimethylsilyloxy-substituted dioxetanes. Tetrahedron Lett. 1987, 28, 1155–1158. [Google Scholar] [CrossRef]
- Schaap, A.P.; Handley, R.S.; Giri, B.P. Chemical and enzymatic triggering of 1,2-dioxetanes. 1: Aryl esterase-catalyzed chemiluminescence from a naphthyl acetate-substituted dioxetane. Tetrahedron Lett. 1987, 28, 935–938. [Google Scholar] [CrossRef]
- Schaap, A.P.; Sandison, M.D.; Handley, R.S. Chemical and enzymatic triggering of 1,2-dioxetanes. 3: Alkaline phosphatase-catalyzed chemiluminescence from an aryl phosphate-substituted dioxetane. Tetrahedron Lett. 1987, 28, 1159–1162. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J. Nanozyme-based luminescence detection. Luminescence 2020, 35, 1185–1194. [Google Scholar] [CrossRef]
- Bronstein, I.; Edwards, B.; Sparks, A. Improved Chemiluminescent 1, 2-Dioxetanes. Patent WO/1994/026726, 24 November 1994. [Google Scholar]
- Ciuffreda, P.; Loseto, A.; Manzocchi, A.; Santaniello, E. Lipolytic activity of porcine pancreas lipase on fatty acid esters of dialkylglycerols: A structural basis for the design of new substrates for the assay of pancreatic lipases activity. Chem. Phys. Lipids 2001, 111, 105–110. [Google Scholar] [CrossRef]
- Ciuffreda, P.; Casati, S.; Loseto, A.; Santaniello, E. Spectrophotometric Assay of Lipase Activity: A New 4-nitrophenyl Ester of a Dialkylglycerol Suitable as a Chromogenic Substrate of Pseudomonas cepacia Lipase. Biocatal. Biotransform. 2003, 21, 123–127. [Google Scholar] [CrossRef]
- Lauria, S.; Casati, S.; Ciuffreda, P. Synthesis and characterization of a new fluorogenic substrate for monoacylglycerol lipase and application to inhibition studies. Anal. Bioanal. Chem. 2015, 407, 8163–8167. [Google Scholar] [CrossRef]
- Miceli, M.; Casati, S.; Ottria, R.; Di Leo, S.; Eberini, I.; Palazzolo, L.; Parravicini, C.; Ciuffreda, P. Set-Up and Validation of a High Throughput Screening Method for Human Monoacylglycerol Lipase (MAGL) Based on a New Red Fluorescent Probe. Molecules 2019, 24, 2241. [Google Scholar] [CrossRef]
- Miceli, M.; Casati, S.; Allevi, P.; Berra, S.; Ottria, R.; Rota, P.; Branchini, B.R.; Ciuffreda, P. A New Ultrasensitive Bioluminescence-Based Method for Assaying Monoacylglycerol Lipase. Int. J. Mol. Sci. 2021, 22, 6148. [Google Scholar] [CrossRef]
- Rota, P.; Papini, N.; La Rocca, P.; Montefiori, M.; Cirillo, F.; Piccoli, M.; Scurati, R.; Olsen, L.; Allevi, P.; Anastasia, L. Synthesis and chemical characterization of several perfluorinated sialic acid glycals and evaluation of their In Vitro antiviral activity against Newcastle disease virus. MedChemComm 2017, 8, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Rota, P.; La Rocca, P.; Piccoli, M.; Montefiori, M.; Cirillo, F.; Olsen, L.; Orioli, M.; Allevi, P.; Anastasia, L. Potent Inhibitors against Newcastle Disease Virus Hemagglutinin-Neuraminidase. ChemMedChem 2018, 13, 236–240. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, P.; Rota, P.; Piccoli, M.; Cirillo, F.; Ghiroldi, A.; Franco, V.; Allevi, P.; Anastasia, L. 2β-3,4-Unsaturated sialic acid derivatives: Synthesis optimization, and biological evaluation as Newcastle disease virus hemagglutinin-neuraminidase inhibitors. Bioorg. Med. Chem. 2020, 28, 115563. [Google Scholar] [CrossRef] [PubMed]
- Eilon-Shaffer, T.; Roth-Konforti, M.; Eldar-Boock, A.; Satchi-Fainaro, R.; Shabat, D. Ortho-Chlorination of phenoxy 1,2-dioxetane yields superior chemiluminescent probes for In Vitro and In Vivo imaging. Org. Biomol. Chem. 2018, 16, 1708–1712. [Google Scholar] [CrossRef]
- Green, O.; Eilon, T.; Hananya, N.; Gutkin, S.; Bauer, C.R.; Shabat, D. Opening a Gateway for Chemiluminescence Cell Imaging: Distinctive Methodology for Design of Bright Chemiluminescent Dioxetane Probes. ACS Cent. Sci. 2017, 3, 349–358. [Google Scholar] [CrossRef]
- Gilham, D.; Lehner, R. Techniques to measure lipase and esterase activity In Vitro. Methods 2005, 36, 139–147. [Google Scholar] [CrossRef]
- Jaeger, K.-E.; Kovacic, F. Determination of Lipolytic Enzyme Activities. In Pseudomonas Methods and Protocols; Filloux, A., Ramos, J.-L., Eds.; Springer: New York, NY, USA, 2014; pp. 111–134. [Google Scholar]
- Reetz, M.T. Biocatalysis in Organic Chemistry and Biotechnology: Past, Present, and Future. J. Am. Chem. Soc. 2013, 135, 12480–12496. [Google Scholar] [CrossRef]
- Mendes, A.A.; Oliveira, P.C.; de Castro, H.F. Properties and biotechnological applications of porcine pancreatic lipase. J. Mol. Catal. B: Enzym. 2012, 78, 119–134. [Google Scholar] [CrossRef]
- Koo, J.-Y.; Schuster, G.B. Chemically initiated electron exchange luminescence. A new chemiluminescent reaction path for organic peroxides. J. Am. Chem. Soc. 1977, 99, 6107–6109. [Google Scholar] [CrossRef]
- Koo, J.-Y.; Schuster, G.B. Chemiluminescence of diphenoyl peroxide. Chemically initiated electron exchange luminescence. A new general mechanism for chemical production of electronically excited states. J. Am. Chem. Soc. 1978, 100, 4496–4503. [Google Scholar] [CrossRef]
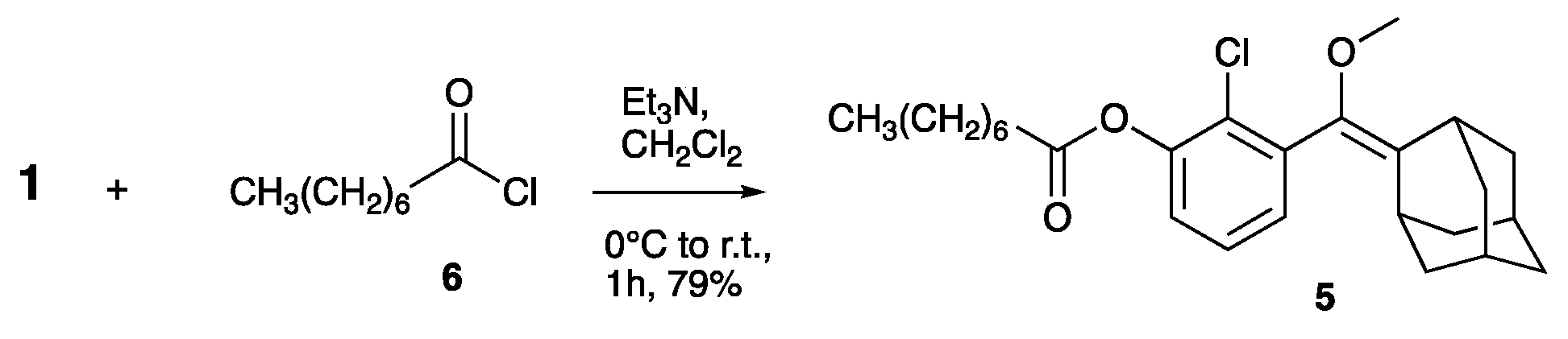

| Entry | Reagent | Product | Time (h) | Yield (%) a |
|---|---|---|---|---|
| 1 | 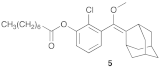 | 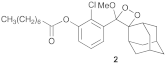 | 1.5 | 67 |
| 2 | 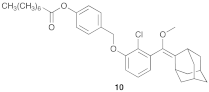 | 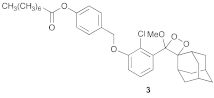 | 1.5 | 74 |
| 3 | 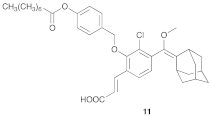 | 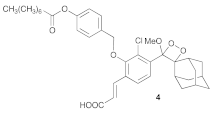 | 3.5 | 52 (79%) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Rocca, P.; Mingione, A.; Casati, S.; Ottria, R.; Allevi, P.; Ciuffreda, P.; Rota, P. Small-Molecules as Chemiluminescent Probes to Detect Lipase Activity. Int. J. Mol. Sci. 2022, 23, 9039. https://doi.org/10.3390/ijms23169039
La Rocca P, Mingione A, Casati S, Ottria R, Allevi P, Ciuffreda P, Rota P. Small-Molecules as Chemiluminescent Probes to Detect Lipase Activity. International Journal of Molecular Sciences. 2022; 23(16):9039. https://doi.org/10.3390/ijms23169039
Chicago/Turabian StyleLa Rocca, Paolo, Alessandra Mingione, Silvana Casati, Roberta Ottria, Pietro Allevi, Pierangela Ciuffreda, and Paola Rota. 2022. "Small-Molecules as Chemiluminescent Probes to Detect Lipase Activity" International Journal of Molecular Sciences 23, no. 16: 9039. https://doi.org/10.3390/ijms23169039
APA StyleLa Rocca, P., Mingione, A., Casati, S., Ottria, R., Allevi, P., Ciuffreda, P., & Rota, P. (2022). Small-Molecules as Chemiluminescent Probes to Detect Lipase Activity. International Journal of Molecular Sciences, 23(16), 9039. https://doi.org/10.3390/ijms23169039








