The Joint Influence of Tl+ and Thiol-Modifying Agents on Rat Liver Mitochondrial Parameters In Vitro
Abstract
1. Introduction
2. Results
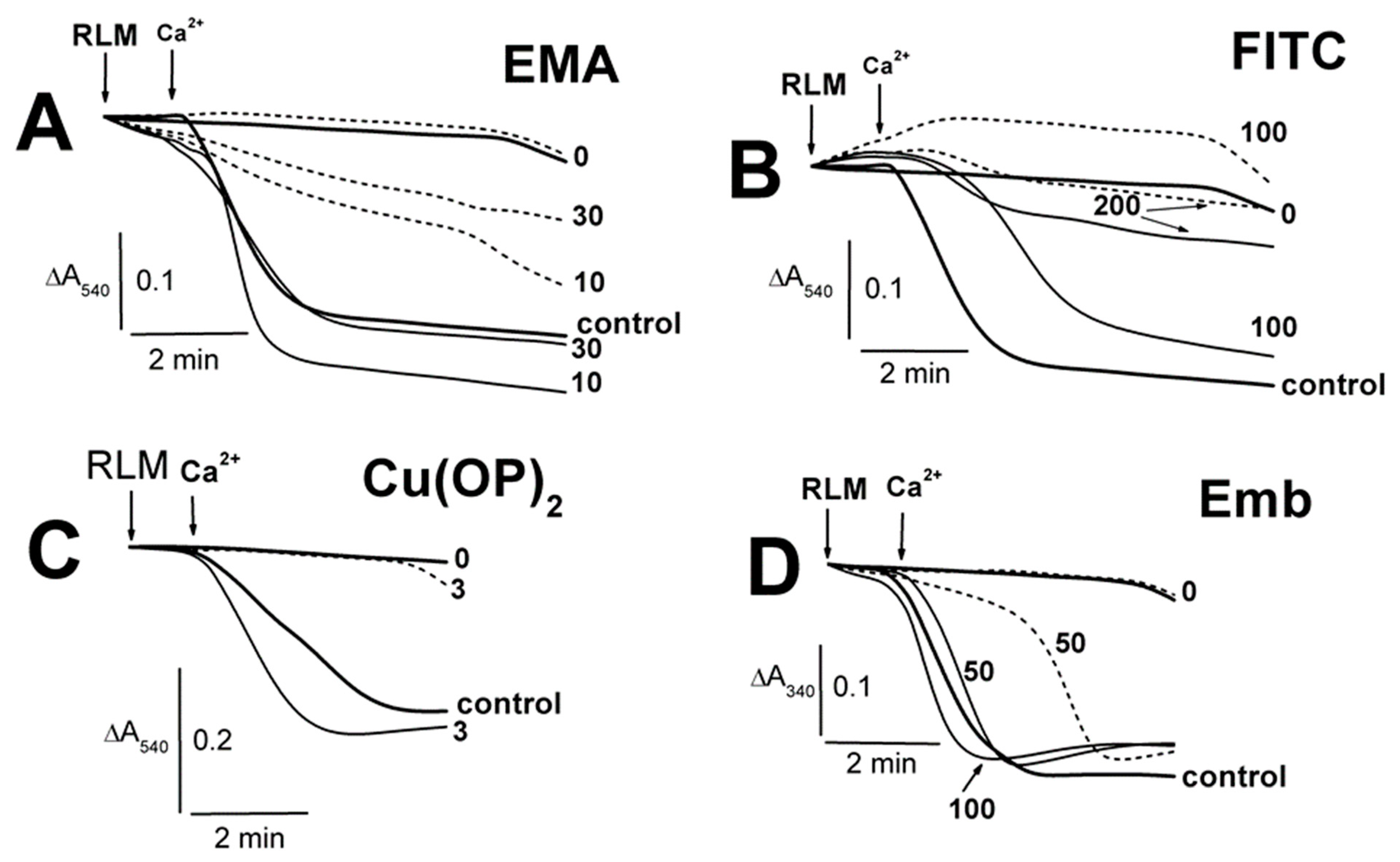
2.1. Effects of Tl+ and Thiol-Modifying Agents on the Swelling of Succinate-Energized Rat Liver Mitochondria
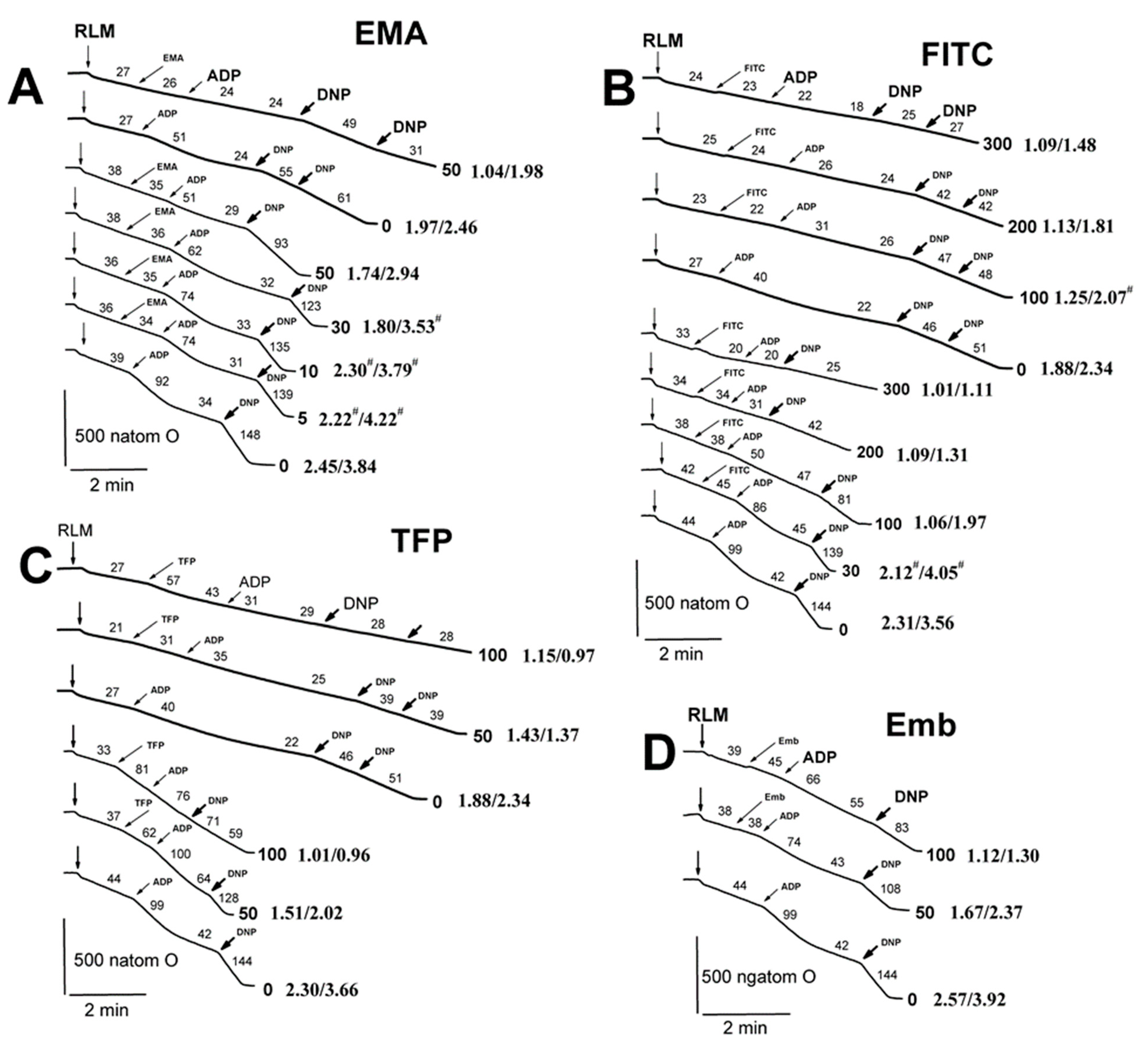
2.2. Effects of Tl+ and Thiol-Modifying Agents on Respiration and ΔΨmito of Succinate-Energized Rat Liver Mitochondria
2.3. The Joint Effects of Tl+ and Thiol-Modifying Agents on the SH Group Content in Rat Liver Mitochondria
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics
4.2. Chemicals
4.3. Mitochondrial Isolation
4.4. Swelling of Mitochondria
4.5. Oxygen Consumption Assay
4.6. Mitochondrial Membrane Potential
4.7. Determination of Protein Thiol Content
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3UDNP respiration | 2,4-uncoupled respiration |
| 3UFCCP respiration | Carbonyl cyanide-p-trifluoromethoxy phenylhydrazone-uncoupled respiration |
| ΔΨmito | Inner mitochondrial membrane potential |
| ANT | Adenine nucleotide translocase |
| BHM | Bovine heart mitochondria |
| CAT | Carboxyatractyloside |
| CsA | Cyclosporine A |
| Cu(OP)2 | Cu(o-phenanthroline)2 |
| CyP-D | Cyclophilin D |
| Diam | Diamide |
| DIDS | 4,4′-diisothiocyanostilbene-2,2′-disulfonate |
| DNP | 2,4-dinitrophenol |
| DTNB | 5,5-dithio-bis-nitrobenzoic acid |
| DTT | Dithiothreitol |
| EDTA | Ethylenediaminetetraacetic acid |
| EGTA | Ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid |
| EMA | Eosin-5-maleimide |
| Emb | 2,5-dihydroxy-3-undecyl-1,4-benzoquinone-embeline |
| FITC | Fluorescein isothiocyanate |
| IMM | Inner mitochondrial membrane |
| MPTP | Mitochondrial permeability transition pore |
| MSL | Mersalyl |
| NEM | n-ethylmaleimide |
| PAO | Phenylarsine oxide |
| PiC | Mitochondrial phosphate carrier |
| Pi | Inorganic phosphate |
| RBM | Rat brain mitochondria |
| RCR | Respiratory control ratio |
| RHM | Rat heart mitochondria |
| RKM | Rat kidney mitochondria |
| RLM | Reactive oxygen species |
| ROS | Rat liver mitochondria |
| SDS | Sodium dodecyl sulfate |
| SMP | Submitochondrial particle |
| tBHP | Tert-butyl hydroperoxide |
| TFP | Trifluoperazine |
| TMA | Thiol-modifying agent |
| VDAC | Voltage-dependent anion channel |
References
- Haworth, R.A.; Hunter, D.R. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 1979, 195, 460–467. [Google Scholar] [CrossRef]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Brenner, C. The adenine nucleotide translocase: A central component of the mitochondrial permeability transition pore and key player in cell death. Curr. Med. Chem. 2003, 10, 1507–1525. [Google Scholar] [CrossRef]
- Brustovetsky, N. The Role of Adenine Nucleotide Translocase in the Mitochondrial Permeability Transition. Cells. 2020, 9, 2686. [Google Scholar] [CrossRef]
- Giorgio, V.; Guo, L.; Bassot, C.; Petronilli, V.; Bernardi, P. Calcium and regulation of the mitochondrial permeability transition. Cell Calcium. 2018, 70, 56–63. [Google Scholar] [CrossRef]
- Halestrap, A.P. The c ring of the F1Fo ATP synthase forms the mitochondrial permeability transition pore: A critical appraisal. Front. Oncol. 2014, 4, 234. [Google Scholar] [CrossRef][Green Version]
- Richardson, A.P.; Halestrap, A.P. Quantification of active mitochondrial permeability transition pores using GNX-4975 inhibitor titrations provides insights into molecular identity. Biochem. J. 2016, 473, 1129–1140. [Google Scholar] [CrossRef]
- Mnatsakanyan, N.; Jonas, E.A. ATP synthase c-subunit ring as the channel of mitochondrial permeability transition: Regulator of metabolism in development and degeneration. J. Mol. Cell. Cardiol. 2020, 144, 109–118. [Google Scholar] [CrossRef]
- Nesci, S.; Trombetti, F.; Ventrella, V.; Pagliarani, A. From the Ca2+-activated F1FO-ATPase to the mitochondrial permeability transition pore: An overview. Biochimie 2018, 152, 85–93. [Google Scholar] [CrossRef]
- Morciano, G.; Naumova, N.; Koprowski, P.; Valente, S.; Sardão, V.A.; Potes, Y.; Rimessi, A.; Wieckowski, M.R.; Oliveira, P.J. The mitochondrial permeability transition pore: An evolving concept critical for cell life and death. Biol. Rev. Camb. Philos. Soc. 2021, 96, 2489–2521. [Google Scholar] [CrossRef]
- Carraro, M.; Checchetto, V.; Szabó, I.; Bernardi, P. F-ATP synthase and the permeability transition pore: Fewer doubts, more certainties. FEBS Lett. 2019, 593, 1542–1553. [Google Scholar] [CrossRef]
- Beutner, G.; Alavian, K.N.; Jonas, E.A.; Porter, G.A., Jr. The Mitochondrial Permeability Transition Pore and ATP Synthase. Handb. Exp. Pharmacol. 2017, 240, 21–46. [Google Scholar] [CrossRef]
- Bernardi, P.; Carraro, M.; Lippe, G. The mitochondrial permeability transition: Recent progress and open questions. FEBS Lett. 2021. [Google Scholar] [CrossRef]
- Ichas, F.; Mazat, J.P. From calcium signaling to cell death: Two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim. Biophys. Acta. 1998, 1366, 33–50. [Google Scholar] [CrossRef]
- Bround, M.J.; Bers, D.M.; Molkentin, J.D. A 20/20 view of ANT function in mitochondrial biology and necrotic cell death. J. Mol. Cell. Cardiol. 2020, 144, A3–A13. [Google Scholar] [CrossRef]
- Bernardi, P.; Veronese, P.; Petronilli, V. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. I. Evidence for two separate Me2+ binding sites with opposing effects on the pore open probability. J. Biol. Chem. 1993, 268, 1005–1010. [Google Scholar] [CrossRef]
- Chávez, E.; Zazueta, C.; García, N.; Martínez-Abundis, E.; Pavón, N.; Hernández-Esquivel, L. Titration of cardiolipin by either 10-N-nonyl acridine orange or acridine orange sensitizes the adenine nucleotide carrier to permeability transition. J Bioenerg Biomembr. 2008, 40, 77–84. [Google Scholar] [CrossRef]
- García, N.; Pavón, N.; Chávez, E. The effect of N-ethylmaleimide on permeability transition as induced by carboxyatractyloside, agaric acid, and oleate. Cell. Biochem. Biophys. 2008, 51, 81–87. [Google Scholar] [CrossRef]
- Chávez, E.; Buelna-Chontal, M.; Macías-López, A.; Hernández-Esquivel, L.; Correa, F.; Pavón, N. Interaction of agaric acid with the adenine nucleotide translocase induces mitochondrial oxidative stress. Biochem. Res. Int. 2020, 2020, 5253108. [Google Scholar] [CrossRef]
- García, N.; Zazueta, C.; Pavón, N.; Chávez, E. Agaric acid induces mitochondrial permeability transition through its interaction with the adenine nucleotide translocase. Its dependence on membrane fluidity. Mitochondrion 2005, 5, 272–281. [Google Scholar] [CrossRef]
- García, N.; Hernández-Esquivel, L.; Zazueta, C.; Martínez-Abundis, E.; Pavón, N.; Chávez, E. Induction of mitochondrial permeability transition by the DNA-intercalating cationic dye ethidium bromide. J. Biochem. 2009, 146, 887–894. [Google Scholar] [CrossRef]
- Korotkov, S.M.; Saris, N.E. Influence of Tl+ on mitochondrial permeability transition pore in Ca2+-loaded rat liver mitochondria. J. Bioenerg. Biomembr. 2011, 43, 149–162. [Google Scholar] [CrossRef]
- Korotkov, S.M.; Brailovskaya, I.V.; Kormilitsyn, B.N.; Furaev, V.V. Tl+ showed negligible interaction with inner membrane sulfhydryl groups of rat liver mitochondria, but formed complexes with matrix proteins. J. Biochem. Mol. Toxicol. 2014, 28, 149–156. [Google Scholar] [CrossRef]
- Korotkov, S.M.; Konovalova, S.A.; Brailovskaya, I.V.; Saris, N.E. To involvement the conformation of the adenine nucleotide translocase in opening the Tl+-induced permeability transition pore in Ca2+-loaded rat liver mitochondria. Toxicol. In Vitro 2016, 32, 320–332. [Google Scholar] [CrossRef]
- Korotkov, S.M.; Konovalova, S.A.; Brailovskaya, I.V. Diamide accelerates opening of the Tl+-induced permeability transition pore in Ca2+-loaded rat liver mitochondria. Biochem. Biophys. Res. Commun. 2015, 468, 360–364. [Google Scholar] [CrossRef]
- Korotkov, S.M.; Konovalova, S.A.; Nesterov, V.P.; Brailovskaya, I.V. Mersalyl prevents the Tl+-induced permeability transition pore opening in the inner membrane of Ca2+-loaded rat liver mitochondria. Biochem. Biophys. Res. Commun. 2018, 495, 1716–1721. [Google Scholar] [CrossRef]
- McStay, G.P.; Clarke, S.J.; Halestrap, A.P. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem. J. 2002, 367 Pt 2, 541–548. [Google Scholar] [CrossRef]
- Majima, E.; Koike, H.; Hong, Y.M.; Shinohara, Y.; Terada, H. Characterization of cysteine residues of mitochondrial ADP/ATP carrier with the SH-reagents eosin 5-maleimide and N-ethylmaleimide. J. Biol. Chem. 1993, 268, 22181–22187. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Woodfield, K.Y.; Connern, C.P. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J. Biol. Chem. 1997, 272, 3346–3354. [Google Scholar] [CrossRef]
- García, N.; Zazueta, C.; Carrillo, R.; Correa, F.; Chávez, E. Copper sensitizes the mitochondrial permeability transition to carboxytractyloside and oleate. Mol. Cell. Biochem. 2000, 209, 119–123. [Google Scholar] [CrossRef]
- Houstĕk, J.; Pedersen, P.L. Adenine nucleotide and phosphate transport systems of mitochondria. Relative location of sulfhydryl groups based on the use of the novel fluorescent probe eosin-5-maleimide. J. Biol. Chem. 1985, 260, 6288–6295. [Google Scholar] [CrossRef]
- Majima, E.; Ishida, M.; Miki, S.; Shinohara, Y.; Terada, H. Specific labeling of the bovine heart mitochondrial phosphate carrier with fluorescein 5-isothiocyanate: Roles of Lys185 and putative adenine nucleotide recognition site in phosphate transport. J. Biol. Chem. 2001, 276, 9792–9799. [Google Scholar] [CrossRef]
- Kozlova, M.V.; Gramadskii, K.B.; Solodovnikova, I.M.; Krasinskaya, I.P.; Vinogradov, A.V.; Yaguzhinskii, L.S. Detection and functional role of local H+ activity gradients on the inner mitochondrial membrane labeled with a covalently bound pH probe. Biophysics 2003, 48, 417–426. [Google Scholar]
- Krasinskaya, I.P.; Korshunov, S.S.; OYu, K.; Yaguzhinsky, L.S. The immobilized matrix buffer controls the rate of mitochondrial respiration in state 3P according to chance. Biochemistry 1997, 62, 364–370. [Google Scholar]
- Joy, B.; Sivadasan, R.; Abraham, T.E.; John, M.; Sobhan, P.K.; Seervi, M. Lysosomal destabilization and cathepsin B contributes for cytochrome c release and caspase activation in embelin-induced apoptosis. Mol. Carcinog. 2010, 49, 324–336. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Yuan, S.; Wang, Z.; Tang, F.; Nie, R.; Weng, J.; Ma, L.; Tang, B. Embelin-induced MCF-7 breast cancer cell apoptosis and blockade of MCF-7 cells in the G2/M phase via the mitochondrial pathway. Oncol. Lett. 2013, 5, 1005–1009. [Google Scholar] [CrossRef]
- Sumalatha, K.; Gowda, M.; Meenakshisundaram, S. ROS-mediated induction of apoptosis by benzoquinone embelin in human colon adenocarcinoma cells HT-29. J. Complement. Integr. Med. 2017, 14, j-jcim. [Google Scholar] [CrossRef]
- Petronilli, V.; Sileikyte, J.; Zulian, A.; Dabbeni-Sala, F.; Jori, G.; Gobbo, S.; Tognon, G.; Nikolov, P.; Bernardi, P.; Ricchelli, F. Switch from inhibition to activation of the mitochondrial permeability transition during hematoporphyrin-mediated photooxidative stress. Unmasking pore-regulating external thiols. Biochim Biophys Acta 2009, 1787, 897–904. [Google Scholar] [CrossRef]
- Ricchelli, F.; Sileikytė, J.; Bernardi, P. Shedding light on the mitochondrial permeability transition. Biochim. Biophys. Acta 2011, 1807, 482–490. [Google Scholar] [CrossRef]
- Costantini, P.; Colonna, R.; Bernardi, P. Induction of the mitochondrial permeability transition by N-ethylmaleimide depends on secondary oxidation of critical thiol groups. Potentiation by copper-ortho-phenanthroline without dimerization of the adenine nucleotide translocase. Biochim. Biophys. Acta 1998, 1365, 385–392. [Google Scholar] [CrossRef]
- Zazueta, C.; Reyes-Vivas, H.; Zafra, G.; Sánchez, C.A.; Vera, G.; Chávez, E. Mitochondrial permeability transition as induced by cross-linking of the adenine nucleotide translocase. Int. J. Biochem. Cell Biol. 1998, 30, 517–527. [Google Scholar] [CrossRef]
- Šileikytè, J.; Petronilli, V.; Zulian, A.; Dabbeni-Sala, F.; Tognon, G.; Nikolov, P.; Bernardi, P.; Ricchelli, F. Regulation of the inner membrane mitochondrial permeability transition by the outer membrane translocator protein (peripheral benzodiazepine receptor). J. Biol. Chem. 2011, 286, 1046–1053. [Google Scholar] [CrossRef]
- Shinohara, Y.; Bandou, S.; Kora, S.; Kitamura, S.; Inazumi, S.; Terada, H. Cationic uncouplers of oxidative phosphorylation are inducers of mitochondrial permeability transition. FEBS Lett. 1998, 428, 89–92. [Google Scholar] [CrossRef]
- Majima, E.; Ikawa, K.; Takeda, M.; Hashimoto, M.; Shinohara, Y.; Terada, H. Translocation of loops regulates transport activity of mitochondrial ADP/ATP carrier deduced from formation of a specific intermolecular disulfide bridge catalyzed by copper-o-phenanthroline. J. Biol. Chem. 1995, 270, 387–391. [Google Scholar] [CrossRef]
- Shinohara, Y.; Terada, H. Possible involvement of the 29 kDa protein in H+-ATPase in the action of cationic uncoupler of oxidative phosphorylation. Effect of the (o-phenanthroline)2-Cu2+ complex as a cationic uncoupler. Biochim. Biophys. Acta 1987, 890, 387–391. [Google Scholar] [CrossRef]
- Walter, L.; Nogueira, V.; Leverve, X.; Heitz, M.P.; Bernardi, P.; Fontaine, E. Three classes of ubiquinone analogs regulate the mitochondrial permeability transition pore through a common site. J. Biol. Chem. 2000, 275, 29521–29527. [Google Scholar] [CrossRef]
- Nantes, I.L.; Rodrigues, T.; Caires, A.C.; Cunha, R.L.; Pessoto, F.S.; Yokomizo, C.H.; Araujo-Chaves, J.C.; Faria, P.A.; Santana, D.P.; dos Santos, C.G. Specific effects of reactive thiol drugs on mitochondrial bioenergetics. J. Bioenerg. Biomembr. 2011, 43, 11–18. [Google Scholar] [CrossRef]
- Müller, M.; Krebs, J.J.; Cherry, R.J.; Kawato, S. Rotational diffusion of the ADP/ATP translocator in the inner membrane of mitochondria and in proteoliposomes. J. Biol. Chem. 1984, 259, 3037–3043. [Google Scholar] [CrossRef]
- Pavón, N.; Correa, F.; Buelna-Chontal, M.; Hernández-Esquivel, L.; Chávez, E. Ebselen induces mitochondrial permeability transition because of its interaction with adenine nucleotide translocase. Life Sci. 2015, 139, 108–113. [Google Scholar] [CrossRef]
- González-González, J.; Nájera-Lara, M.; López-Ramírez, V.; Ramírez-Vázquez, J.A.; Segoviano-Garfias, J.J.N. Spectrophotometric determination of the formation constants of calcium(II) complexes with 2,2′-bipyridyl and 1,10-phenanthroline in acetonitrile. Resour.-Effic. Technol. 2016, 2, 240–246. [Google Scholar] [CrossRef]
- Banks, C.V.; Bystroff, R.I. Stability orders in transition metal-1,l o-phenanthroline complexes. J. Am. Chem. Soc. 1959, 81, 6153–6158. [Google Scholar] [CrossRef]
- Korotkov, S.M. Effects of Tl+ on ion permeability, membrane potential and respiration of isolated rat liver mitochondria. J. Bioenerg. Biomembr. 2009, 41, 277–287. [Google Scholar] [CrossRef]
- Korotkov, S.M.; Emel’yanova, L.V.; Yagodina, O.V. Inorganic Phosphate Stimulates the Toxic Effects of Tl+ in Rat Liver Mitochondria. J. Biochem. Mol. Toxicol. 2008, 22, 148–157. [Google Scholar] [CrossRef]
- Korotkov, S.M.; Skulskii, I.A.; Glazunov, V.V. Cd2+ effects on respiration and swelling of rat liver mitochondria were modified by monovalent cations. J. Inorg. Biochem. 1998, 70, 17–23. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; Vercesi, A.E.; Castilho, R.F. Mitochondrial membrane protein thiol reactivity with N-ethylmaleimide or mersalyl is modified by Ca2+: Correlation with mitochondrial permeability transition. Biochim. Biophys. Acta 1997, 1318, 395–402. [Google Scholar] [CrossRef]
- Correa, F.; Pavón, N.; Buelna-Chontal, M.; Chiquete-Félix, N.; Hernández-Esquivel, L.; Chávez, E. Calcium Induces Mitochondrial Oxidative Stress Because of its Binding to Adenine Nucleotide Translocase. Cell Biochem. Biophys. 2018, 76, 445–450. [Google Scholar] [CrossRef]
- Majima, E.; Shinohara, Y.; Yamaguchi, N.; Hong, Y.M.; Terada, H. Importance of loops of mitochondrial ADP/ATP carrier for its transport activity deduced from reactivities of its cysteine residues with the sulfhydryl reagent eosin-5-maleimide. Biochemistry 1994, 33, 9530–9536. [Google Scholar] [CrossRef]
- Pereira, R.S.; Bertocchi, A.P.; Vercesi, A.E. Protective effect of trifluoperazine on the mitochondrial damage induced by Ca2+ plus prooxidants. Biochem. Pharmacol. 1992, 44, 1795–1801. [Google Scholar] [CrossRef]
- Avisetti, D.R.; Amireddy, N.; Kalivendi, S.V. The mitochondrial effects of embelin are independent of its MAP kinase regulation: Role of p53 in conferring selectivity towards cancer cells. Mitochondrion 2019, 46, 158–163. [Google Scholar] [CrossRef]
- Kanno, T.; Sato, E.E.; Muranaka, S.; Fujita, H.; Fujiwara, T.; Utsumi, T.; Inoue, M.; Utsumi, K. Oxidative stress underlies the mechanism for Ca2+-induced permeability transition of mitochondria. Free Radic. Res. 2004, 38, 27–35. [Google Scholar] [CrossRef]
- Cruz, T.S.; Faria, P.A.; Santana, D.P.; Ferreira, J.C.; Oliveira, V.; Nascimento, O.R.; Cerchiaro, G.; Curti, C.; Nantes, I.L.; Rodrigues, T. On the mechanisms of phenothiazine-induced mitochondrial permeability transition: Thiol oxidation, strict Ca2+ dependence, and cyt c release. Biochem. Pharmacol. 2010, 80, 1284–1295. [Google Scholar] [CrossRef]
- Rodrigues, T.; Santos, A.C.; Pigoso, A.A.; Mingatto, F.E.; Uyemura, S.A.; Curti, C. Thioridazine interacts with the membrane of mitochondria acquiring antioxidant activity toward apoptosis--potentially implicated mechanisms. Br. J. Pharmacol. 2002, 136, 136–142. [Google Scholar] [CrossRef]
- Broekemeier, K.M.; Pfeiffer, D.R. Inhibition of the mitochondrial permeability transition by cyclosporin A during long time frame experiments: Relationship between pore opening and the activity of mitochondrial phospholipases. Biochemistry 1995, 34, 16440–16449. [Google Scholar] [CrossRef]
- Korotkov, S.M. Effects of Tl+ on the inner membrane thiol groups, respiration, and swelling in succinate-energized rat liver mitochondria were modified by thiol reagents. Biometals 2021, 34, 987–1006. [Google Scholar] [CrossRef]
- Korotkov, S.M. Mitochondria as a Key Intracellular Target of Thallium Toxicity; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–261. Available online: https://www.elsevier.com/books/mitochondria-as-a-key-intracellular-target-of-thallium-toxicity/korotkov/978-0-323-95531-7 (accessed on 1 June 2022).
- Korotkov, S.M.; Brailovskaya, I.V.; Shumakov, A.R.; Emelyanova, L.V. Closure of mitochondrial potassium channels favors opening of the Tl+-induced permeability transition pore in Ca2+-loaded rat liver mitochondria. J. Bioenerg. Biomembr. 2015, 47, 243–254. [Google Scholar] [CrossRef]
- Waldmeier, P.C.; Feldtrauer, J.J.; Qian, T.; Lemasters, J.J. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporine derivative NIM811. Mol. Pharmacol. 2002, 62, 22–29. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korotkov, S.M.; Novozhilov, A.V. The Joint Influence of Tl+ and Thiol-Modifying Agents on Rat Liver Mitochondrial Parameters In Vitro. Int. J. Mol. Sci. 2022, 23, 8964. https://doi.org/10.3390/ijms23168964
Korotkov SM, Novozhilov AV. The Joint Influence of Tl+ and Thiol-Modifying Agents on Rat Liver Mitochondrial Parameters In Vitro. International Journal of Molecular Sciences. 2022; 23(16):8964. https://doi.org/10.3390/ijms23168964
Chicago/Turabian StyleKorotkov, Sergey M., and Artemy V. Novozhilov. 2022. "The Joint Influence of Tl+ and Thiol-Modifying Agents on Rat Liver Mitochondrial Parameters In Vitro" International Journal of Molecular Sciences 23, no. 16: 8964. https://doi.org/10.3390/ijms23168964
APA StyleKorotkov, S. M., & Novozhilov, A. V. (2022). The Joint Influence of Tl+ and Thiol-Modifying Agents on Rat Liver Mitochondrial Parameters In Vitro. International Journal of Molecular Sciences, 23(16), 8964. https://doi.org/10.3390/ijms23168964





