Immunofluorescent Evidence for Nuclear Localization of Aromatase in Astrocytes in the Rat Central Nervous System
Abstract
1. Introduction
2. Results
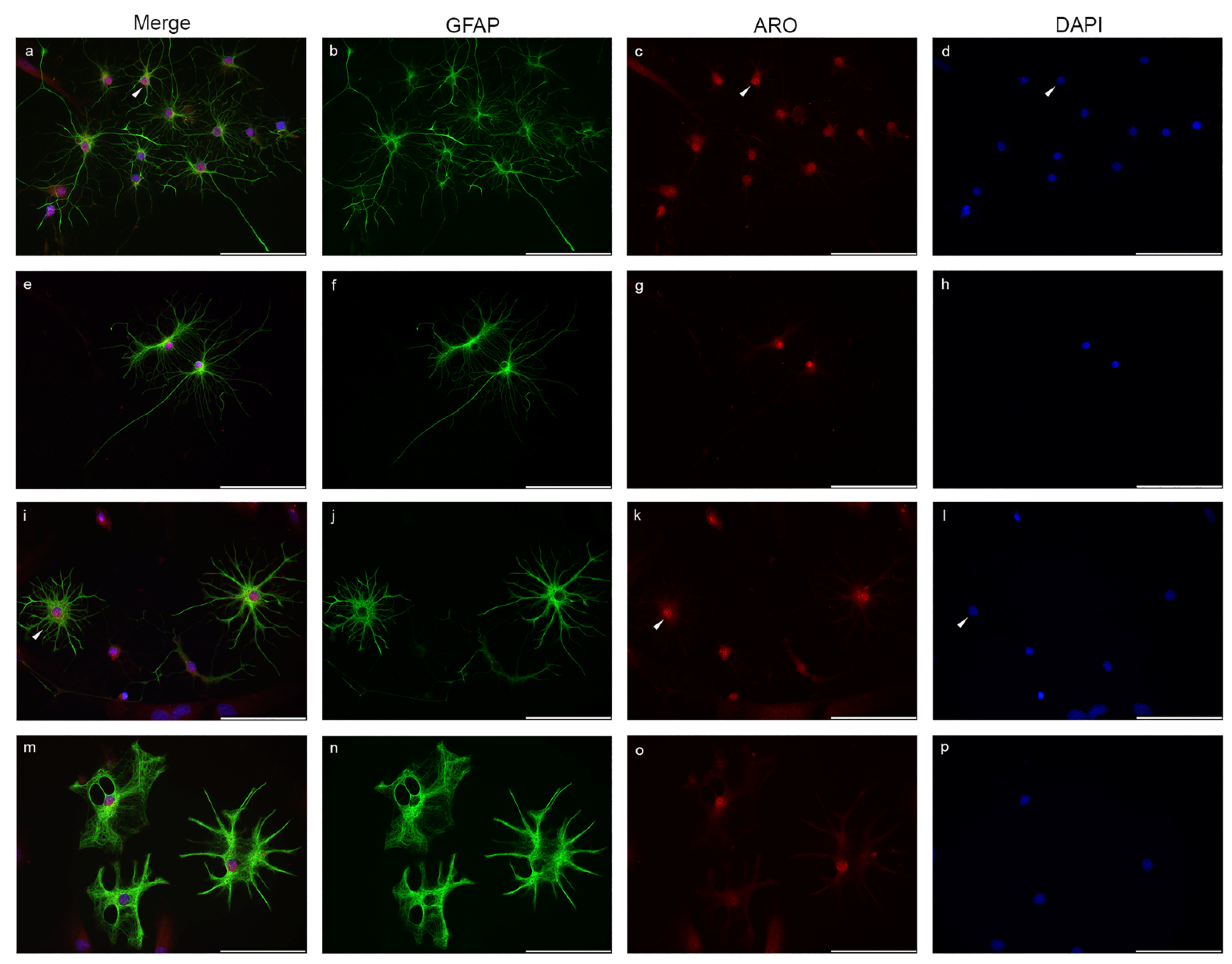
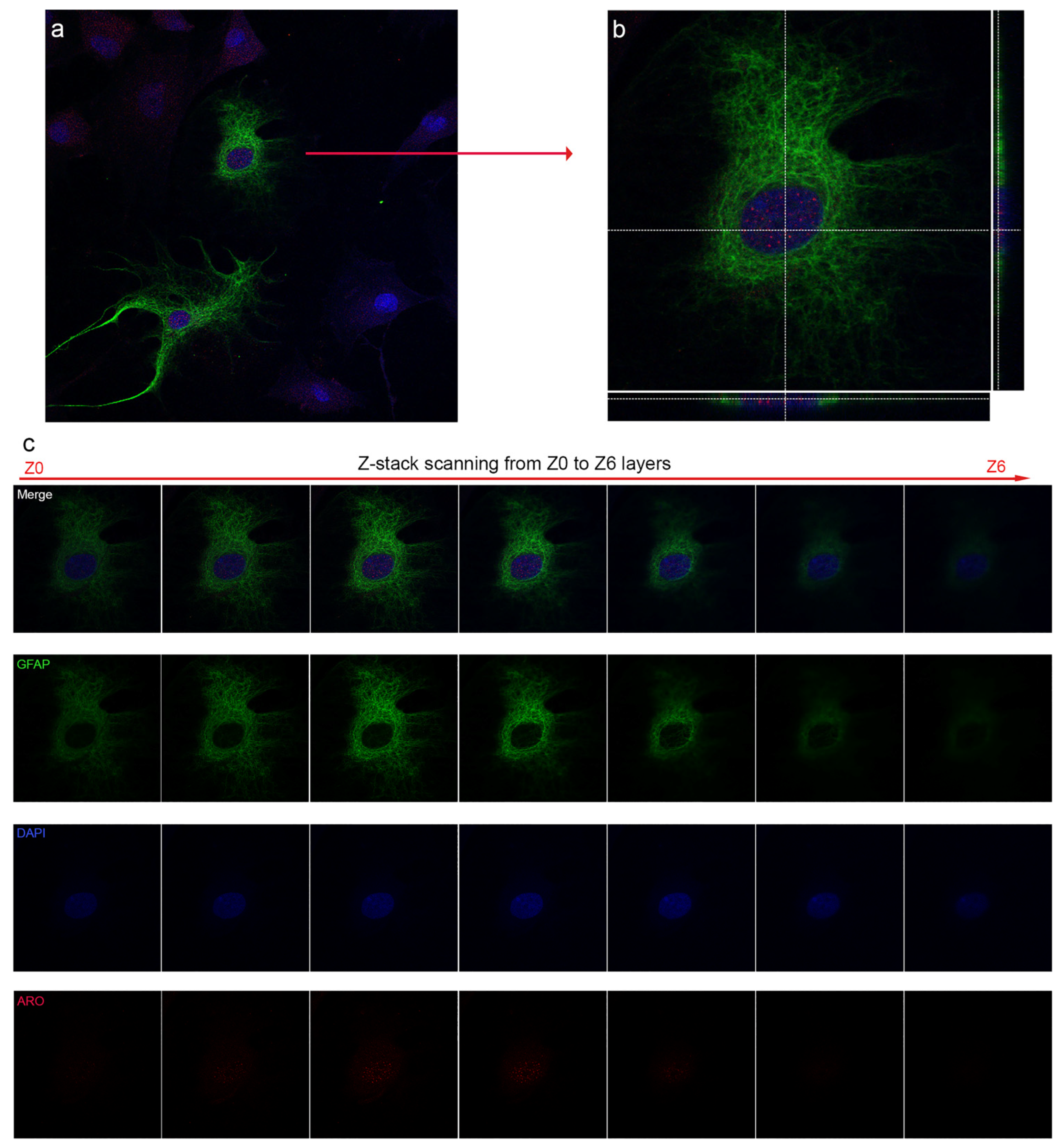
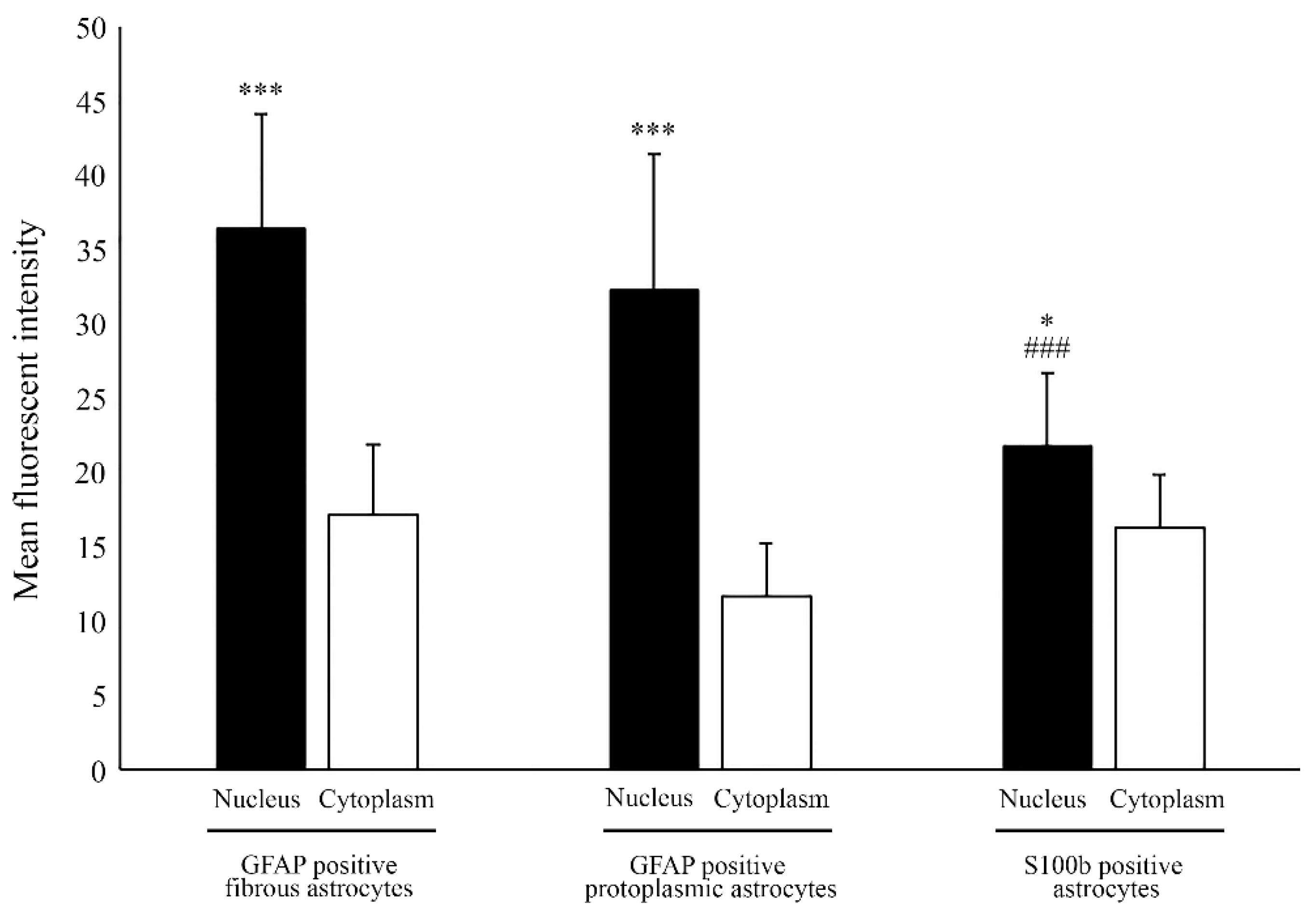
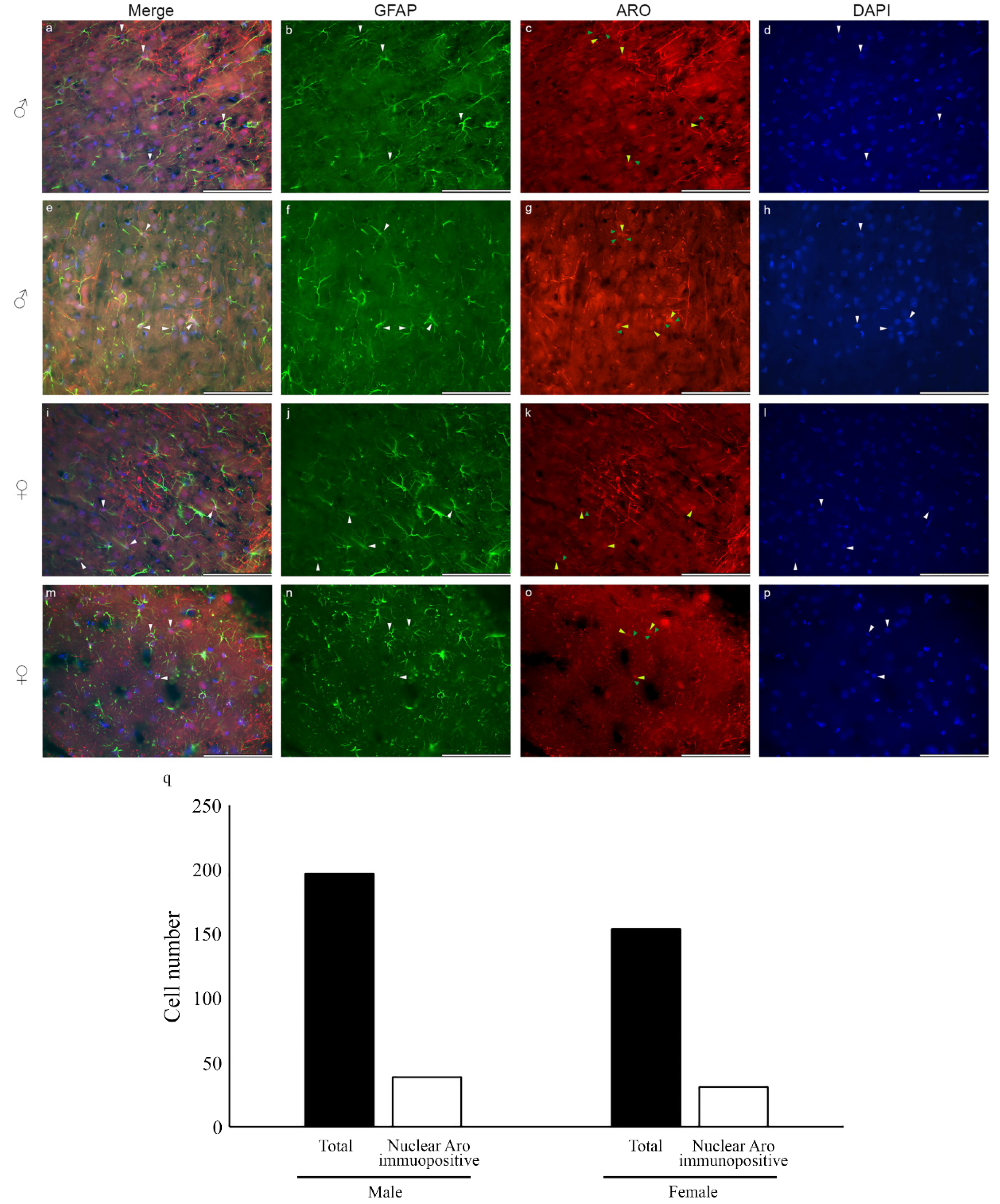
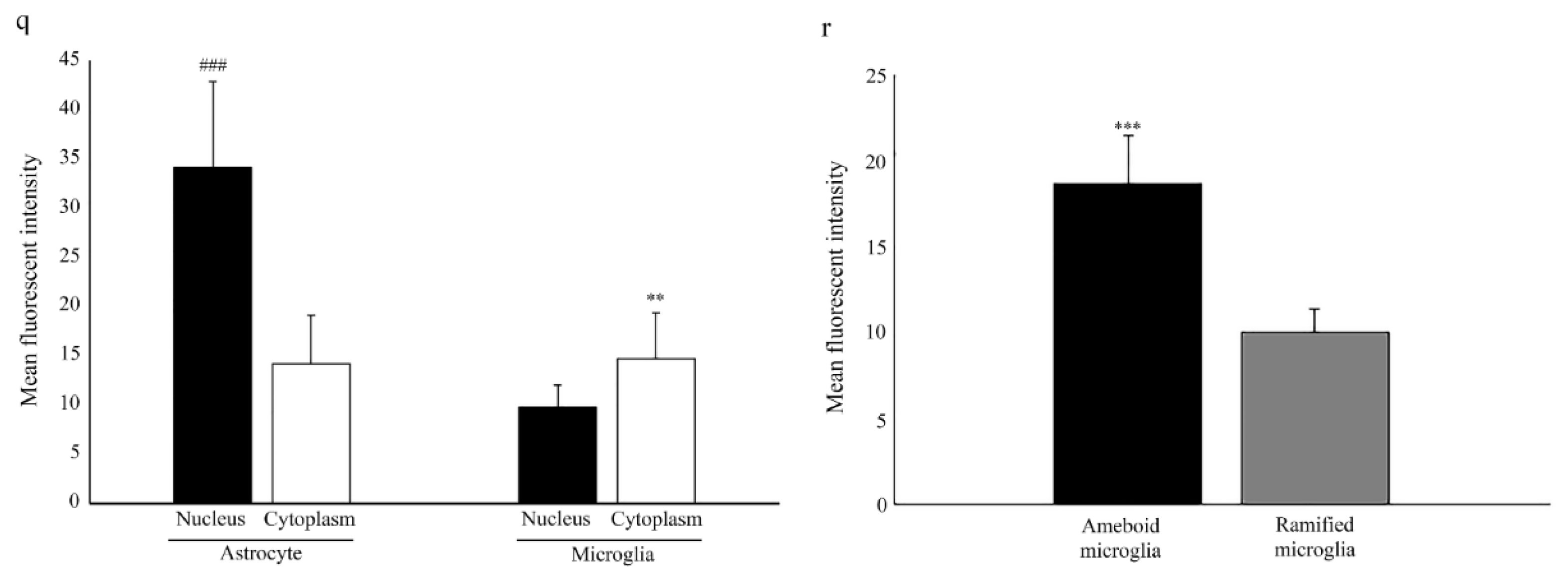
2.1. Nuclear Localization of Aromatase in Astrocytes
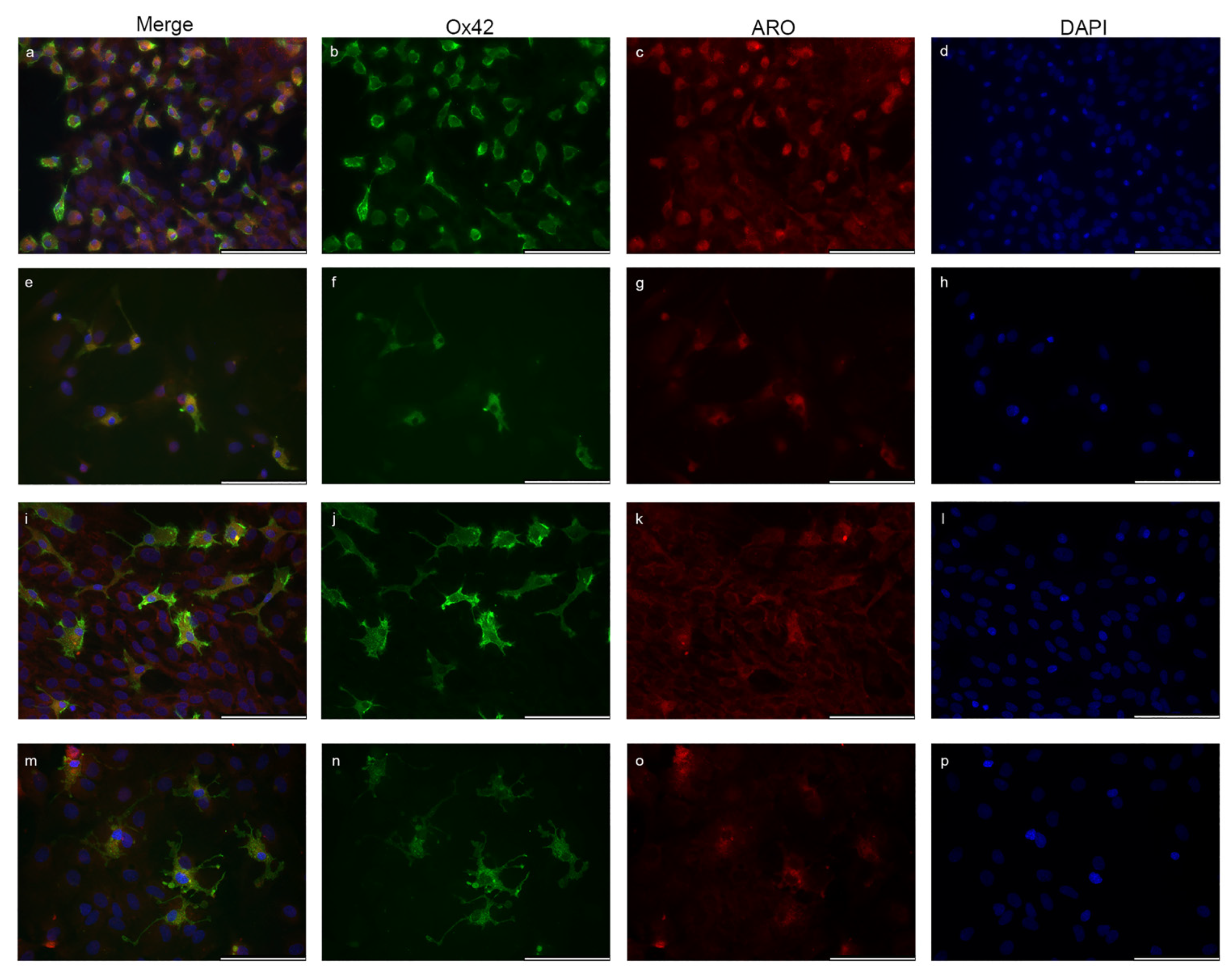
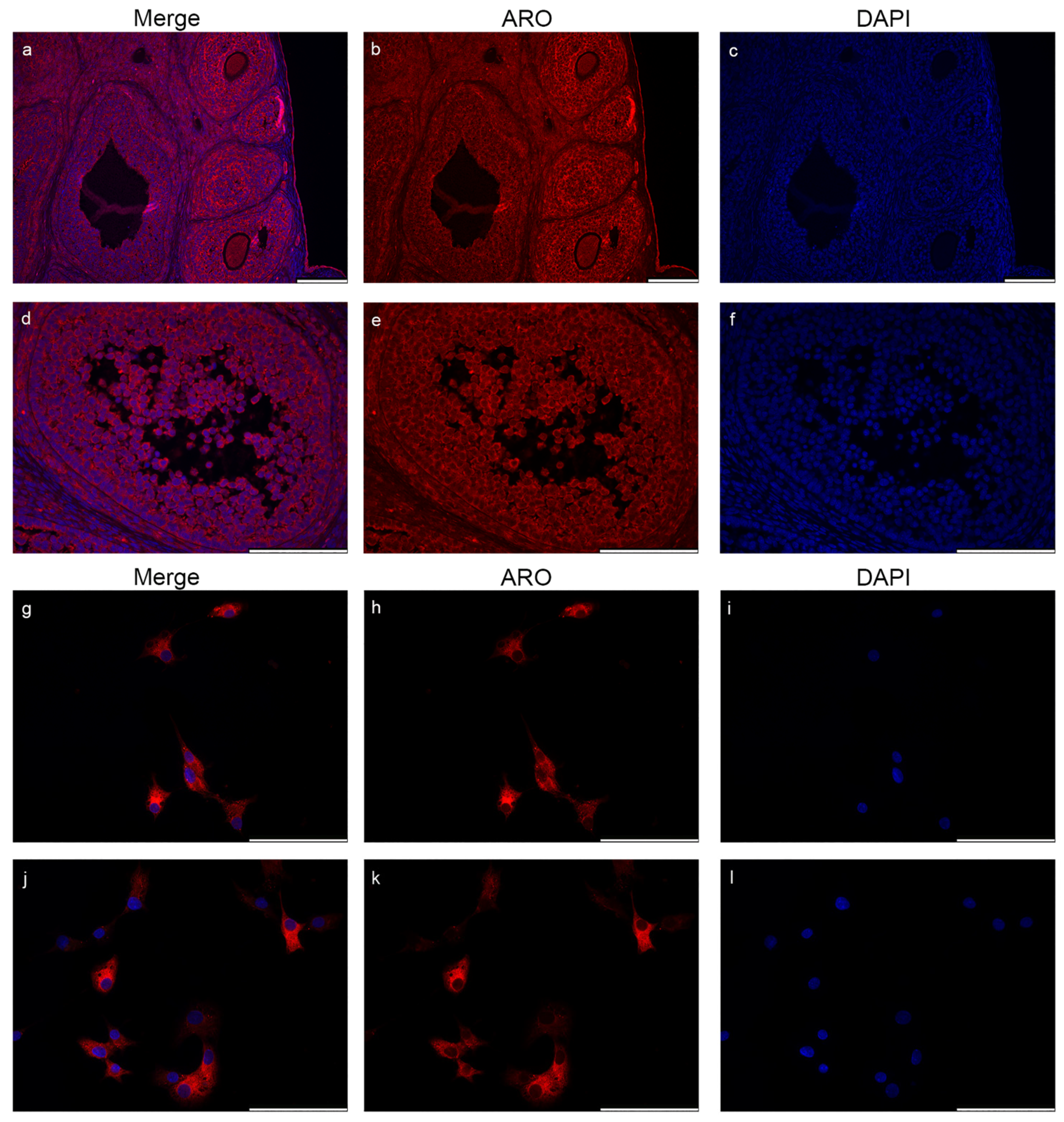
2.2. Microglia Cells Only Show Cytoplasmic Aromatase Positivity
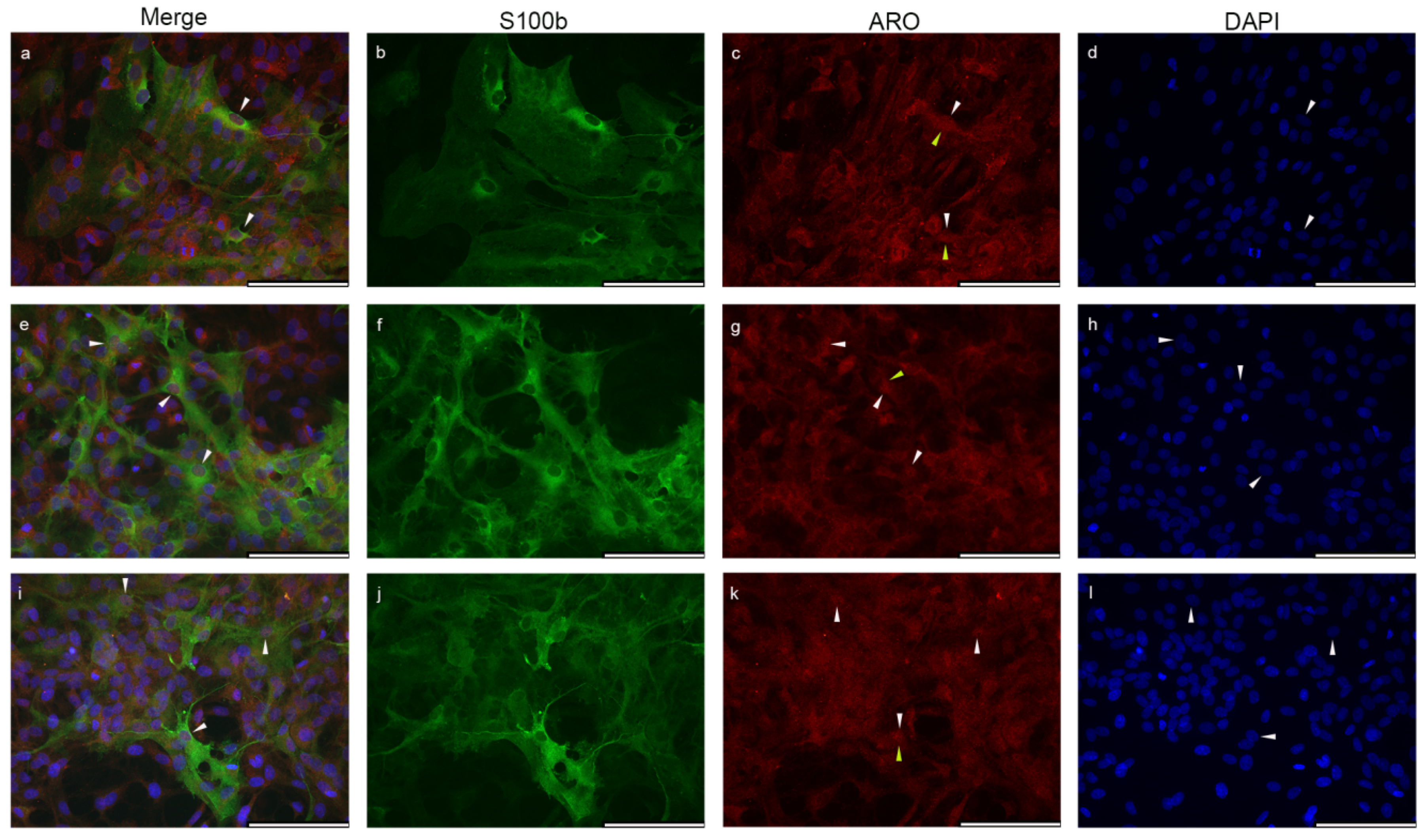
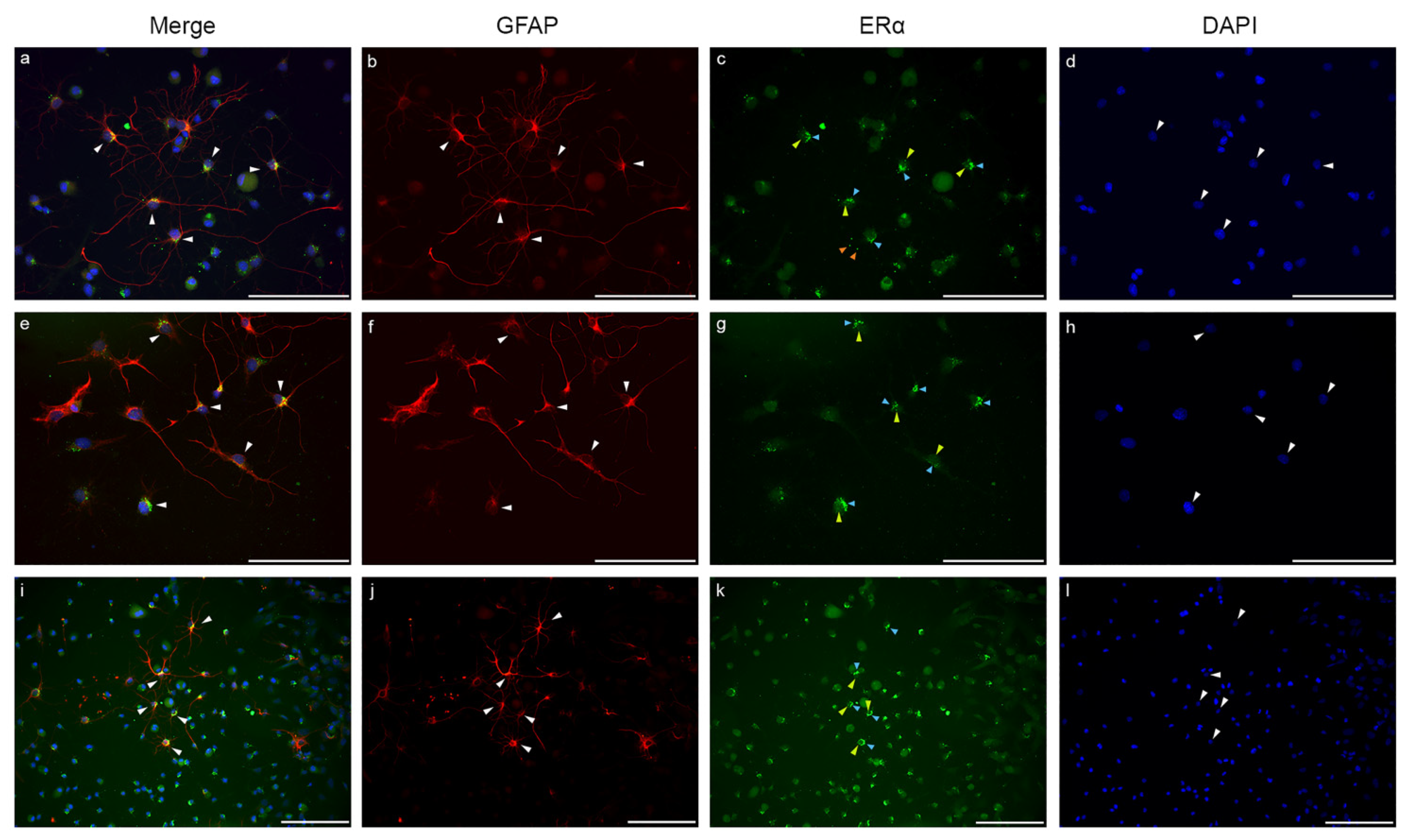
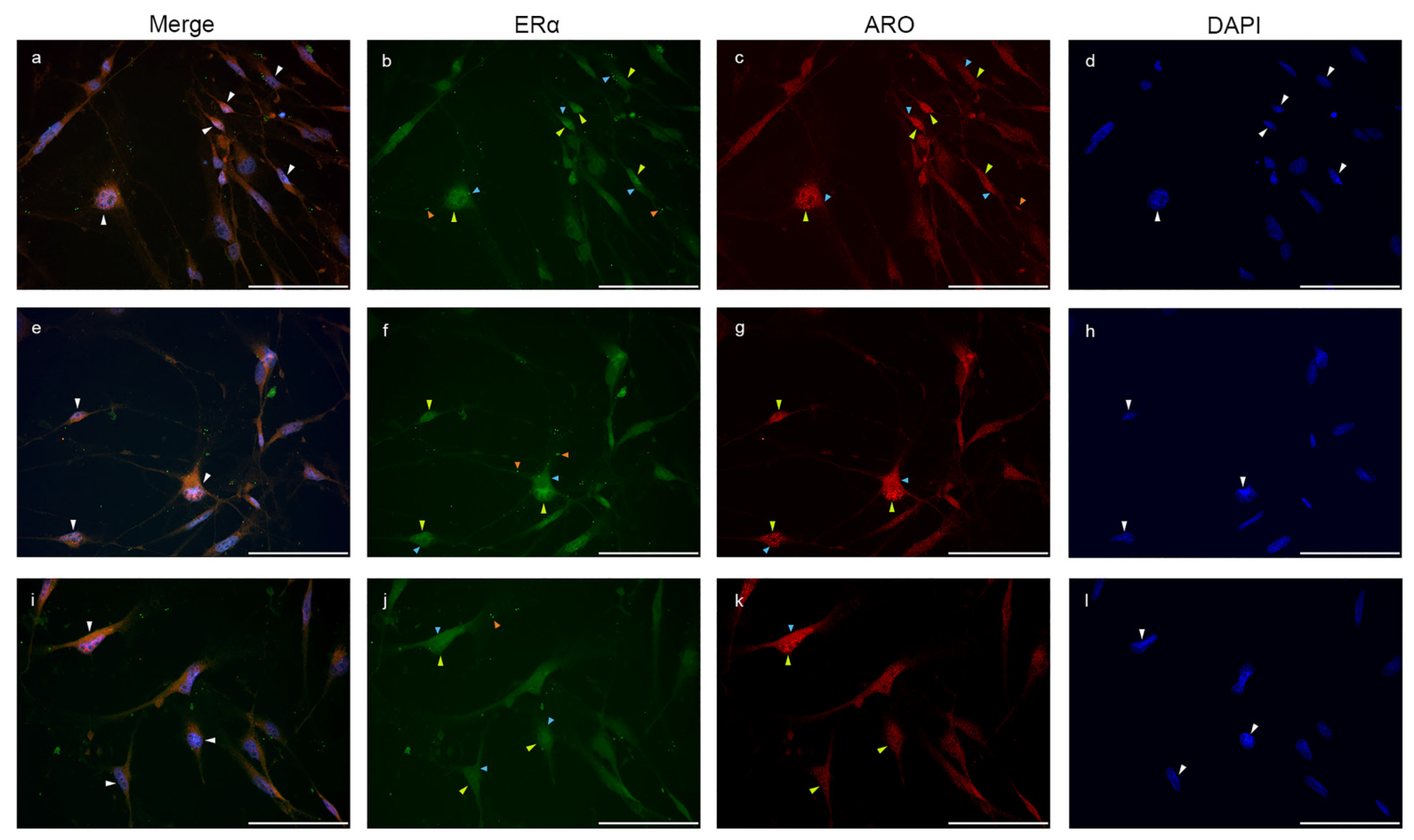
2.3. Estrogen Receptor Alpha Is Strongly Expressed in Astrocytes
2.4. Colocalization of Nuclear Aromatase and Estrogen Receptor Alpha
2.5. Nuclear Aromatase Expression Is Not Present in the Ovarian Cells and Tissue
3. Discussion
4. Materials and Methods
4.1. Housing and Handling of the Animals
4.2. Preparation of Mixed Primary Cortical Cultures and Glia Enriched Subcultures
4.3. Preparation of Human Granulosa Cell Culture
4.4. Immunocytochemistry
4.5. Immunostaining of Frozen Brain Tissue Sections
4.6. Immunostaining of Formalin-Fixed, Paraffin-Embedded Ovary Tissue Sections
4.7. Image Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Ovejero, D.; Azcoitia, I.; Doncarlos, L.L.; Melcangi, R.C.; Garcia-Segura, L.M. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res. Rev. 2005, 48, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Azcoitia, I.; Sierra, A.; Veiga, S.; Honda, S.; Harada, N.; Garcia-Segura, L.M. Brain aromatase is neuroprotective. J. Neurobiol. 2001, 47, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, C.J.; Duncan, K.A.; Walters, B.J. Neuroprotective actions of brain aromatase. Front. Neuroendocrinol. 2009, 30, 106–118. [Google Scholar] [CrossRef]
- Krentzel, A.A.; Willett, J.A.; Johnson, A.G.; Meitzen, J. Estrogen receptor alpha, G-protein coupled estrogen receptor 1, and aromatase: Developmental, sex, and region-specific differences across the rat caudate-putamen, nucleus accumbens core and shell. J. Comp. Neurol. 2020, 529, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Villa, A.; Della Torre, S.; Crippa, V.; Rusmini, P.; Cristofani, R.; Galbiati, M.; Maggi, A.; Poletti, A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr Rev. 2020, 41, 273–319. [Google Scholar] [CrossRef]
- Hong, Y.; Li, H.; Yuan, Y.C.; Chen, S. Molecular characterization of aromatase. Ann. N. Y. Acad Sci. 2009, 1155, 112–120. [Google Scholar] [CrossRef]
- Di Nardo, G.; Zhang, C.; Marcelli, A.G.; Gilardi, G. Molecular and Structural Evolution of Cytochrome P450 Aromatase. Int J. Mol. Sci. 2021, 22, 631. [Google Scholar] [CrossRef]
- Azcoitia, I.; Yague, J.G.; Garcia-Segura, L.M. Estradiol synthesis within the human brain. Neuroscience 2011, 191, 139–147. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, S.; Bossers, K.; Van der Bilt, S.; Agrapart, V.; Morales, R.R.; Frajese, G.V.; Swaab, D.F. Neurosteroid biosynthetic pathways changes in prefrontal cortex in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Jakab, R.L.; Horvath, T.L.; Leranth, C.; Harada, N.; Naftolin, F. Aromatase immunoreactivity in the rat brain: Gonadectomy-sensitve hypothalamic neurons and an unresponsive “limbic ring” of the lateral septum-bed nucleus-amygdala complex. J. Steroid Bioch. Mol. Biol. 1993, 44, 481–498. [Google Scholar] [CrossRef]
- Ishunina, T.A.; van Beurden, D.; van der Meulen, G.; Unmehopa, U.A.; Hol, E.M.; Huitinga, I.; Swaab, D.F. Diminished aromatase immunoreactivity in the hypothalamus, but not in the basal forebrain nuclei in Alzheimer’s disease. Neurobiol. Aging 2005, 26, 173–194. [Google Scholar] [CrossRef]
- Yague, J.G.; Munoz, A.; de Monasterio-Schrader, P.; DeFelipe, J.; Garcia-Segura, L.M. Aromatase expression in the human temporal cortex. Neuroscience 2006, 138, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Hoyk, Z.; Csakvari, E.; Gyenes, A.; Siklos, L.; Harada, N.; Parducz, A. Aromatase and estrogen receptor beta expression in the rat olfactory bulb: Neuroestrogen action in the first relay station of the olfactory pathway? Acta Neurobiol. Exp. 2014, 74, 1–14. [Google Scholar]
- Yague, J.G.; Lavaque, E.; Carretero, J.; Azcoitia, I.; Garcia-Segura, L.M. Aromatase, the enzyme responsible for estrogen biosynthesis, is expressed by human and rat glioblastomas. Neurosci. Lett. 2004, 368, 279–284. [Google Scholar] [CrossRef]
- Hojo, Y.; Hattori, T.; Enami, T.; Furukawa, A.; Suzuki, K.; Ishii, H.; Mukai, H.; Morrison, J.H.; Janssen, W.G.M.; Kominami, S.; et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 865–870. [Google Scholar] [CrossRef]
- Takahashi, K.; Bergström, M.; Frandberg, P.; Vesström, E.L.; Watanabe, Y.; Langström, B. Imaging of aromatase distribution in rat and rhesus monkey brains with [11C]vorozole. Nucl. Med. Biol. 2006, 33, 599–605. [Google Scholar] [CrossRef]
- Biegon, A.; Kim, S.W.; Alexoff, D.L.; Jayne, M.; Carter, P.; Hubbard, B.; King, P.; Logan, J.; Muench, L.; Pareto, D.; et al. Unique distribution of aromatase in the human brain: In vivo studies with PET and [N-methyl-11C]vorozole. Synapse 2010, 64, 801–807. [Google Scholar] [CrossRef]
- Roselli, C.F. Brain aromatase: Roles in reproduction and neuroprotection. J. Steroid. Bioch. Mol. Biol. 2007, 106, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.M. Aromatase in the brain: Not just for reproduction anymore. J. Neuroendocrinol. 2008, 20, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.M.; Veiga, S.; Sierra, A.; Melcangi, R.C.; Azcoitia, I. Aromatase: A neuroprotective enzyme. Prog. Neurobiol. 2003, 71, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Brocca, M.E.; Garcia-Segura, L.M. Non-reproductive Functions of Aromatase in the Central Nervous System under Physiological and Pathological Conditions. Cell. Mol. Neurobiol. 2019, 39, 473–481. [Google Scholar] [CrossRef]
- Saldanha, C.J.; Remage-Healey, L.; Schlinger, B.A. Synaptocrine signaling: Steroid synthesis and action at the synapse. Endocr. Rev. 2011, 32, 532–549. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Li, R.; Hu, Y. The alternative noncoding exons 1 of aromatase (Cyp19) gene modulate gene expression in a posttranscriptional manner. Endocrinology 2009, 150, 3301–3307. [Google Scholar] [CrossRef]
- Bogan, R.L.; Murphy, M.J.; Stouffer, R.L.; Hennebold, J.D. Systemic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol. Endocrinol. 2008, 22, 1260–1273. [Google Scholar] [CrossRef][Green Version]
- Bogan, R.L.; Murphy, M.J.; Hennebold, J.D. Dynamic changes in gene expression that occur during the period of spontaneous functional regression in the Rhesus macaque corpus luteum. Endocrinology 2009, 150, 1521–1529. [Google Scholar] [CrossRef]
- Pignatti, E.; Casarini, L.; Scaltriti, S.; Wistuba, J.; Schatt, S.; Rossi, A.; Lachhab, A.; Taliani, E.; Carani, C.; Simoni, M. Aromatase expression in human peripheral blood leucocytes (PBLs) and in various tissues in primates: Studies in elderly humans and cynomolgus monkeys. J. Med. Primat. 2012, 41, 372–383. [Google Scholar] [CrossRef]
- Ganesan, S.; Keating, A.F. Ovarian mitochondrial and oxidative stress proteins are altered by glyphosate exposure in mice. Toxicol. Appl. Pharmacol. 2021, 402, 115116. [Google Scholar] [CrossRef]
- Ganesan, S.; McGuire, B.C.; Keating, A.F. Absence of glyphosate-induced effects on ovarian folliculogenesis and steroidogenesis. Reprod. Toxicol. 2021, 96, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Evrard, H.C.; Harada, N.; Balthazart, J. Immunocytochemical localization of aromatase in sensory and integrating nuclei of the hindbrain in Japanese quail (Coturnix japonica). J. Comp. Neurol. 2004, 473, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Balthazart, J.; Choleris, E.; Remage-Healey, L. Steroids and the brain: 50 years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Horm. Behav. 2018, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, M.A.; Diz-Chaves, Y.; Santos-Galindo, M.; Bellini, M.J.; Garcia-Segura, L.M. Selective oestrogen receptor modulators decrease the inflammatory response of glial cells. J. Neuroendocrinol. 2012, 24, 183–190. [Google Scholar] [CrossRef]
- Sárvári, M.; Hrabovszky, E.; Kalló, I.; Solymosi, N.; Likó, I.; Berchtold, N.; Cotman, C.; Liposits, Z. Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: Rat and human studies identify strikingly similar changes. J. Neuroinflamm. 2012, 9, 264. [Google Scholar] [CrossRef]
- Liu, N.J.; Murugaiyan, V.; Storman, E.M.; Schnell, S.A.; Wessendorf, M.W.; Gintzler, A.R. Estrogens synthesized and acting within a spinal oligomer suppress spinal endomorphin 2 antinociception: Ebb and flow over the rat reproductive cycle. Pain 2017, 158, 1903–1914. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Giraud, S.N.; Caron, C.M.; Pham-Dinh, D.; Kitabgi, P.; Nicot, A.B. Estradiol inhibits ongoing autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 8416–8421. [Google Scholar] [CrossRef]
- Storman, E.M.; Liu, N.J.; Wessendorf, M.W.; Gintzler, A.R. Physical Linkage of Estrogen Receptor α and Aromatase in Rat: Oligocrine and Endocrine Actions of CNS-Produced Estrogens. Endocrinology 2018, 159, 2683–2697. [Google Scholar] [CrossRef]
- Osawa, Y.; Higashiyama, T.; Shimazu, Y.; Yarborough, C. Multiple functions of aromatase and the active site structure; aromatase is the placental estrogen 2-hydroxylase. J. Steroid. Bioch. Mol. Biol. 1993, 44, 469–480. [Google Scholar] [CrossRef]
- Kata, D.; Földesi, I.; Feher, L.Z.; Jr Hackler, L.; Puskas, L.G.; Gulya, K. Rosuvastatin enhances anti-inflammatory and inhibits pro-inflammatory functions in cultured microglial cells. Neuroscience 2016, 314, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Kata, D.; Földesi, I.; Feher, L.Z., Jr.; Hackler, L.; Puskas, L.G.; Gulya, K. A novel pleiotropic effect of aspirin: Beneficial regulation of pro- and anti-inflammatory mechanisms in microglial cells. Brain Res. Bull. 2017, 132, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Földesi, I.; Breckwoldt, M.; Neulen, J. Oestradiol production by luteinized human granulosa cells: Evidence of the stimulatory action of recombinant human follicle stimulating hormone. Hum. Reprod. 1998, 13, 1455–1460. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, R.M.; Ginger, L.A.; Kosila, N.; Elkins, N.D.; Essary, B.; McManaman, J.L.; Repine, J.E. Mononuclear phagocyte xanthine oxidoreductase contributes to cytokine-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 2004, 30, 479–490. [Google Scholar] [CrossRef]
- Avila-Rodriguez, M.; Garcia-Segura, L.M.; Hidalgo-Lanussa, O.; Baez, E.; Gonzalez, J.; Barreto, G.E. Tibolone protects astrocytic cells from glucose deprivation through a mechanism involving estrogen receptor beta and the upregulation of neuroglobin expression. Mol. Cell Endoocrinol. 2016, 433, 35–46. [Google Scholar] [CrossRef]
- Shiow, L.R.; Favrais, G.; Schirmer, L.; Schang, A.L.; Cipriani, S.; Andres, C.; Wright, J.N.; Nobuta, H.; Fleiss, B.; Gressens, P.; et al. Reactive astrocyte COX2-PGE2 production inhibits oligodendrocyte maturation in neonatal white matter injury. Glia 2017, 65, 2024–2037. [Google Scholar] [CrossRef]
- Kruminis-Kaszkiel, E.; Osowski, A.; Bejer-Oleńska, E.; Dziekoński, M.; Wojtkiewicz, J. Differentiation of Human Mesenchymal Stem Cells from Wharton’s Jelly Towards Neural Stem Cells Using A Feasible and Repeatable Protocol. Cells 2020, 9, 739. [Google Scholar] [CrossRef]
- Raponi, E.; Agenes, F.; Delphin, C.; Assard, N.; Baudier, J.; Legraverend, C.; Deloulme, J.C. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia 2007, 55, 165–177. [Google Scholar] [CrossRef]
- Legradi, A.; Dulka, K.; Jancsó, G.; Gulya, K. Orofacial skin inflammation increases the number of macrophages in the maxillary subregion of the rat trigeminal ganglion in a corticosteroid-reversible manner. Cell Tissue Res. 2020, 382, 551–561. [Google Scholar] [CrossRef]

| Species | Cell Type/Area | Localization of Aro | Method | Reference |
|---|---|---|---|---|
| Rat (Sprague-Dawley, adult, male, female) | Neurons/hypothalamus Neurons/subcortical telencephalic areas | Granular immunoreactivity in the somata and axon-like processes, but no staining in dendrites in hypothalamic cells Homogeneous but patchy labelling in the somata and dendritic processes in the subcortical areas Glia cells were immunonegative! There was no sexual dimorphism in the distribution and intensity of the staining No indication of nuclear localization | Immunostaining (a purified polyclonal rabbit-anti human placental Aro; In-house antibody) | [13] |
| Rat (Wistar, adult, male) | Pyramidal neurons/CA1-CA3 Granule cells/dentate gyrus Astrocytes/stratum radiatum and oriens Oligodendrocytes/hippocampus | Cell body, dendrites in CA3 pyramidal neurons, axon terminals and dendritic spines of principal cells, pre- and postsynaptic compartments Most of the glia cells were lacking P450arom immunoreactivity No indication of nuclear localization | Immunohistochemistry (a anti-P450arom IgG; In-house antibody) Postembedding immunogold labelling | [18] |
| Rat (Wistar, adult, male) | Neurons/olfactory bulb | Immunoreactivity mainly in the somata and also in cellular processes in juxtaglomerular neurons Weaker immunoreactivity in the cytoplasm (surrounding the nuclei) of mitral/tufted cells No indication of nuclear localization | Immunohistochemistry (b rabbit anti-ARO polyclonal antibody-epitope corresponding to amino acids 209–503 mapping at the C-terminus of human CYP19. Commercial antibody, Santa Cruz Biotechnology; Antibody registry ID: AB_2088681) | [16] |
| Rat (Sprague-Dawley newborn, adult, male, female) | Nucleus caudatus/putamen Nucleus accumbens core and shell | Immunopositivity in the cytoplasm but empty nucleus No sexual dimorphism in the Aro immunopositivity No indication of nuclear localization | Immunohistochemistry (c monoclonal anti-aromatase, Residue 376–390 human p450, clone H4, Commercial antibody, BioRad; Antibody registry ID: AB_566942) | [4] |
| Human (male, female) | Neurons and glia cells/cholinergic basal forebrain nuclei, hypothalamic nuclei | Immunoreactivity in the processes and the soma in granular cytoplasmic pattern accumulating around the nucleus and near plasmalemma (staining the endoplasmic reticulum) in neurons Immunopositivity in glia cells (astrocytes, oligodendrocytes, ependymal and choroid plexus cells) but no data about the subcellular localization No indication of nuclear localization | Immunohistochemistry (d polyclonal rabbit antibody raised against the 20 amino acid peptide coupled to thyroglobulin by glutaraldehyde corresponding to the N-terminus of human Aro; In-house antibody) | [14] |
| Human (male, female) | Neurons/lateral temporal neocortex Astrocytes/ lateral temporal neocortex | Immunoreactivity in the perikaryon and proximal processes Immunoreactivity located in the soma and processes No indication of nuclear localization | Immunohistochemistry (antibody A: rabbit polyclonal AB recognizes human, rat, bovine, mouse, chicken Aro. Commercial antibody, Acris Antibody) (e antibody B: rabbit polyclonal AB generated from 15 amino acid peptic corresponding to residues 488–502 of mouse Aro. In-house antibody) | [15] |
| Human (male, female) | Whole brain | Distribution volume values followed the rank order: thalamus > amygdala > preoptic area > medulla > cortex > putamen > cerebellum > white matter No information about the subcellular distribution of aromatase No indication of nuclear localization | Positron emission tomography (PET) (with radiolabelled aromatase inhibitor[N-methyl-11C]vorozole) | [20] |
| Japanese quail (adult, male) | Neurons/hindbrain | Immunoreactive perikarya with immunonegative nuclei in neurons Immunoreactive fibres with punctate structures were also observed in neurons No indication of nuclear localization | Immunohistochemistry (polyclonal affinity-purified rabbit-anti quail recombinant antibody; In-house antibody) | [32] |
| Name/Order Number (Manufacturer) Antibody Registry ID | Host/Clonality | Immunogen | Reactivity | Dilution | Citation |
|---|---|---|---|---|---|
| Aromatase NB100-1596 (Novus Biologicals) AB_10000919 | Rabbit/Polyclonal | C-terminal portion of the human aromatase protein (between residues 400–502) | Human, Mouse, Rat, Primate, Bovine, Rabbit | 1:100 | [31] |
| CD11b/c (OX42) MA1-90756 (Thermo Scientific/Invitrogen) AB_2280688 | Mouse/Monoclonal | Rat peritoneal macrophages | Rat | 1:100 | [44] |
| Estrogen receptor alpha MA1-310 (Thermo Scientific/Invitrogen) AB_325422 | Mouse/Monoclonal | Synthetic peptide corresponding to the residues E(247) V G M M K G G I R K D R R G(261) of the ER alpha DNA binding domain | Bovine, Human, Human, Mouse, Primate, Rat | 1:100 | [45] |
| Glial fibrillary acidic protein (GFAP) MA5-12023 (Thermo Scientific/Invitrogen) AB_10984338 | Mouse/Monoclonal | Glial fibrillary acidic protein | Chicken, Human, Pig, Rat | 1:100 | [46] |
| Glial fibrillary acidic protein (GFAP) PA1-10019 (Thermo Scientific/Invitrogen) AB_1074611 | Rabbit/Polyclonal | Full-length recombinant human GFAP protein | Bovine, Horse, Human, Mouse, Pig, Rat | 1:1000 | [47] |
| S100β 287 011 (Synaptic Systems) AB_2814881 | Mouse/Monoclonal | Recombinant protein corresponding to AA 1 to 92 from rat S100B | Mouse, Rat | 1:600 | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kata, D.; Gróf, I.; Hoyk, Z.; Ducza, E.; Deli, M.A.; Zupkó, I.; Földesi, I. Immunofluorescent Evidence for Nuclear Localization of Aromatase in Astrocytes in the Rat Central Nervous System. Int. J. Mol. Sci. 2022, 23, 8946. https://doi.org/10.3390/ijms23168946
Kata D, Gróf I, Hoyk Z, Ducza E, Deli MA, Zupkó I, Földesi I. Immunofluorescent Evidence for Nuclear Localization of Aromatase in Astrocytes in the Rat Central Nervous System. International Journal of Molecular Sciences. 2022; 23(16):8946. https://doi.org/10.3390/ijms23168946
Chicago/Turabian StyleKata, Diána, Ilona Gróf, Zsófia Hoyk, Eszter Ducza, Mária A. Deli, István Zupkó, and Imre Földesi. 2022. "Immunofluorescent Evidence for Nuclear Localization of Aromatase in Astrocytes in the Rat Central Nervous System" International Journal of Molecular Sciences 23, no. 16: 8946. https://doi.org/10.3390/ijms23168946
APA StyleKata, D., Gróf, I., Hoyk, Z., Ducza, E., Deli, M. A., Zupkó, I., & Földesi, I. (2022). Immunofluorescent Evidence for Nuclear Localization of Aromatase in Astrocytes in the Rat Central Nervous System. International Journal of Molecular Sciences, 23(16), 8946. https://doi.org/10.3390/ijms23168946







