Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part II)
Abstract
1. Introduction
2. Betulinic Acid
2.1. Anticancer Activity
2.2. Anti-Inflammatory Activity
2.3. Antidiabetic Activity
2.4. Cardioprotective Activity
2.5. Lipolytic Activity
2.6. Antibacterial and Antiparasitic Activity
2.7. Effect against T2-Mycotoxin Induced Toxicity
2.8. Other Biological Activities
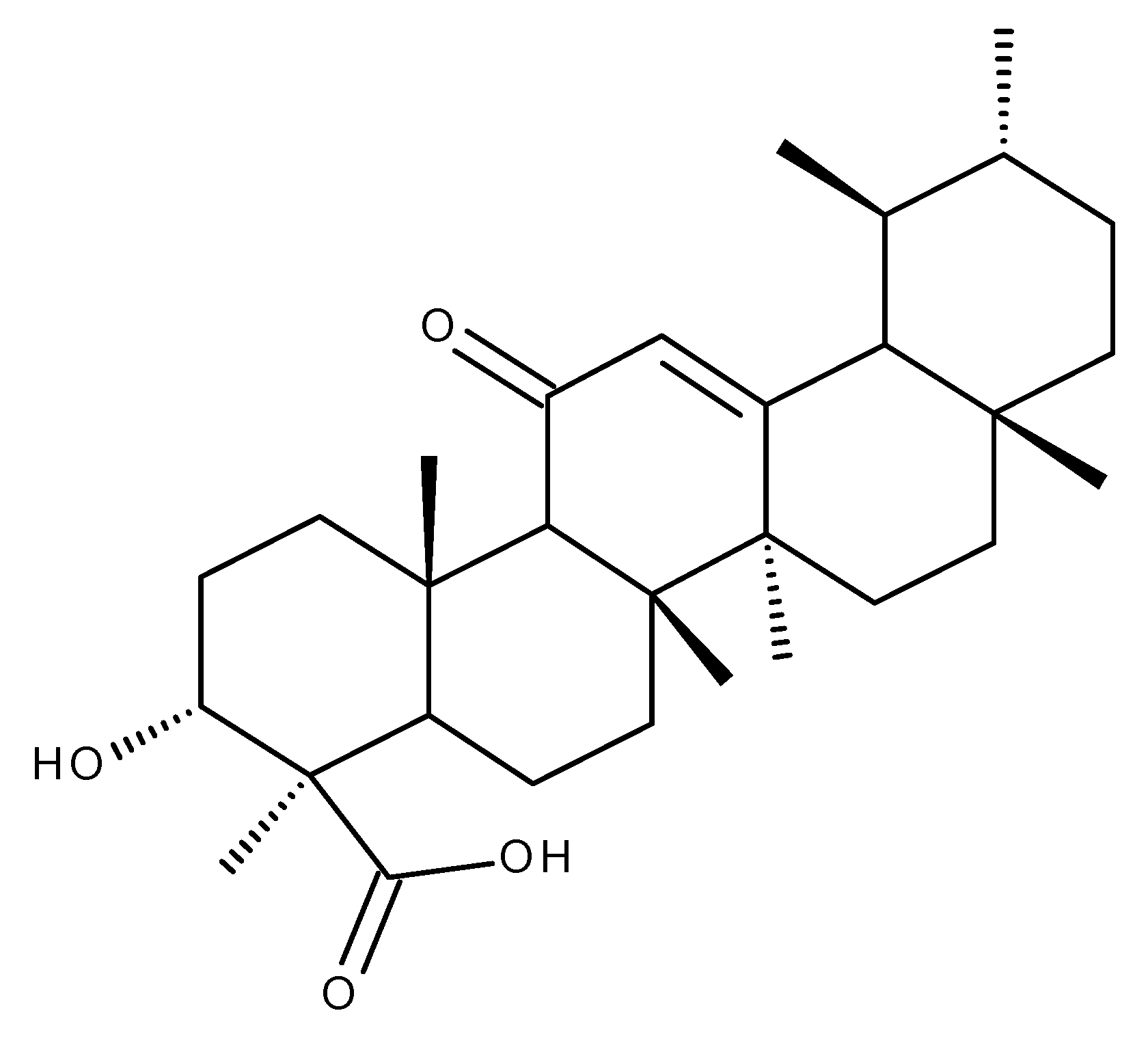
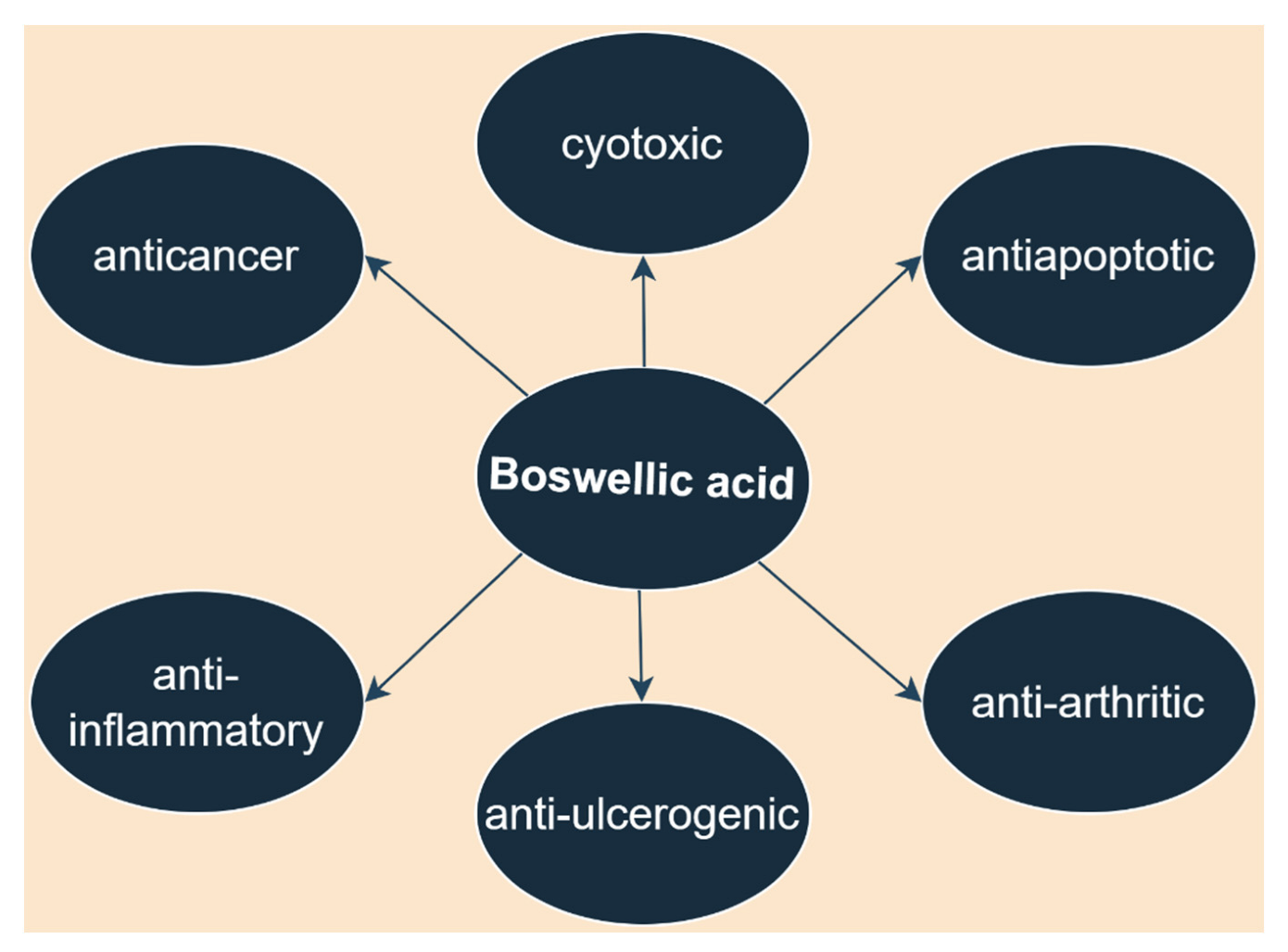
3. Boswellic Acid
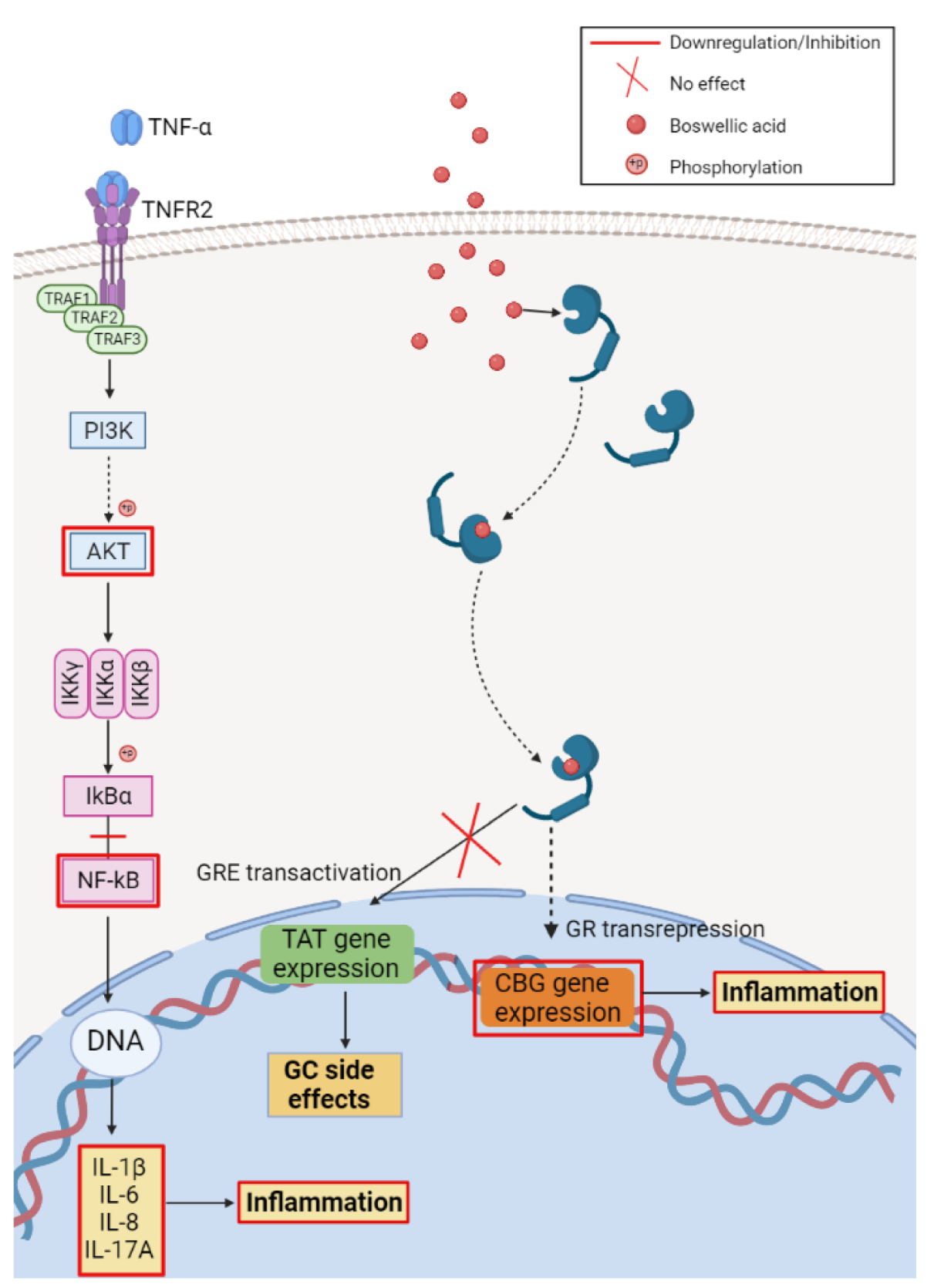
3.1. Anti-Inflammatory Activity
3.2. Antidiabetic Activity
3.3. Anti-Infectious Activity
3.4. Neuroprotective Activity
3.5. Other Biological Activities
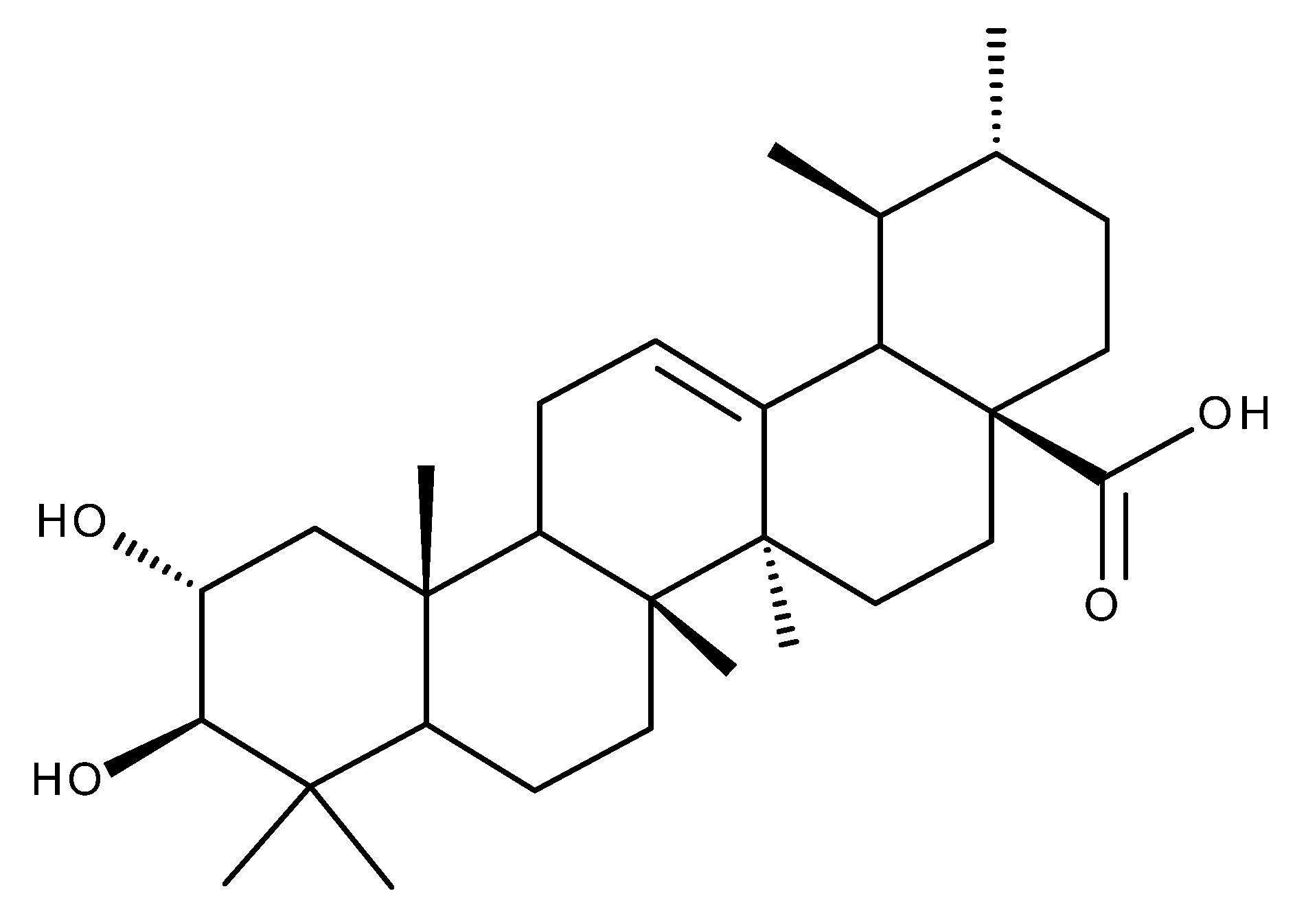
4. Corosolic Acid
4.1. Anticancer Activity
4.2. Antidiabetic Activity
4.3. Cardioprotective Activity
4.4. Other Biological Activities
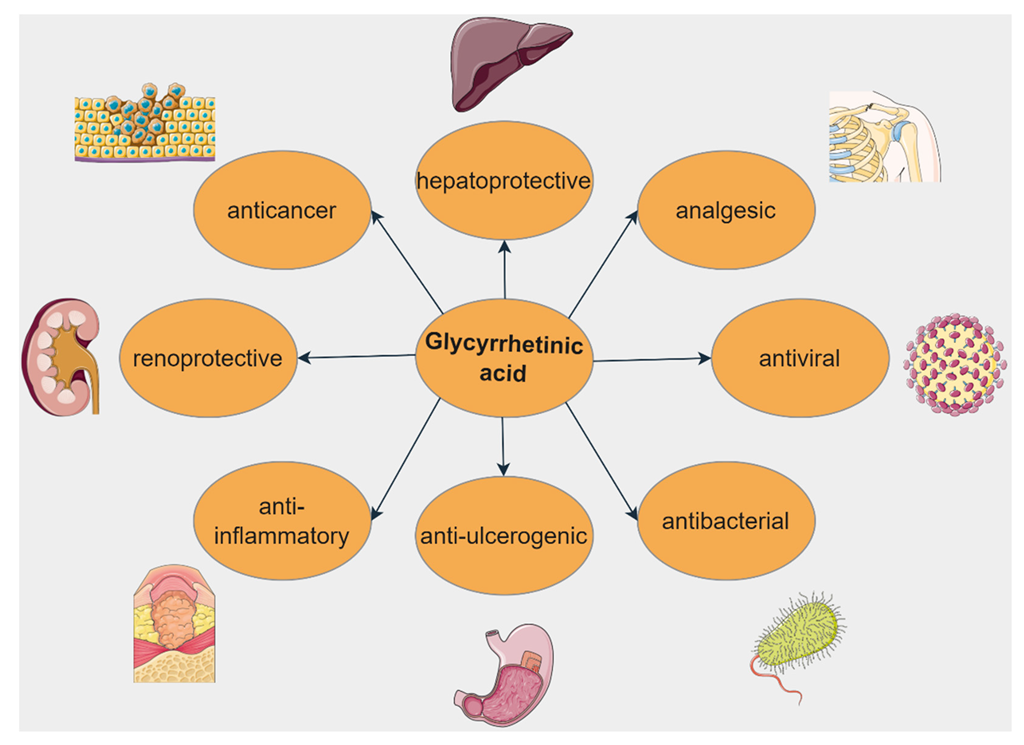
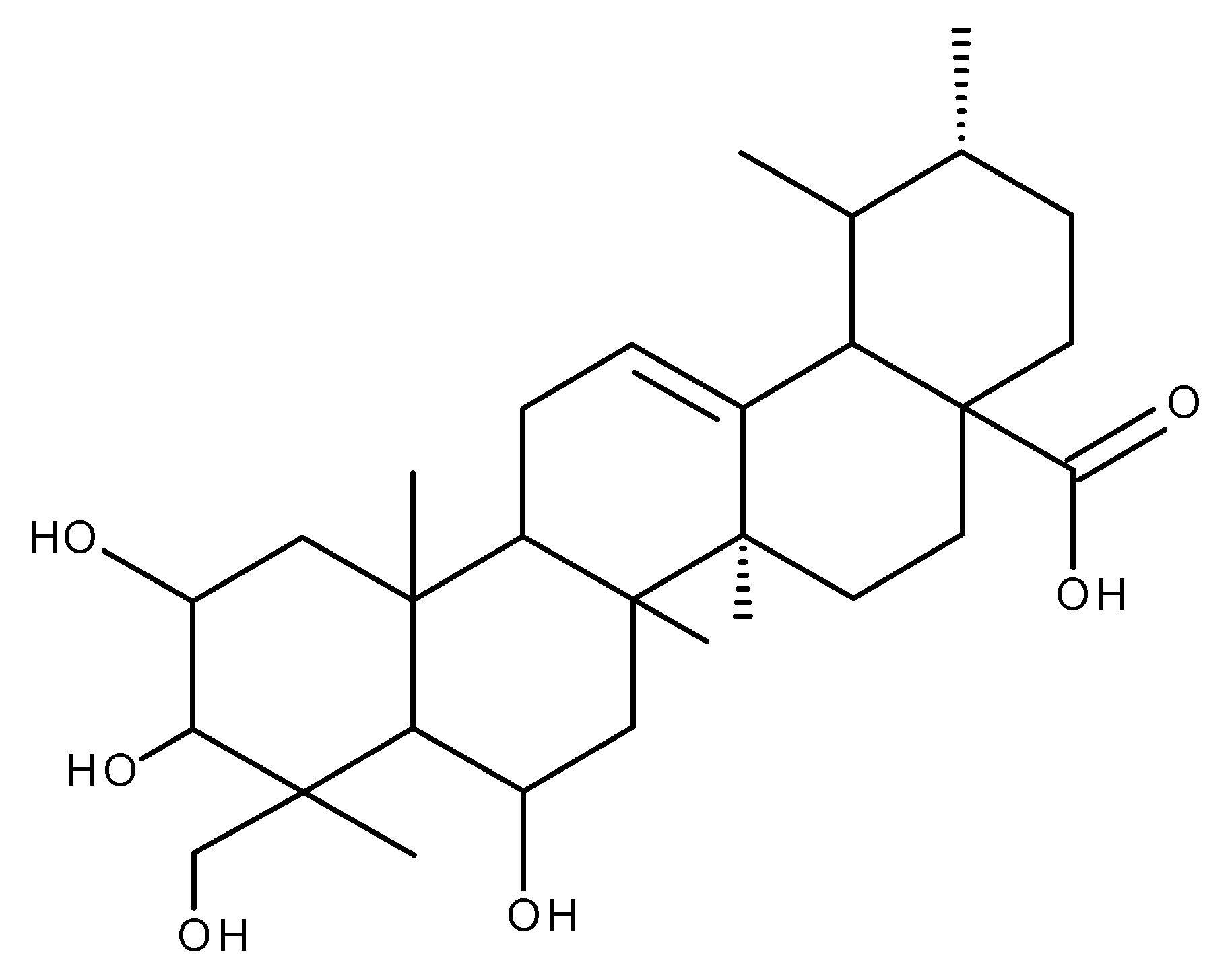
5. Glycyrrhetinic Acid
5.1. Anticancer Activity
5.2. Hepatoprotective Activity
5.3. Anti-Inflammatory Activity
5.4. Antibiotic Activity
5.5. Antidiabetic Activity
5.6. Renoprotective Activity
5.7. Cardioprotective Activity
5.8. Other Biological Activities
6. Madecassic Acid
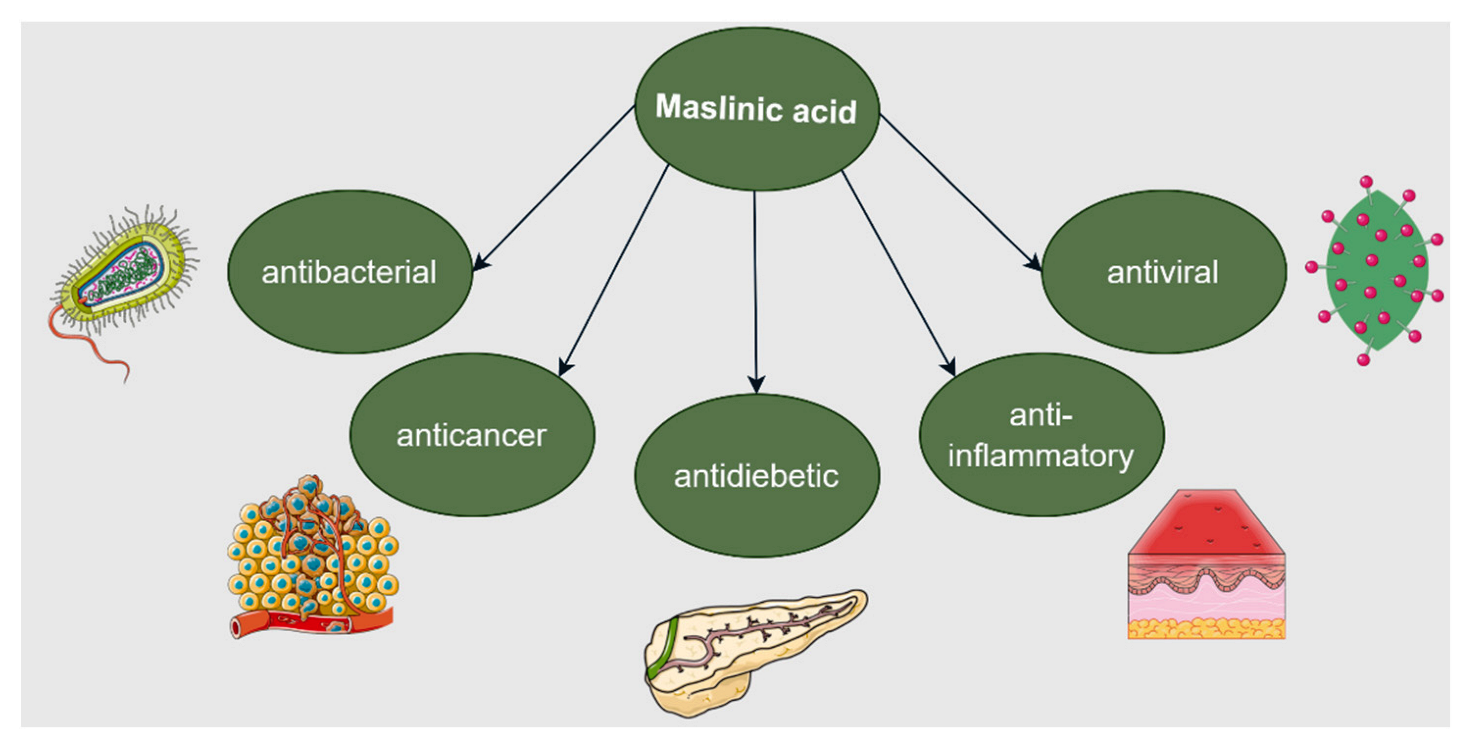
7. Maslinic Acid
7.1. Anticancer Activity
7.2. Anti-Inflammatory Activity
7.3. Cardioprotective Activity
7.4. Antidiabetic Activity
7.5. Neuroprotective Activity
7.6. Hepatoprotective Activity
7.7. Renoprotective Activity
7.8. Other Biological Activities
8. Pomolic Acid
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Petrovska, B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.L.; Abraham, A.; LaBella, A.L.; Abbot, P.; Rokas, A.; Capra, J.A. The influence of evolutionary history on human health and disease. Nat. Rev. Genet. 2021, 22, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Gedda, M.R.; Tiwari, V.K.; Singh, S.P.; Singh, R.K. Limitations of Current Therapeutic Options, Possible Drug Targets and Scope of Natural Products in Control of Leishmaniasis. Mini-Rev. Med. Chem. 2017, 18, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Yavari, B.; Mahjub, R.; Saidijam, M.; Raigani, M.; Soleimani, M. The Potential Use of Peptides in Cancer Treatment. Curr. Protein Pept. Sci. 2018, 19, 759–770. [Google Scholar] [CrossRef]
- Singh, V.K.; Arora, D.; Ansari, M.I.; Sharma, P.K. Phytochemicals based chemopreventive and chemotherapeutic strategies and modern technologies to overcome limitations for better clinical applications. Phyther. Res. 2019, 33, 3064–3089. [Google Scholar] [CrossRef]
- Fatima, M.; Iqubal, M.K.; Iqubal, A.; Kaur, H.; Gilani, S.J.; Rahman, M.H.; Ahmadi, A.; Rizwanullah, M. Current Insight into the Therapeutic Potential of Phytocompounds and their Nanoparticle-Based Systems for Effective Management of Lung Cancer. Anticancer. Agents Med. Chem. 2022, 22, 668–686. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, K.B.; Dhiman, M.; Arora, S.; Jaitak, V. New pentacyclic triterpene from Potentilla atrosanguinea Lodd. as anticancer agent for breast cancer targeting estrogen receptor—α. Nat. Prod. Res. 2021, 35, 1–6. [Google Scholar] [CrossRef]
- Ghante, M.H.; Jamkhande, P.G. Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms. J. Pharmacopuncture 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Kumar, P.; Bhadauria, A.S.; Singh, A.K.; Saha, S. Betulinic acid as apoptosis activator: Molecular mechanisms, mathematical modeling and chemical modifications. Life Sci. 2018, 209, 24–33. [Google Scholar] [CrossRef]
- Pereira, M.X.G.; Hammes, A.S.O.; Vasconcelos, F.C.; Pozzo, A.R.; Pereira, T.H.; Caffarena, E.R.; Gattass, C.R.; Maia, R.C. Antitumor Effect of Pomolic Acid in Acute Myeloid Leukemia Cells Involves Cell Death, Decreased Cell Growth and Topoisomerases Inhibition. Anticancer. Agents Med. Chem. 2019, 18, 1457–1468. [Google Scholar] [CrossRef]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.-P.; Zhang, X.-H.; Sun, L.-N.; Xing, W.-F.; Wang, Y.; Sun, S.-Y.; Ma, M.-Y.; Cheng, Z.-P.; Wu, Z.-D.; Xing, C.; et al. Corosolic acid and its structural analogs: A systematic review of their biological activities and underlying mechanism of action. Phytomedicine 2021, 91, 153696. [Google Scholar] [CrossRef] [PubMed]
- Hodon, J.; Borkova, L.; Pokorny, J.; Kazakova, A.; Urban, M. Design and synthesis of pentacyclic triterpene conjugates and their use in medicinal research. Eur. J. Med. Chem. 2019, 182, 111653. [Google Scholar] [CrossRef] [PubMed]
- Prodea, A.; Mioc, A.; Banciu, C.; Trandafirescu, C.; Milan, A.; Racoviceanu, R.; Ghiulai, R.; Mioc, M.; Soica, C. The Role of Cyclodextrins in the Design and Development of Triterpene-Based Therapeutic Agents. Int. J. Mol. Sci. 2022, 23, 736. [Google Scholar] [CrossRef] [PubMed]
- Kaps, A.; Gwiazdoń, P.; Chodurek, E. Nanoformulations for Delivery of Pentacyclic Triterpenoids in Anticancer Therapies. Molecules 2021, 26, 1764. [Google Scholar] [CrossRef] [PubMed]
- Marrero, A.D.; Quesada, A.R.; Martínez-Poveda, B.; Medina, M.Á. Antiangiogenic Phytochemicals Constituent of Diet as Promising Candidates for Chemoprevention of Cancer. Antioxidants 2022, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Renda, G.; Gökkaya, İ.; Şöhretoğlu, D. Immunomodulatory properties of triterpenes. Phytochem. Rev. 2022, 21, 537–563. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Mioc, M.; Milan, A.; Malița, D.; Mioc, A.; Prodea, A.; Racoviceanu, R.; Ghiulai, R.; Cristea, A.; Căruntu, F.; Șoica, C. Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part I). Int. J. Mol. Sci. 2022, 23, 7740. [Google Scholar] [CrossRef]
- Farcas, C.G.; Dehelean, C.; Pinzaru, I.A.; Mioc, M.; Socoliuc, V.; Moaca, E.-A.; Avram, S.; Ghiulai, R.; Coricovac, D.; Pavel, I.; et al. Thermosensitive Betulinic Acid-Loaded Magnetoliposomes: A Promising Antitumor Potential for Highly Aggressive Human Breast Adenocarcinoma Cells under Hyperthermic Conditions. Int. J. Nanomed. 2020, 15, 8175–8200. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, N.; Liu, Y.; Cheng, M.-S. Recent progress on betulinic acid and its derivatives as antitumor agents: A mini review. Chin. J. Nat. Med. 2021, 19, 641–647. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Nedopekina, D.A.; Gubaidullin, R.R.; Davletshin, E.V.; Tukhbatullin, A.A.; D’yakonov, V.A.; Yunusbaeva, M.M.; Dzhemileva, L.U.; Dzhemilev, U.M. Pentacyclic triterpene acid conjugated with mitochondria-targeting cation F16: Synthesis and evaluation of cytotoxic activities. Med. Chem. Res. 2021, 30, 940–951. [Google Scholar] [CrossRef]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef] [PubMed]
- Yaozu, Z.; Liu, Y.; Zhao, H.; Peng, P.; Tingbao, Z.; Jincao, C. Betulinic acid inhibits glioma cell viability by downregulation of NF-κB and enhancement of apoptosis. Trop. J. Pharm. Res. 2021, 19, 2545–2551. [Google Scholar] [CrossRef]
- Shen, H.; Liu, L.; Yang, Y.; Xun, W.; Wei, K.; Zeng, G. Betulinic Acid Inhibits Cell Proliferation in Human Oral Squamous Cell Carcinoma via Modulating ROS-Regulated p53 Signaling. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 1141–1152. [Google Scholar] [CrossRef]
- Yuan, D.-Y.; Meng, Z.; Xu, K.; Li, Q.-F.; Chen, C.; Li, K.-Y.; Zhang, B. Betulinic acid increases radiosensitization of oral squamous cell carcinoma through inducing Sp1 sumoylation and PTEN expression. Oncol. Rep. 2017, 38, 2360–2368. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Ge, L.; Zhao, Y.; Zhu, K.; Chen, Z.; Wu, Q.; Xin, Y.; Guo, J. Betulinic acid targets drug-resistant human gastric cancer cells by inducing autophagic cell death, suppresses cell migration and invasion, and modulates the ERK/MEK signaling pathway. Acta Biochim. Pol. 2021, 69, 25–30. [Google Scholar] [CrossRef]
- Liu, W.; Li, S.; Qu, Z.; Luo, Y.; Chen, R.; Wei, S.; Yang, X.; Wang, Q. Betulinic acid induces autophagy-mediated apoptosis through suppression of the PI3K/AKT/mTOR signaling pathway and inhibits hepatocellular carcinoma. Am. J. Transl. Res. 2019, 11, 6952–6964. [Google Scholar]
- Chen, F.; Zhong, Z.; Tan, H.Y.; Guo, W.; Zhang, C.; Cheng, C.; Wang, N.; Ren, J.; Feng, Y. Suppression of lncRNA MALAT1 by betulinic acid inhibits hepatocellular carcinoma progression by targeting IAPs via miR-22-3p. Clin. Transl. Med. 2020, 10, e190. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, H.; Weng, M.; Wang, C.; Sun, L. Chemopreventive effect of Betulinic acid via mTOR -Caspases/Bcl2/Bax apoptotic signaling in pancreatic cancer. BMC Complement. Med. Ther. 2020, 20, 178. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 21–86. [Google Scholar]
- Ghosh, P.; Mandal, A.; Ghosh, J.; Pal, C.; Nanda, A.K. Synthesis of bioactive 28-hydroxy-3-oxolup-20(29)-en-30-al with antileukemic activity. J. Asian Nat. Prod. Res. 2012, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Binaschi, M.; Zunino, F.; Capranico, G. Mechanism of action of DNA topoisomerase inhibitors. Stem Cells 1995, 13, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Li, M.; Huang, X.; Xie, W.; Xiang, W.; Yao, P. Combination of betulinic acid and chidamide inhibits acute myeloid leukemia by suppression of the HIF1α pathway and generation of reactive oxygen species. Oncotarget 2017, 8, 94743–94758. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Jeong, J.-W.; Han, M.H.; Lee, H.; Kim, G.-Y.; Jin, S.; Park, J.-H.; Kwon, H.J.; Kim, B.W.; Choi, Y.H. The anti-cancer effect of betulinic acid in u937 human leukemia cells is mediated through ROS-dependent cell cycle arrest and apoptosis. Animal Cells Syst. 2021, 25, 119–127. [Google Scholar] [CrossRef]
- Shen, M.; Hu, Y.; Yang, Y.; Wang, L.; Yang, X.; Wang, B.; Huang, M. Betulinic Acid Induces ROS-Dependent Apoptosis and S-Phase Arrest by Inhibiting the NF- κ B Pathway in Human Multiple Myeloma. Oxid. Med. Cell. Longev. 2019, 2019, 5083158. [Google Scholar] [CrossRef]
- Kutkowska, J.; Strzadala, L.; Rapak, A. Hypoxia increases the apoptotic response to betulinic acid and betulin in human non-small cell lung cancer cells. Chem. Biol. Interact. 2021, 333, 109320. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, Q.; Ma, Y.-B.; Ding, L.; Xu, X.-L.; Wei, D.-F.; Wei, L.; Zhang, J.-W. Betulinic acid induces apoptosis and ultrastructural changes in MDA-MB-231 breast cancer cells. Ultrastruct. Pathol. 2018, 42, 49–54. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, P.; Wang, N.; Wang, S.; Yang, B.; Li, M.; Chen, J.; Situ, H.; Xie, M.; Lin, Y.; et al. Betulinic Acid Suppresses Breast Cancer Metastasis by Targeting GRP78-Mediated Glycolysis and ER Stress Apoptotic Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 8781690. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.R.; Kang, K.S.; Ko, Y.; Pang, C.; Yamabe, N.; Kim, K.H. Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis. Biomolecules 2019, 9, 257. [Google Scholar] [CrossRef]
- Xu, T.; Pang, Q.; Wang, Y.; Yan, X. Betulinic acid induces apoptosis by regulating PI3K/Akt signaling and mitochondrial pathways in human cervical cancer cells. Int. J. Mol. Med. 2017, 40, 1669–1678. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cho, H.-S.; Ban, H.S.; Nakamura, H. Suppression of HIF-1α accumulation by betulinic acid through proteasome activation in hypoxic cervical cancer. Biochem. Biophys. Res. Commun. 2020, 523, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hwangbo, H.; Kim, M.Y.; Ji, S.Y.; Kim, D.H.; Lee, H.; Kim, G.-Y.; Moon, S.-K.; Leem, S.-H.; Yun, S.J.; et al. Betulinic Acid Restricts Human Bladder Cancer Cell Proliferation In Vitro by Inducing Caspase-Dependent Cell Death and Cell Cycle Arrest, and Decreasing Metastatic Potential. Molecules 2021, 26, 1381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, N.; Zhou, X.; Wang, F.; Cai, H.; Huang, S.H.; Chen, X.; Hu, Z.; Jin, X. Betulinic acid induces autophagy-dependent apoptosis via Bmi-1/ROS/AMPK-mTOR-ULK1 axis in human bladder cancer cells. Aging 2021, 13, 21251–21267. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Shafaei, A.; Majid, A.S.A.; Ezzat, M.O.; Dahham, S.S.; Ahamed, M.B.K.; Oon, C.E.; Majid, A.M.S.A. Mesua ferrea stem bark extract induces apoptosis and inhibits metastasis in human colorectal carcinoma HCT 116 cells, through modulation of multiple cell signalling pathways. Chin. J. Nat. Med. 2017, 15, 505–5014. [Google Scholar] [CrossRef]
- Zeng, A.; Hua, H.; Liu, L.; Zhao, J. Betulinic acid induces apoptosis and inhibits metastasis of human colorectal cancer cells in vitro and in vivo. Bioorg. Med. Chem. 2019, 27, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, K.; Zhang, C.; Zhang, W.; Xu, Q.; Wang, Y.; Zhang, Y.; Li, Y.; Zhang, Y.; Zhu, H.; et al. Overaccumulation of p53-mediated autophagy protects against betulinic acid-induced apoptotic cell death in colorectal cancer cells. Cell Death Dis. 2017, 8, e3087. [Google Scholar] [CrossRef]
- Goswami, P.; Paul, S.; Banerjee, R.; Kundu, R.; Mukherjee, A. Betulinic acid induces DNA damage and apoptosis in SiHa cells. Mutat. Res. Toxicol. Environ. Mutagen. 2018, 828, 1–9. [Google Scholar] [CrossRef]
- Feki-Tounsi, M.; Hamza-Chaffai, A. Cadmium as a possible cause for bladder cancer: A review of accumulated evidence. Environ. Sci. Pollut. Res. 2014, 21, 10561–10573. [Google Scholar] [CrossRef]
- Fan, R.; Hu, P.; Wang, Y.; Lin, H.; Su, K.; Feng, X.; Wei, L.; Yang, F. Betulinic acid protects mice from cadmium chloride-induced toxicity by inhibiting cadmium-induced apoptosis in kidney and liver. Toxicol. Lett. 2018, 299, 56–66. [Google Scholar] [CrossRef]
- Ou, Z.; Zhao, J.; Zhu, L.; Huang, L.; Ma, Y.; Ma, C.; Luo, C.; Zhu, Z.; Yuan, Z.; Wu, J.; et al. Anti-inflammatory effect and potential mechanism of betulinic acid on λ-carrageenan-induced paw edema in mice. Biomed. Pharmacother. 2019, 118, 109347. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Q. Betulinic acid inhibits cell proliferation, migration, and inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes. J. Cell. Biochem. 2019, 120, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gong, Z.; Li, X.; Ma, Q.; Wu, M.; Liu, D.; Deng, L.; Pan, D.; Liu, Q.; Wei, Z.; et al. Betulinic acid inhibits the migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Int. Immunopharmacol. 2019, 67, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kun-Liu; Wang, J.-Y.; Zhang, L.; Pan, Y.-Y.; Chen, X.-Y.; Yuan, Y. Effects of betulinic acid on synovial inflammation in rats with collagen-induced arthritis. Int. J. Immunopathol. Pharmacol. 2020, 34, 205873842094507. [Google Scholar] [CrossRef]
- Zhou, Z.; Choi, J.-W.; Shin, J.Y.; Kim, D.-U.; Kweon, B.; Oh, H.; Kim, Y.-C.; Song, H.-J.; Bae, G.-S.; Park, S.-J. Betulinic Acid Ameliorates the Severity of Acute Pancreatitis via Inhibition of the NF-κB Signaling Pathway in Mice. Int. J. Mol. Sci. 2021, 22, 6871. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Lu, C.; Chen, H.; Deng, J.; Yan, Y.; Xu, Y.-Y.; Liu, H.; Huang, H.; Wei, J.; et al. Betulinic acid suppresses Th17 response and ameliorates psoriasis-like murine skin inflammation. Int. Immunopharmacol. 2019, 73, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, C.; Zhou, H.; Feng, Y.; Tang, F.; Hoi, M.P.M.; He, C.; Ma, D.; Zhao, C.; Lee, S.M.Y. Inhibitory Effects of Betulinic Acid on LPS-Induced Neuroinflammation Involve M2 Microglial Polarization via CaMKKβ-Dependent AMPK Activation. Front. Mol. Neurosci. 2018, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Jung, J.; Jang, D.S.; Kim, J.; Kim, J.H. Induction of Cell Death by Betulinic Acid through Induction of Apoptosis and Inhibition of Autophagic Flux in Microglia BV-2 Cells. Biomol. Ther. 2017, 25, 618–624. [Google Scholar] [CrossRef]
- Chen, Y.; Jinag, W.; Chen, Y.; Chen, X.-L.; Chen, W.; Li, F.-T.; Li, Y.-J.; Yao, X. Protective effect of betulinic acid for treating unpredictable chronic mild stress-induced depression in mice by inhibiting brain RIP140 activation. Int. J. Clin. Exp. Med. 2017, 10, 16492–16498. [Google Scholar]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wang, R.; Tan, H. Role of Pyroptosis in Cardiovascular Diseases and its Therapeutic Implications. Int. J. Biol. Sci. 2019, 15, 1345–1357. [Google Scholar] [CrossRef]
- Al Mamun, A.; Wu, Y.; Monalisa, I.; Jia, C.; Zhou, K.; Munir, F.; Xiao, J. Role of pyroptosis in spinal cord injury and its therapeutic implications. J. Adv. Res. 2021, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, H.; Zhuang, R.; Zhang, H.; Wang, Y.; Hu, X.; Xu, Y.; Li, J.; Li, Y.; Wang, X.; et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int. J. Biol. Sci. 2021, 17, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, X.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. New Insights into the Inhibition Mechanism of Betulinic Acid on α-Glucosidase. J. Agric. Food Chem. 2018, 66, 7065–7075. [Google Scholar] [CrossRef] [PubMed]
- Gomes Castro, A.J.; Cazarolli, L.H.; Bretanha, L.C.; Sulis, P.M.; Rey Padilla, D.P.; Aragón Novoa, D.M.; Dambrós, B.F.; Pizzolatti, M.G.; Mena Barreto Silva, F.R. The potent insulin secretagogue effect of betulinic acid is mediated by potassium and chloride channels. Arch. Biochem. Biophys. 2018, 648, 20–26. [Google Scholar] [CrossRef]
- Birgani, G.A.; Ahangarpour, A.; Khorsandi, L.; Moghaddam, H.F. Anti-Diabetic effect of betulinic acid on streptozotocin-nicotinamide induced diabetic male mouse model. Braz. J. Pharm. Sci. 2018, 54, e17171. [Google Scholar] [CrossRef]
- Song, T.-J.; Park, C.-H.; In, K.-R.; Kim, J.-B.; Kim, J.H.; Kim, M.; Chang, H.J. Antidiabetic effects of betulinic acid mediated by the activation of the AMP-activated protein kinase pathway. PLoS ONE 2021, 16, e0249109. [Google Scholar] [CrossRef]
- Bai, Y.-Y.; Yan, D.; Zhou, H.-Y.; Li, W.-X.; Lou, Y.-Y.; Zhou, X.-R.; Qian, L.-B.; Xiao, C. Betulinic acid attenuates lipopolysaccharide-induced vascular hyporeactivity in the rat aorta by modulating Nrf2 antioxidative function. Inflammopharmacology 2020, 28, 165–174. [Google Scholar] [CrossRef]
- Zhu, L.; Yi, X.; Zhao, J.; Yuan, Z.; Wen, L.; Pozniak, B.; Obminska-Mrukowicz, B.; Tian, Y.; Tan, Z.; Wu, J.; et al. Betulinic acid attenuates dexamethasone-induced oxidative damage through the JNK-P38 MAPK signaling pathway in mice. Biomed. Pharmacother. 2018, 103, 499–508. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, F.-X.; Zang, S.-T.; Yang, Q.-F. Betulinic acid prevents high glucose-induced expression of extracellular matrix protein in cardiac fibroblasts by inhibiting the TGF-β1/Smad signaling pathway. Mol. Med. Rep. 2017, 16, 6320–6325. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell. Mol. Biol. 2012, 298, 229–317. [Google Scholar]
- Lu, P.; Zhang, C.; Zhang, X.; Li, H.; Luo, A.; Tian, Y.; Xu, H. Down-Regulation of NOX4 by betulinic acid protects against cerebral ischemia-reperfusion in mice. Curr. Med. Sci. 2017, 37, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hao, K. Protective Action of Betulinic Acid on Cerebral Ischemia/Reperfusion Injury through Inflammation and Energy Metabolic Homeostasis. Appl. Sci. 2020, 10, 2578. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, X.; Wang, J.; Mang, J.; Xu, Z. Betulinic Acid Ameliorates Cerebral Injury in Middle Cerebral Artery Occlusion Rats through Regulating Autophagy. ACS Chem. Neurosci. 2021, 12, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, T.; Liu, F. Betulinic acid alleviates myocardial hypoxia/reoxygenation injury via inducing Nrf2/HO-1 and inhibiting p38 and JNK pathways. Eur. J. Pharmacol. 2018, 838, 53–59. [Google Scholar] [CrossRef]
- Yoon, J.J.; Lee, Y.J.; Han, B.H.; Choi, E.S.; Kho, M.C.; Park, J.H.; Ahn, Y.M.; Kim, H.Y.; Kang, D.G.; Lee, H.S. Protective effect of betulinic acid on early atherosclerosis in diabetic apolipoprotein-E gene knockout mice. Eur. J. Pharmacol. 2017, 796, 224–232. [Google Scholar] [CrossRef]
- Senamontree, S.; Lakthan, T.; Charoenpanich, P.; Chanchao, C.; Charoenpanich, A. Betulinic acid decreases lipid accumulation in adipogenesis-induced human mesenchymal stem cells with upregulation of PGC-1α and UCP-1 and post-transcriptional downregulation of adiponectin and leptin secretion. PeerJ 2021, 9, e12321. [Google Scholar] [CrossRef]
- Kim, K.-D.; Jung, H.-Y.; Ryu, H.G.; Kim, B.; Jeon, J.; Yoo, H.Y.; Park, C.H.; Choi, B.-H.; Hyun, C.-K.; Kim, K.-T.; et al. Betulinic acid inhibits high-fat diet-induced obesity and improves energy balance by activating AMPK. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Brusotti, G.; Montanari, R.; Capelli, D.; Cattaneo, G.; Laghezza, A.; Tortorella, P.; Loiodice, F.; Peiretti, F.; Bonardo, B.; Paiardini, A.; et al. Betulinic acid is a PPARγ antagonist that improves glucose uptake, promotes osteogenesis and inhibits adipogenesis. Sci. Rep. 2017, 7, 5777. [Google Scholar] [CrossRef]
- Savova, M.S.; Vasileva, L.V.; Mladenova, S.G.; Amirova, K.M.; Ferrante, C.; Orlando, G.; Wabitsch, M.; Georgiev, M.I. Ziziphus jujuba Mill. leaf extract restrains adipogenesis by targeting PI3K/AKT signaling pathway. Biomed. Pharmacother. 2021, 141, 111934. [Google Scholar] [CrossRef]
- Mohsen G, A.-M.; Abu-Taweel, G.M.; Rajagopal, R.; Sun-Ju, K.; Kim, H.-J.; Kim, Y.O.; Mothana, R.A.; Kadaikunnan, S.; Khaled, J.M.; Siddiqui, N.A.; et al. Betulinic acid lowers lipid accumulation in adipocytes through enhanced NCoA1–PPARγ interaction. J. Infect. Public Health 2019, 12, 726–732. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, H.; Tong, L.; Fang, Q.; Xiang, M.; Han, L.; Jin, L.; Yang, J.; Qian, Z.; Ning, G.; et al. Betulinic acid improves nonalcoholic fatty liver disease through YY1/FAS signaling pathway. FASEB J. 2020, 34, 13033–13048. [Google Scholar] [CrossRef] [PubMed]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Sousa, P.L.; da Silva Souza, R.O.; Tessarolo, L.D.; de Menezes, R.R.P.P.B.; Sampaio, T.L.; Canuto, J.A.; Martins, A.M.C. Betulinic acid induces cell death by necrosis in Trypanosoma cruzi. Acta Trop. 2017, 174, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, C.; Liu, J.; Chen, J.; Huang, C.; Wang, J.; Liang, Z.; Wen, L.; Yi, J.; Yuan, Z. Betulinic Acid Attenuates T-2-Toxin-Induced Testis Oxidative Damage Through Regulation of the JAK2/STAT3 Signaling Pathway in Mice. Biomolecules 2019, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yi, X.; Ma, C.; Luo, C.; Kong, L.; Lin, X.; Gao, X.; Yuan, Z.; Wen, L.; Li, R.; et al. Betulinic Acid Attenuates Oxidative Stress in the Thymus Induced by Acute Exposure to T-2 Toxin via Regulation of the MAPK/Nrf2 Signaling Pathway. Toxins 2020, 12, 540. [Google Scholar] [CrossRef]
- Kong, L.; Zhu, L.; Yi, X.; Huang, Y.; Zhao, H.; Chen, Y.; Yuan, Z.; Wen, L.; Wu, J.; Yi, J. Betulinic Acid Alleviates Spleen Oxidative Damage Induced by Acute Intraperitoneal Exposure to T-2 Toxin by Activating Nrf2 and Inhibiting MAPK Signaling Pathways. Antioxidants 2021, 10, 158. [Google Scholar] [CrossRef]
- Luo, C.; Huang, C.; Zhu, L.; Kong, L.; Yuan, Z.; Wen, L.; Li, R.; Wu, J.; Yi, J. Betulinic Acid Ameliorates the T-2 Toxin-Triggered Intestinal Impairment in Mice by Inhibiting Inflammation and Mucosal Barrier Dysfunction through the NF-κB Signaling Pathway. Toxins 2020, 12, 794. [Google Scholar] [CrossRef]
- Ou, Z.; Zhu, L.; Huang, C.; Ma, C.; Kong, L.; Lin, X.; Gao, X.; Huang, L.; Wen, L.; Liang, Z.; et al. Betulinic acid attenuates cyclophosphamide-induced intestinal mucosa injury by inhibiting the NF-κB/MAPK signalling pathways and activating the Nrf2 signalling pathway. Ecotoxicol. Environ. Saf. 2021, 225, 112746. [Google Scholar] [CrossRef]
- Biswas, R.; Chanda, J.; Kar, A.; Mukherjee, P.K. Tyrosinase inhibitory mechanism of betulinic acid from Dillenia indica. Food Chem. 2017, 232, 689–696. [Google Scholar] [CrossRef]
- Kaundal, M.; Deshmukh, R.; Akhtar, M. Protective effect of betulinic acid against intracerebroventricular streptozotocin induced cognitive impairment and neuronal damage in rats: Possible neurotransmitters and neuroinflammatory mechanism. Pharmacol. Rep. 2018, 70, 540–548. [Google Scholar] [CrossRef]
- Sutariya, B.; Taneja, N.; Saraf, M. Betulinic acid, isolated from the leaves of Syzygium cumini (L.) Skeels, ameliorates the proteinuria in experimental membranous nephropathy through regulating Nrf2/NF-κB pathways. Chem. Biol. Interact. 2017, 274, 124–137. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, R.; Lingaraju, M.C.; Kumar, D.; Mathesh, K.; Telang, A.G.; Singh, T.U.; Kumar, D. Betulinic acid attenuates renal fibrosis in rat chronic kidney disease model. Biomed. Pharmacother. 2017, 89, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bao, G.; ALyafeai, E.; Ding, J.; Li, S.; Sheng, S.; Shen, Z.; Jia, Z.; Lin, C.; Zhang, C.; et al. Betulinic Acid Enhances the Viability of Random-Pattern Skin Flaps by Activating Autophagy. Front. Pharmacol. 2019, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Zhao, M.; Cai, X.; Wang, Y.; Zhang, L.; Yao, H.; Liu, M.; Yang, H.; Xu, M.; Li, H.; et al. Betulinic Acid Inhibits Endometriosis Through Suppression of Estrogen Receptor β Signaling Pathway. Front. Endocrinol. 2020, 11, 604648. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ali, I.; Wang, D.; Hakkim, F.L.; Westermann, B.; Rashan, L.; Ahmed, I.; Green, I.R. Boswellic acids: Privileged structures to develop lead compounds for anticancer drug discovery. Expert Opin. Drug Discov. 2021, 16, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, J.; Sun, Y.; Liang, Y.; Zhao, J.; Zou, H.; Zhang, T.; Ren, L. GR-mediated anti-inflammation of α-boswellic acid: Insights from in vitro and in silico studies. Food Chem. Toxicol. 2021, 155, 112379. [Google Scholar] [CrossRef]
- Khan, A.; Khan, I.; Halim, S.A.; Rehman, N.U.; Karim, N.; Ahmad, W.; Khan, M.; Csuk, R.; Al-Harrasi, A. Anti-Diabetic potential of β-boswellic acid and 11-keto-β-boswellic acid: Mechanistic insights from computational and biochemical approaches. Biomed. Pharmacother. 2022, 147, 112669. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Pengzong, Z.; Yuanmin, L.; Xiaoming, X.; Shang, D.; Wei, X.; Zhigang, L.; Dongzhou, D.; Wenjing, Y.; Jianbiao, Y.; Yang, X.; et al. Wound Healing Potential of the Standardized Extract of Boswellia serrata on Experimental Diabetic Foot Ulcer via Inhibition of Inflammatory, Angiogenetic and Apoptotic Markers. Planta Med. 2019, 85, 657–669. [Google Scholar] [CrossRef]
- Staton, C.A.; Valluru, M.; Hoh, L.; Reed, M.W.R.; Brown, N.J. Angiopoietin-1, angiopoietin-2 and Tie-2 receptor expression in human dermal wound repair and scarring. Br. J. Dermatol. 2010, 163, 920–927. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Bir, S.C.; Kevil, C.G. Endothelial Dysfunction and Diabetes: Effects on Angiogenesis, Vascular Remodeling, and Wound Healing. Int. J. Vasc. Med. 2012, 2012, 918267. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Das Mahapatra, A.; Banerjee, S.; Kar, A.; Ojha, D.; Mukherjee, P.K.; Chattopadhyay, D. Boswellia serrata oleo-gum-resin and β-boswellic acid inhibits HSV-1 infection in vitro through modulation of NF-κB and p38 MAP kinase signaling. Phytomedicine 2018, 51, 94–103. [Google Scholar] [CrossRef]
- Santoro, M.G. NF-κB and virus infection: Who controls whom. EMBO J. 2003, 22, 2552–2560. [Google Scholar] [CrossRef]
- McLean, T.I.; Bachenheimer, S.L. Activation of cJUN N-Terminal Kinase by Herpes Simplex Virus Type 1 Enhances Viral Replication. J. Virol. 1999, 73, 8415–8426. [Google Scholar] [CrossRef] [PubMed]
- Marefati, N.; Khamse, S.; Mansouri, S.; Hosseini, M.; Anaeigoudari, A. Effects of Boswellia serrata resin on central nervous system: A mini review. Physiol. Pharmacol. 2021, 25, 288–295. [Google Scholar] [CrossRef]
- Shasaltaneh, M.D.; Naghdi, N.; Ramezani, S.; Alizadeh, L.; Riazi, G.H. Protection of Beta Boswellic Acid against Streptozotocin-induced Alzheimer’s Model by Reduction of Tau Phosphorylation Level and Enhancement of Reelin Expression. Planta Med. 2022, 88, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Lane-Donovan, C.; Herz, J. The ApoE receptors Vldlr and Apoer2 in central nervous system function and disease. J. Lipid Res. 2017, 58, 1036–1043. [Google Scholar] [CrossRef]
- Förster, E.; Bock, H.H.; Herz, J.; Chai, X.; Frotscher, M.; Zhao, S. Emerging topics in Reelin function. Eur. J. Neurosci. 2010, 31, 1511–1518. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ebrahimpour, S.; Fazeli, M.; Mehri, S.; Taherianfard, M. Boswellic Acid Improves Cognitive Function in a Rat Model Through Its Antioxidant Activity. J. Pharmacopuncture 2017, 20, 10–17. [Google Scholar] [CrossRef]
- Afzal, M.; Alzarea, S.I.; Mohsin Qua, A.; Kazmi, I.; Zafar, A.; Imam, F.; Al-Harb, N.O.; Saad Alhar, K.; Alruwaili, N.K. Boswellic Acid Attenuates Scopolamine-Induced Neurotoxicity and Dementia in Rats: Possible Mechanism of Action. Int. J. Pharmacol. 2021, 17, 499–505. [Google Scholar] [CrossRef]
- Yahyapour, R.; Amini, P.; Rezapour, S.; Cheki, M.; Rezaeyan, A.; Farhood, B.; Shabeeb, D.; Musa, A.E.; Fallah, H.; Najafi, M. Radiation-Induced inflammation and autoimmune diseases. Mil. Med. Res. 2018, 5, 9. [Google Scholar] [CrossRef]
- Thabet, N.M.; Abdel-Rafei, M.K.; Moustafa, E.M. Boswellic acid protects against Bisphenol-A and gamma radiation induced hepatic steatosis and cardiac remodelling in rats: Role of hepatic PPAR-α/P38 and cardiac Calcineurin-A/NFATc1/P38 pathways. Arch. Physiol. Biochem. 2022, 128, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, J.; Yan, W.; Zhou, K.; Cao, Y.; Cai, W. p38α MAPK antagonizing JNK to control the hepatic fat accumulation in pediatric patients onset intestinal failure. Cell Death Dis. 2017, 8, e3110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, H.; An, Y.; Shen, K.; Yu, L. Biological effects of corosolic acid as an anti-inflammatory, anti-metabolic syndrome and anti-neoplasic natural compound (Review). Oncol. Lett. 2020, 21, 84. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, J.; Lee, M.; Cha, E.; Kim, S.; Sul, J. Corosolic acid reduces 5-FU chemoresistance in human gastric cancer cells by activating AMPK. Mol. Med. Rep. 2018, 18, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.-L.; Li, H.-L.; Li, Y.-C.; Liu, Z.-W.; Guo, X.-H.; Cheng, Y.-J. CRA (Crosolic Acid) isolated from Actinidia valvata Dunn.Radix induces apoptosis of human gastric cancer cell line BGC823 in vitro via down-regulation of the NF-κB pathway. Food Chem. Toxicol. 2017, 105, 475–485. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Chen, Y.; Qiao, X.; Zhao, S.; Li, P.; Liu, J.; Wen, X.; Yang, J. Corosolic acid inhibits colorectal cancer cells growth as a novel HER2/HER3 heterodimerization inhibitor. Br. J. Pharmacol. 2021, 178, 1475–1491. [Google Scholar] [CrossRef] [PubMed]
- Gespach, C. Increasing Potential of HER3 Signaling in Colon Cancer Progression and Therapy. Clin. Cancer Res. 2012, 18, 917–919. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, Y.; Wang, Z.; Xu, X.; Li, Y.; Ma, L.; Wang, J.; Yu, Y. Corosolic acid inhibits cancer progression by decreasing the level of CDK19-mediated O-GlcNAcylation in liver cancer cells. Cell Death Dis. 2021, 12, 889. [Google Scholar] [CrossRef]
- Jia, M.; Xiong, Y.; Li, M.; Mao, Q. Corosolic Acid Inhibits Cancer Progress through Inactivating YAP in Hepatocellular Carcinoma. Oncol. Res. 2020, 28, 371–383. [Google Scholar] [CrossRef]
- Woo, S.; Seo, S.; Min, K.; Im, S.-S.; Nam, J.-O.; Chang, J.-S.; Kim, S.; Park, J.-W.; Kwon, T. Corosolic Acid Induces Non-Apoptotic Cell Death through Generation of Lipid Reactive Oxygen Species Production in Human Renal Carcinoma Caki Cells. Int. J. Mol. Sci. 2018, 19, 1309. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, H.; Wang, Y.; Zhao, A.; Zhu, Z.; Bao, X.; Sun, Y.; Li, L.; Zhang, Q. Corosolic acid, a natural triterpenoid, induces ER stress-dependent apoptosis in human castration resistant prostate cancer cells via activation of IRE-1/JNK, PERK/CHOP and TRIB3. J. Exp. Clin. Cancer Res. 2018, 37, 210. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-W.; Kao, S.-H.; Yang, S.-F.; Jhang, S.-W.; Lin, Y.-C.; Chen, C.-M.; Hsieh, Y.-H. Corosolic Acid Attenuates the Invasiveness of Glioblastoma Cells by Promoting CHIP-Mediated AXL Degradation and Inhibiting GAS6/AXL/JAK Axis. Cells 2021, 10, 2919. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, X.; Yao, Y.; Yang, M.; Zhou, F.; Zhu, L. Corosolic acid induces cell cycle arrest and cell apoptosis in human retinoblastoma Y-79 cells via disruption of MELK-FoxM1 signaling. Oncol. Rep. 2018, 39, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef]
- Peng, C.; Zhu, Y.; Zhang, W.; Liao, Q.; Chen, Y.; Zhao, X.; Guo, Q.; Shen, P.; Zhen, B.; Qian, X.; et al. Regulation of the Hippo-YAP Pathway by Glucose Sensor O-GlcNAcylation. Mol. Cell 2017, 68, 591–604.e5. [Google Scholar] [CrossRef]
- Trachoomtham, D.; Alexandre, J.; Huang, P.Q. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.; Varol, A.; Thakral, F.; Yerer, M.; Sak, K.; Varol, M.; Jain, A.; Khan, M.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef]
- Nakada, M.; Kita, D.; Teng, L.; Pyko, I.V.; Watanabe, T.; Hayashi, Y.; Hamada, J. Receptor Tyrosine Kinases: Principles and Functions in Glioma Invasion. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; pp. 151–178. [Google Scholar]
- Hutterer, M.; Knyazev, P.; Abate, A.; Reschke, M.; Maier, H.; Stefanova, N.; Knyazeva, T.; Barbieri, V.; Reindl, M.; Muigg, A.; et al. Axl and Growth Arrest–Specific Gene 6 Are Frequently Overexpressed in Human Gliomas and Predict Poor Prognosis in Patients with Glioblastoma Multiforme. Clin. Cancer Res. 2008, 14, 130–138. [Google Scholar] [CrossRef]
- Koo, C.-Y.; Muir, K.W.; Lam, E.W.-F. FOXM1: From cancer initiation to progression and treatment. Biochim. Biophys. Acta—Gene Regul. Mech. 2012, 1819, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, Y.; Wang, C.; Sun, Z.; Li, L.; Cheng, S.; Zhou, W. Overexpression of PCK1 Gene Antagonizes Hepatocellular Carcinoma Through the Activation of Gluconeogenesis and Suppression of Glycolysis Pathways. Cell. Physiol. Biochem. 2018, 47, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, G.; Peng, W.; Xu, Y.; Zhang, Y.; Ge, Y.; Jing, Y.; Gong, Z. Corosolic acid isolated from Eriobotrya japonica leaves reduces glucose level in human hepatocellular carcinoma cells, zebrafish and rats. Sci. Rep. 2019, 9, 4388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-P.; Che, Y.; Zhou, H.; Meng, Y.-Y.; Wu, H.-M.; Jin, Y.-G.; Wu, Q.-Q.; Wang, S.-S.; Yuan, Y. Corosolic acid inhibits adipose tissue inflammation and ameliorates insulin resistance via AMPK activation in high-fat fed mice. Phytomedicine 2016, 23, 181–190. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Zimmermann, K.; Baldinger, J.; Mayerhofer, B.; Atanasov, A.G.; Dirsch, V.M.; Heiss, E.H. Activated AMPK boosts the Nrf2/HO-1 signaling axis—A role for the unfolded protein response. Free Radic. Biol. Med. 2015, 88, 417–426. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Che, Y.; Zhou, H.; Meng, Y.-Y.; Wu, H.-M.; Jin, Y.-G.; Wu, Q.-Q.; Wang, S.-S.; Yuan, Y. Corosolic acid attenuates cardiac fibrosis following myocardial infarction in mice. Int. J. Mol. Med. 2020, 45, 1425–1435. [Google Scholar] [CrossRef]
- Che, Y.; Wang, Z.; Yuan, Y.; Zhou, H.; Wu, H.; Wang, S.; Tang, Q. By restoring autophagic flux and improving mitochondrial function, corosolic acid protects against Dox-induced cardiotoxicity. Cell Biol. Toxicol. 2022, 38, 451–467. [Google Scholar] [CrossRef]
- Xu, G.; Huang, K.; Zhou, J. Hepatic AMP Kinase as a Potential Target for Treating Nonalcoholic Fatty Liver Disease: Evidence from Studies of Natural Products. Curr. Med. Chem. 2018, 25, 889–907. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Feng, W.-J.; Liu, G.-C.; Ma, Q.-Q.; Li, H.-L.; Gao, X.-Y.; Liu, H.-Z.; Piao, G.-C.; Yuan, H.-D. Corosolic Acid Attenuates Hepatic Lipid Accumulation and Inflammatory Response via AMPK/SREBPs and NF-κB/MAPK Signaling Pathways. Am. J. Chin. Med. 2020, 48, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Cha, J.-Y.; Kang, H.S.; Lee, J.-H.; Lee, J.Y.; Park, J.-H.; Bae, J.-H.; Song, D.-K.; Im, S.-S. Corosolic acid ameliorates acute inflammation through inhibition of IRAK-1 phosphorylation in macrophages. BMB Rep. 2016, 49, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ali, I.; Wang, D.; Hakkim, F.L.; Westermann, B.; Ahmed, I.; Ashour, A.M.; Khan, A.; Hussain, A.; Green, I.R.; et al. Glycyrrhetinic acid: A promising scaffold for the discovery of anticancer agents. Expert Opin. Drug Discov. 2021, 16, 1497–1516. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Shamraiz, U.; Saleem, M.; Badshah, A.; Abbas, G.; Rehman, N.U.; Irshad, M. Therapeutic potential of glycyrrhetinic acids: A patent review (2010–2017). Expert Opin. Ther. Pat. 2018, 28, 383–398. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Song, J.; Liu, Q.; Wang, C.; Huang, Z.; Chu, L.; Liang, H.; Zhang, B.; Chen, X. 18β-Glycyrrhetinic-Acid-Mediated unfolded protein response induces autophagy and apoptosis in hepatocellular carcinoma. Sci. Rep. 2018, 8, 9365. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Chen, X.; Zhang, J.; Wang, J. 18β-Glycyrrhetinic acid inhibits migration and invasion of human gastric cancer cells via the ROS/PKC-α/ERK pathway. J. Nat. Med. 2018, 72, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, M.; Park, P.-H.; Song, K. Transcriptional suppression of androgen receptor by 18β-glycyrrhetinic acid in LNCaP human prostate cancer cells. Arch. Pharm. Res. 2020, 43, 433–448. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.; Qiu, R.; Chen, Z.; Chen, Z.; Chen, W. 18β-Glycyrrhetinic acid exhibits potent antitumor effects against colorectal cancer via inhibition of cell proliferation and migration. Int. J. Oncol. 2017, 51, 615–624. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Wang, C.; Xu, W.-T.; Zhang, Y.; Zhang, T.; Xue, H.; Li, Y.-N.; Fu, Z.-R.; Wang, Y.; Jin, C.-H. 18β-Glycyrrhetinic Acid Has Anti-Cancer Effects via Inducing Apoptosis and G2/M Cell Cycle Arrest, and Inhibiting Migration of A549 Lung Cancer Cells. Onco. Targets. Ther. 2021, 14, 5131–5144. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, M.; Huang, K.; Wu, Y.; Ma, Y.; Wang, J.; Ma, J.; Fan, S. Blocking autophagy enhances the apoptotic effect of 18β-glycyrrhetinic acid on human sarcoma cells via endoplasmic reticulum stress and JNK activation. Cell Death Dis. 2017, 8, e3055. [Google Scholar] [CrossRef]
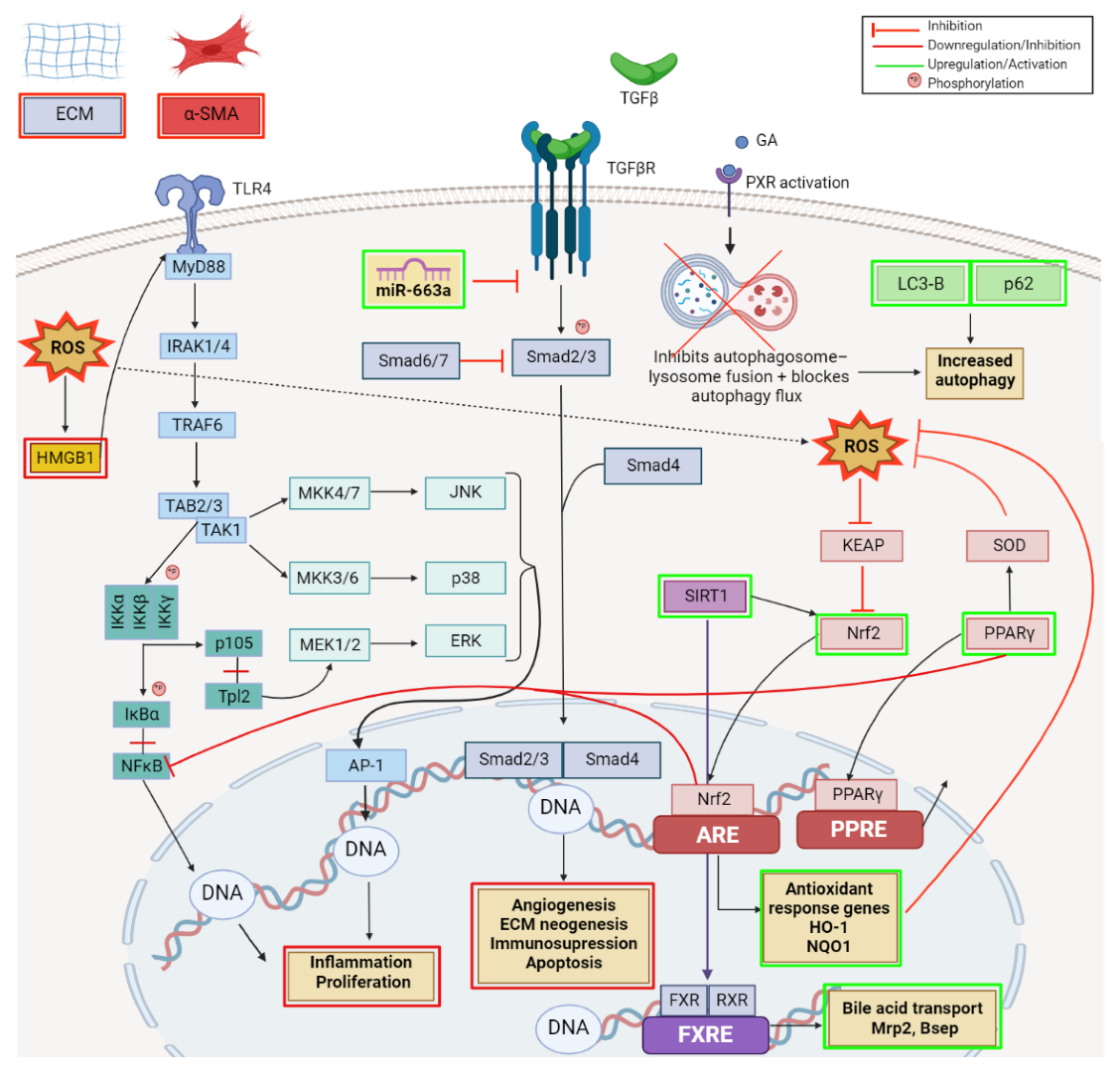
- Guo, X.-X.; Yang, W.-N.; Dong, B.-S.; Shang, J.-W.; Su, S.-B.; Yan, X.-L.; Zhang, H. Glycyrrhetinic Acid -Induced MiR-663a Alleviates Hepatic Stellate Cell Activation by Attenuating the TGF-β/Smad Signaling Pathway. Evid.-Based Complement. Altern. Med. 2020, 2020, 3156267. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, Y.; Ma, T.-T.; Huang, C.; Zhang, L.; Li, J. Participation of miR-200a in TGF-β1-mediated hepatic stellate cell activation. Mol. Cell. Biochem. 2014, 388, 11–23. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, B.; Jiao, A.; Li, F.; Sun, N.; Zhang, G.; Zhang, J. miR-663a inhibits tumor growth and invasion by regulating TGF-β1 in hepatocellular carcinoma. BMC Cancer 2018, 18, 1179. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, H.; Wang, W.; Song, L.; Liu, M.; Cao, Y.; Qi, X.; Sun, J.; Gong, L. Prevention of D-GalN/LPS-induced ALI by 18β-glycyrrhetinic acid through PXR-mediated inhibition of autophagy degradation. Cell Death Dis. 2021, 12, 480. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, L.; Ma, L.; Jiang, R.; Kuang, G.; Li, K.; Tie, H.; Wang, B.; Chen, X.; Xie, T.; et al. Glycyrrhetinic acid prevents acetaminophen-induced acute liver injury via the inhibition of CYP2E1 expression and HMGB1-TLR4 signal activation in mice. Int. Immunopharmacol. 2017, 50, 186–193. [Google Scholar] [CrossRef]
- Jiang, X.; Kuang, G.; Gong, X.; Jiang, R.; Xie, T.; Tie, H.; Wu, S.; Wang, T.; Wan, J.; Wang, B. Glycyrrhetinic acid pretreatment attenuates liver ischemia/reperfusion injury via inhibiting TLR4 signaling cascade in mice. Int. Immunopharmacol. 2019, 76, 105870. [Google Scholar] [CrossRef]
- Shi, X.; Yu, L.; Zhang, Y.; Liu, Z.; Zhang, H.; Zhang, Y.; Liu, P.; Du, P. Glycyrrhetinic acid alleviates hepatic inflammation injury in viral hepatitis disease via a HMGB1-TLR4 signaling pathway. Int. Immunopharmacol. 2020, 84, 106578. [Google Scholar] [CrossRef]
- Wang, H.; Fang, Z.-Z.; Meng, R.; Cao, Y.-F.; Tanaka, N.; Krausz, K.W.; Gonzalez, F.J. Glycyrrhizin and glycyrrhetinic acid inhibits alpha-naphthyl isothiocyanate-induced liver injury and bile acid cycle disruption. Toxicology 2017, 386, 133–142. [Google Scholar] [CrossRef]
- Wu, S.; Cui, S.; Wang, L.; Zhang, Y.; Yan, X.; Lu, H.; Xing, G.; Ren, J.; Gong, L. 18β-Glycyrrhetinic acid protects against alpha-naphthylisothiocyanate-induced cholestasis through activation of the Sirt1/FXR signaling pathway. Acta Pharmacol. Sin. 2018, 39, 1865–1873. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, B.-C.; Ma, Y.-S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Bellussi, L.M.; Cocca, S.; Wang, J.; Passali, G.C.; Hao, X.; Chen, L.; Passali, D. Glycyrrhetinic acid suppressed hmgb1 release by up-regulation of Sirt6 in nasal inflammation. J. Biol. Regul. Homeost. Agents 2017, 31, 269–277. [Google Scholar] [PubMed]
- Su, L.; Wang, Z.; Huang, F.; Lan, R.; Chen, X.; Han, D.; Zhang, L.; Zhang, W.; Hong, J. 18β-Glycyrrhetinic acid mitigates radiation-induced skin damage via NADPH oxidase/ROS/p38MAPK and NF-κB pathways. Environ. Toxicol. Pharmacol. 2018, 60, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-X.; Wink, M. Evidence for Anti-Inflammatory Activity of Isoliquiritigenin, 18β Glycyrrhetinic Acid, Ursolic Acid, and the Traditional Chinese Medicine Plants Glycyrrhiza glabra and Eriobotrya japonica, at the Molecular Level. Medicines 2019, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Mei, L.; Wang, M.; Huang, Q.; Huang, R. Anti-Inflammatory and Pro-apoptotic Effects of 18beta-Glycyrrhetinic Acid In Vitro and In Vivo Models of Rheumatoid Arthritis. Front. Pharmacol. 2021, 12, 681525. [Google Scholar] [CrossRef]
- Zhang, T.; Liao, J.-Y.; Yu, L.; Liu, G.-S. Regulating effect of glycyrrhetinic acid on bronchial asthma smooth muscle proliferation and apoptosis as well as inflammatory factor expression through ERK1/2 signaling pathway. Asian Pac. J. Trop. Med. 2017, 10, 1172–1176. [Google Scholar] [CrossRef]
- Rao, C.; Hong, Z.; Yao, Y.; Zheng, G.; Wang, S. 18-β-glycyrrhetinic Acid Protects against Staphylococcus aureus Infection by Regulating the NF-κB Pathway. Indian J. Pharm. Educ. Res. 2019, 53, s151–s158. [Google Scholar] [CrossRef]
- Wang, X.; Xie, F.; Zhou, X.; Chen, T.; Xue, Y.; Wang, W. 18β-Glycyrrhetinic acid inhibits the apoptosis of cells infected with rotavirus SA11 via the Fas/FasL pathway. Pharm. Biol. 2021, 59, 1096–1103. [Google Scholar] [CrossRef]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Zhang, M.; Wang, Z.; He, X.; Hou, Y.; Bai, G. Glycyrrhetinic Acid Improves Insulin-Response Pathway by Regulating the Balance between the Ras/MAPK and PI3K/Akt Pathways. Nutrients 2019, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tung, Y.; Chen, S.; Yen, G. Novel findings of 18β-glycyrrhetinic acid on sRAGE secretion through inhibition of transient receptor potential canonical channels in high-glucose environment. BioFactors 2019, 45, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gu, M.; Xu, H. Lysosomal Ion Channels as Decoders of Cellular Signals. Trends Biochem. Sci. 2019, 44, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. ROS Promotes Cancer Cell Survival through Calcium Signaling. Cancer Cell 2018, 33, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.M.; Yan, S.D.; Yan, S.F.; Schmidt, A.M. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res. Rev. 2002, 1, 1–15. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, L.; Xia, P.; Zhao, J.; Li, W.; Zhou, Y.; Wei, Q.; Wu, Q.; Wu, Q.; Sun, D.; et al. Glycyrrhetinic Acid Protects Renal Tubular Cells against Oxidative Injury via Reciprocal Regulation of JNK-Connexin 43-Thioredoxin 1 Signaling. Front. Pharmacol. 2021, 12, 619567. [Google Scholar] [CrossRef] [PubMed]
- Prakoura, N.; Kavvadas, P.; Chadjichristos, C.E. Connexin 43: A New Therapeutic Target Against Chronic Kidney Disease. Cell. Physiol. Biochem. 2018, 49, 998–1009. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Wan, H.; Jin, W.; Yu, L.; Zhou, H.; Yang, J. Glycyrrhetinic acid protects H9c2 cells from oxygen glucose deprivation-induced injury through the PI3K/AKt signaling pathway. J. Nat. Med. 2017, 71, 27–35. [Google Scholar] [CrossRef]
- Chu, S.; Wang, W.; Zhang, N.; Liu, T.; Li, J.; Chu, X.; Zuo, S.; Ma, Z.; Ma, D.; Chu, L. Protective effects of 18β-Glycyrrhetinic acid against myocardial infarction: Involvement of PI3K/Akt pathway activation and inhibiting Ca2+ influx via L-type Ca2+ channels. Food Sci. Nutr. 2021, 9, 6831–6843. [Google Scholar] [CrossRef]
- Han, J.; Su, G.; Wang, Y.; Lu, Y.; Zhao, H.; Shuai, X. 18β-Glycyrrhetinic Acid Improves Cardiac Diastolic Function by Attenuating Intracellular Calcium Overload. Curr. Med. Sci. 2020, 40, 654–661. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Zhou, D.; Li, B.; Chen, G.; Li, N. Glycyrrhetinic acid reverses antibiotic-induced intestinal epithelial injury through RNA-binding protein human antigen R (HuR). Phytomedicine 2022, 94, 153836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, M.; Huo, T.; Zhang, Y.; Fang, Y.; Feng, C.; Wang, S.; Jiang, H. Effects of Glycyrrhetinic Acid on GSH Synthesis Induced by Realgar in the Mouse Hippocampus: Involvement of System X (AG)−, System X(C)−, MRP-1, and Nrf2. Mol. Neurobiol. 2017, 54, 3102–3116. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Y.; Cao, Y.; Shi, Z.; Lin, Y.; Chen, Y.; Zhao, H.; Liu, X. Glycyrrhetinic acid alleviates acute lung injury by PI3K/AKT suppressing macrophagic Nlrp3 inflammasome activation. Biochem. Biophys. Res. Commun. 2020, 532, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.; Bhattamisra, S.K.; Chellappan, D.K.; Candasamy, M. Actions and Therapeutic Potential of Madecassoside and Other Major Constituents of Centella asiatica: A Review. Appl. Sci. 2021, 11, 8475. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Wei, Z.; Wei, W.; Zhao, P.; Tong, B.; Xia, Y.; Dai, Y. Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPARγ/AMPK/ACC1 pathway. Cell Death Dis. 2017, 8, e2723. [Google Scholar] [CrossRef]
- Zhang, H.L.; Zheng, Y.J.; Pan, Y.D.; Xie, C.; Sun, H.; Zhang, Y.H.; Yuan, M.Y.; Song, B.L.; Chen, J.F. Regulatory T-cell depletion in the gut caused by integrin β7 deficiency exacerbates DSS colitis by evoking aberrant innate immunity. Mucosal Immunol. 2016, 9, 391–400. [Google Scholar] [CrossRef]
- Endo, Y.; Asou, H.K.; Matsugae, N.; Hirahara, K.; Shinoda, K.; Tumes, D.J.; Tokuyama, H.; Yokote, K.; Nakayama, T. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep. 2015, 12, 1042–1055. [Google Scholar] [CrossRef]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic Acid, a Triterpene from Olive, Affects the Antioxidant and Mitochondrial Status of B16F10 Melanoma Cells Grown under Stressful Conditions. Evid.-Based Complement. Altern. Med. 2015, 2015, 272457. [Google Scholar] [CrossRef]
- Fuentes-Rios, D.; Cepero, A.; García-Castro, M.; Contreras-Cáceres, R.; López-Romero, J.M.; Luque, C.; Cabeza, L.; Melguizo, C.; Prados, J. Synthesis, solubility and antitumor activity of maslinic acid derivatives. Eur. J. Med. Chem. Rep. 2022, 4, 100032. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, X.; Yin, Y. Maslinic Acid Enhances Docetaxel Response in Human Docetaxel-Resistant Triple Negative Breast Carcinoma MDA-MB-231 Cells via Regulating MELK-FoxM1-ABCB1 Signaling Cascade. Front. Pharmacol. 2020, 11, 835. [Google Scholar] [CrossRef]
- Lu, K.-W.; Yang, M.-D.; Peng, S.-F.; Chen, J.-C.; Chen, P.-Y.; Chen, H.-Y.; Lu, T.-J.; Chueh, F.-S.; Lien, J.-C.; Lai, K.-C.; et al. Maslinic Acid Induces DNA Damage and Impairs DNA Repair in Human Cervical Cancer HeLa Cells. Anticancer Res. 2020, 40, 6869–6877. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.; Subramaniam, M.; Fong, L.; Goh, H.; Khoo, S.A.; Lim, Y. Apoptotic and cytostatic actions of maslinic acid in colorectal cancer cells through possible IKK-β inhibition. Asian Pac. J. Trop. Biomed. 2021, 11, 122. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, B.; Li, P.; Wen, X.; Yang, J. Maslinic Acid Inhibits Colon Tumorigenesis by the AMPK–mTOR Signaling Pathway. J. Agric. Food Chem. 2019, 67, 4259–4272. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tang, S.; Zhang, Q. Maslinic acid suppresses the growth of human gastric cells by inducing apoptosis via inhibition of the interleukin-6 mediated Janus kinase/signal transducer and activator of transcription 3 signaling pathway. Oncol. Lett. 2017, 13, 4875–4881. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, H.; Dong, Q.; Hao, X.; Qiao, L. Maslinic acid induces anticancer effects in human neuroblastoma cells mediated via apoptosis induction and caspase activation, inhibition of cell migration and invasion and targeting MAPK/ERK signaling pathway. AMB Express 2020, 10, 104. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, H.; Farooq, A.A.; Nie, B.; Chen, X.; Su, S.; Yuan, R.; Qiao, G.; Li, C.; Li, X.; et al. Maslinic acid induces autophagy by down-regulating HSPA8 in pancreatic cancer cells. Phyther. Res. 2018, 32, 1320–1331. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, L.; Zhang, Y.; Wang, C.; Sun, L. Transcriptome and proteome analysis of the antitumor activity of maslinic acid against pancreatic cancer cells. Aging 2021, 13, 23308–23327. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, J.; Zhou, Z.; Ye, Z.; Chen, J.; Yuan, J.; Cao, F.; Wang, X.; Liu, W.; Yu, W.; et al. Maslinic acid promotes autophagy by disrupting the interaction between Bcl2 and Beclin1 in rat pheochromocytoma PC12 cells. Oncotarget 2017, 8, 74527–74538. [Google Scholar] [CrossRef]
- Shimazu, K.; Fukumitsu, S.; Ishijima, T.; Toyoda, T.; Nakai, Y.; Abe, K.; Aida, K.; Okada, S.; Hino, A. The Anti-Arthritis Effect of Olive-Derived Maslinic Acid in Mice is Due to its Promotion of Tissue Formation and its Anti-Inflammatory Effects. Mol. Nutr. Food Res. 2019, 63, 1800543. [Google Scholar] [CrossRef]
- Mehu, M.; Narasimhulu, C.A.; Singla, D.K. Inflammatory Cells in Atherosclerosis. Antioxidants 2022, 11, 233. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Phang, S.W.; Ooi, B.K.; Ahemad, N.; Yap, W.H. Maslinic acid suppresses macrophage foam cells formation: Regulation of monocyte recruitment and macrophage lipids homeostasis. Vascul. Pharmacol. 2020, 128–129, 106675. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-Q.; Li, J.; Li, X.-W.; Li, Y.-C.; Li, J.; Lin, J.-F. Effect of miR-21/TLR4/NF-κB pathway on myocardial apoptosis in rats with myocardial ischemia-reperfusion. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7928–7937. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Regulation of the Inflammatory Response in Cardiac Repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, M.; Li, Z.; Li, T.; Wang, Y.; Chen, Q.; Wang, Y.; Feng, J.; Yin, X.; Lu, C. Maslinic Acid Attenuates Ischemia/Reperfusion Injury-Induced Myocardial Inflammation and Apoptosis by Regulating HMGB1-TLR4 Axis. Front. Cardiovasc. Med. 2021, 8, 768947. [Google Scholar] [CrossRef]
- Ampofo, E.; Berg, J.J.; Menger, M.D.; Laschke, M.W. Maslinic acid alleviates ischemia/reperfusion-induced inflammation by downregulation of NFκB-mediated adhesion molecule expression. Sci. Rep. 2019, 9, 6119. [Google Scholar] [CrossRef]
- Mwakalukwa, R.; Amen, Y.; Nagata, M.; Shimizu, K. Postprandial Hyperglycemia Lowering Effect of the Isolated Compounds from Olive Mill Wastes—An Inhibitory Activity and Kinetics Studies on α-Glucosidase and α-Amylase Enzymes. ACS Omega 2020, 5, 20070–20079. [Google Scholar] [CrossRef]
- Bae, H.J.; Kim, J.; Kim, J.; Goo, N.; Cai, M.; Cho, K.; Jung, S.Y.; Kwon, H.; Kim, D.H.; Jang, D.S.; et al. The effect of maslinic acid on cognitive dysfunction induced by cholinergic blockade in mice. Br. J. Pharmacol. 2020, 177, 3197–3209. [Google Scholar] [CrossRef]
- Borodinova, A.A.; Salozhin, S.V. Diversity of proBDNF and mBDNF functions in the central nervous system. Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova 2016, 66, 3–23. [Google Scholar]
- Yamauchi, Y.; Ferdousi, F.; Fukumitsu, S.; Isoda, H. Maslinic Acid Attenuates Denervation-Induced Loss of Skeletal Muscle Mass and Strength. Nutrients 2021, 13, 2950. [Google Scholar] [CrossRef]
- Wang, Y.; Diao, B.; Zhong, L.; Lu, B.; Cheng, Y.; Yu, L.; Zhu, L. Maslinic acid protects against lipopolysaccharide/d -galactosamine-induced acute liver injury in mice. Microb. Pathog. 2018, 119, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.; Dai, Y.; Wang, C.; Fang, L.; Huang, W. Maslinic acid protects against obesity-induced nonalcoholic fatty liver disease in mice through regulation of the Sirt1/AMPK signaling pathway. FASEB J. 2019, 33, 11791–11803. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Byon, C.H.; Kim, D.H.; Choi, H.I.; Park, J.S.; Joo, S.Y.; Kim, I.J.; Jung, I.; Bae, E.H.; Ma, S.K.; et al. Renoprotective Effects of Maslinic Acid on Experimental Renal Fibrosis in Unilateral Ureteral Obstruction Model via Targeting MyD88. Front. Pharmacol. 2021, 12, 708575. [Google Scholar] [CrossRef] [PubMed]
- Martín-Navarro, C.M.; López-Arencibia, A.; Sifaoui, I.; Reyes-Batlle, M.; Fouque, E.; Osuna, A.; Valladares, B.; Piñero, J.E.; Héchard, Y.; Maciver, S.K.; et al. Amoebicidal Activity of Caffeine and Maslinic Acid by the Induction of Programmed Cell Death in Acanthamoeba. Antimicrob. Agents Chemother. 2017, 61, e02660-16. [Google Scholar] [CrossRef]
- Kraft, O.; Wiemann, J.; Al-Harrasi, A.; Csuk, R. En route to anti-glioblastoma active pomolic acid. Phytochem. Lett. 2019, 32, 29–32. [Google Scholar] [CrossRef]
- Estrada, O.; Alvarado-Castillo, C.; Fernandez, A.; Lopez, M.; Romero-Vecchione, E.; Vasquez, J.; Mendez, J.; Conde, D.; Cardozo, A. Pomolic Acid Isolated from the Leaves of Licania pittieri Inhibits ADP-and Epinephrine-Induced Platelet Aggregation and has Hypotensive Effect on Rats. Curr. Bioact. Compd. 2009, 5, 219–225. [Google Scholar] [CrossRef]
- Hisham Shady, N.; Youssif, K.A.; Sayed, A.M.; Belbahri, L.; Oszako, T.; Hassan, H.M.; Abdelmohsen, U.R. Sterols and Triterpenes: Antiviral Potential Supported by In-Silico Analysis. Plants 2020, 10, 41. [Google Scholar] [CrossRef]
- Yoo, K.H.; Park, J.-H.; Lee, D.K.; Fu, Y.Y.; Baek, N.I.; Chung, I.S. Pomolic acid induces apoptosis in SK-OV-3 human ovarian adenocarcinoma cells through the mitochondrial-mediated intrinsic and death receptor-induced extrinsic pathways. Oncol. Lett. 2013, 5, 386–390. [Google Scholar] [CrossRef][Green Version]
- Youn, S.H.; Lee, J.S.; Lee, M.S.; Cha, E.Y.; Thuong, P.T.; Kim, J.R.; Chang, E.S. Anticancer Properties of Pomolic Acid-Induced AMP-Activated Protein Kinase Activation in MCF7 Human Breast Cancer Cells. Biol. Pharm. Bull. 2012, 35, 105–110. [Google Scholar] [CrossRef]
- Fernandes, J.; Weinlich, R.; Oliveiracastilho, R.; Coelhokaplan, M.; Amarantemendes, G.; Gattass, C. Pomolic acid triggers mitochondria-dependent apoptotic cell death in leukemia cell line. Cancer Lett. 2005, 219, 49–55. [Google Scholar] [CrossRef]
- Park, J.-H.; Cho, Y.Y.; Yoon, S.W.; Park, B. Suppression of MMP-9 and FAK expression by pomolic acid via blocking of NF-κB/ERK/mTOR signaling pathways in growth factor-stimulated human breast cancer cells. Int. J. Oncol. 2016, 49, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.P.T.P.; Da Graça Rocha, G.; De Queiroz, R.M.; De Araujo Martins, C.; Takiya, C.M.; Gattass, C.R. Pomolic acid induces apoptosis and inhibits multidrug resistance protein MRP1 and migration in glioblastoma cells. Oncol. Rep. 2017, 38, 2525–2534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martins, C.D.A.; Rocha, G.D.G.; Gattass, C.R.; Takiya, C.M. Pomolic acid exhibits anticancer potential against a docetaxel-resistant PC3 prostate cell line. Oncol. Rep. 2019, 42, 328–338. [Google Scholar] [CrossRef] [PubMed]

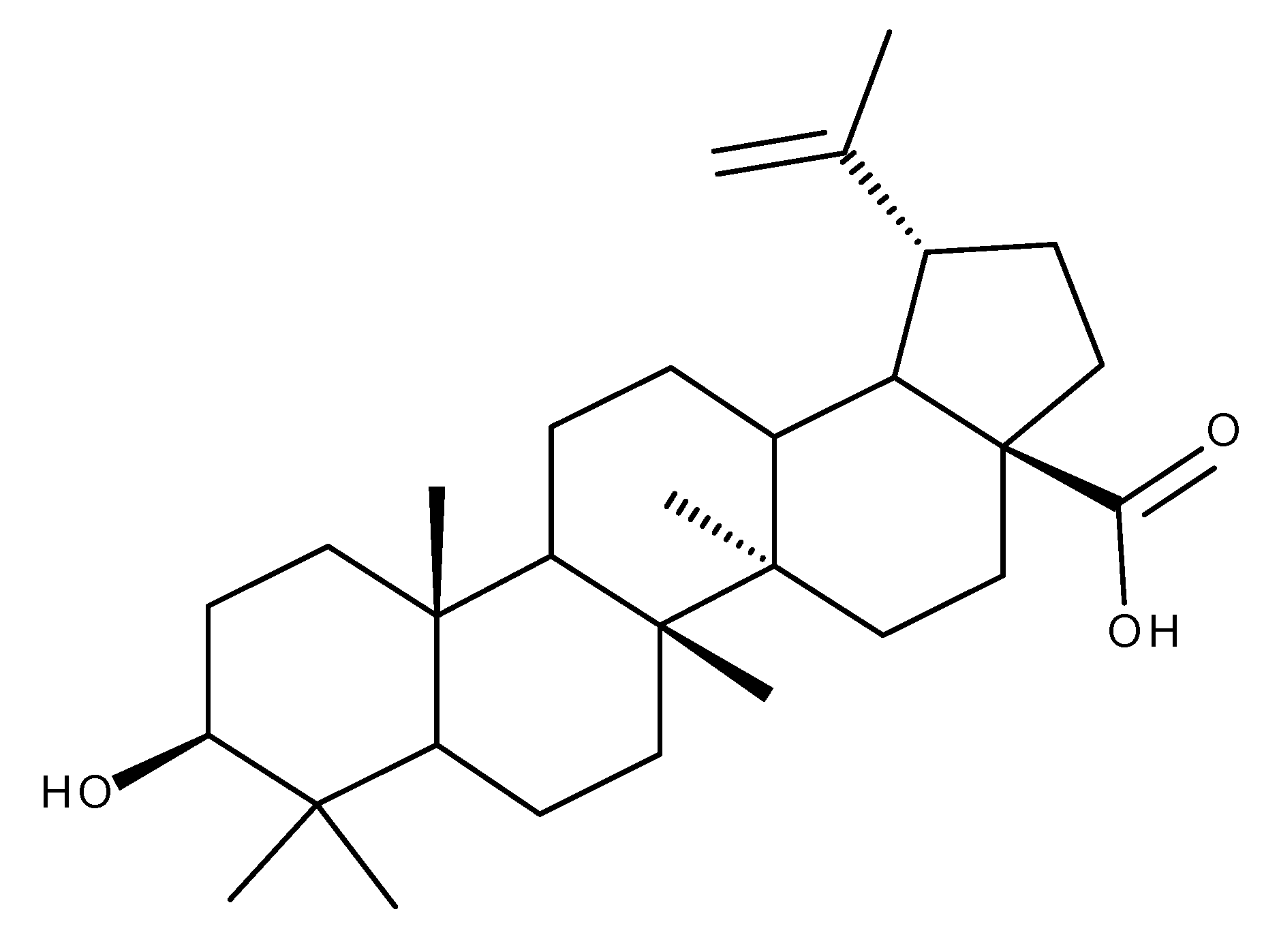
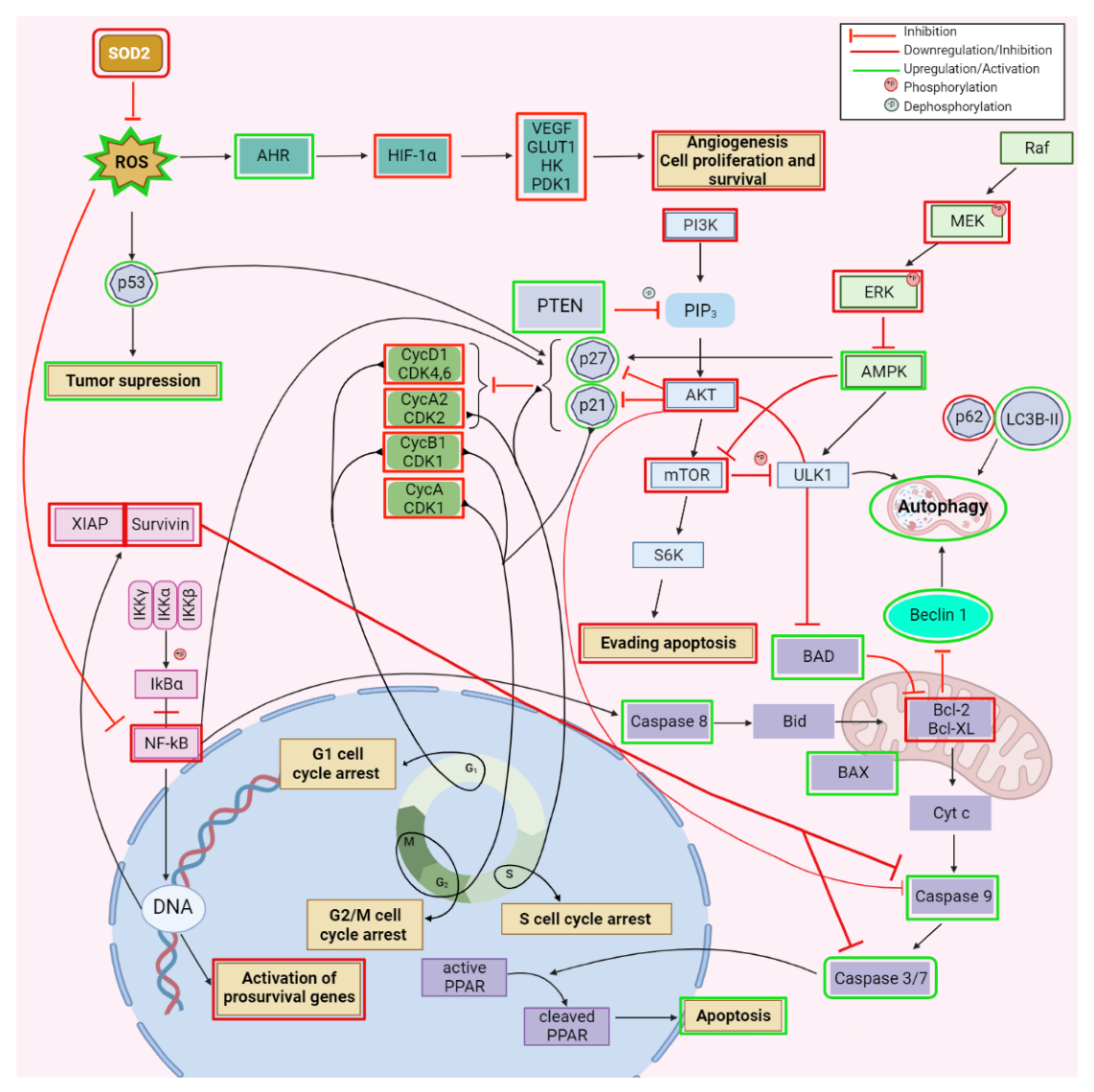
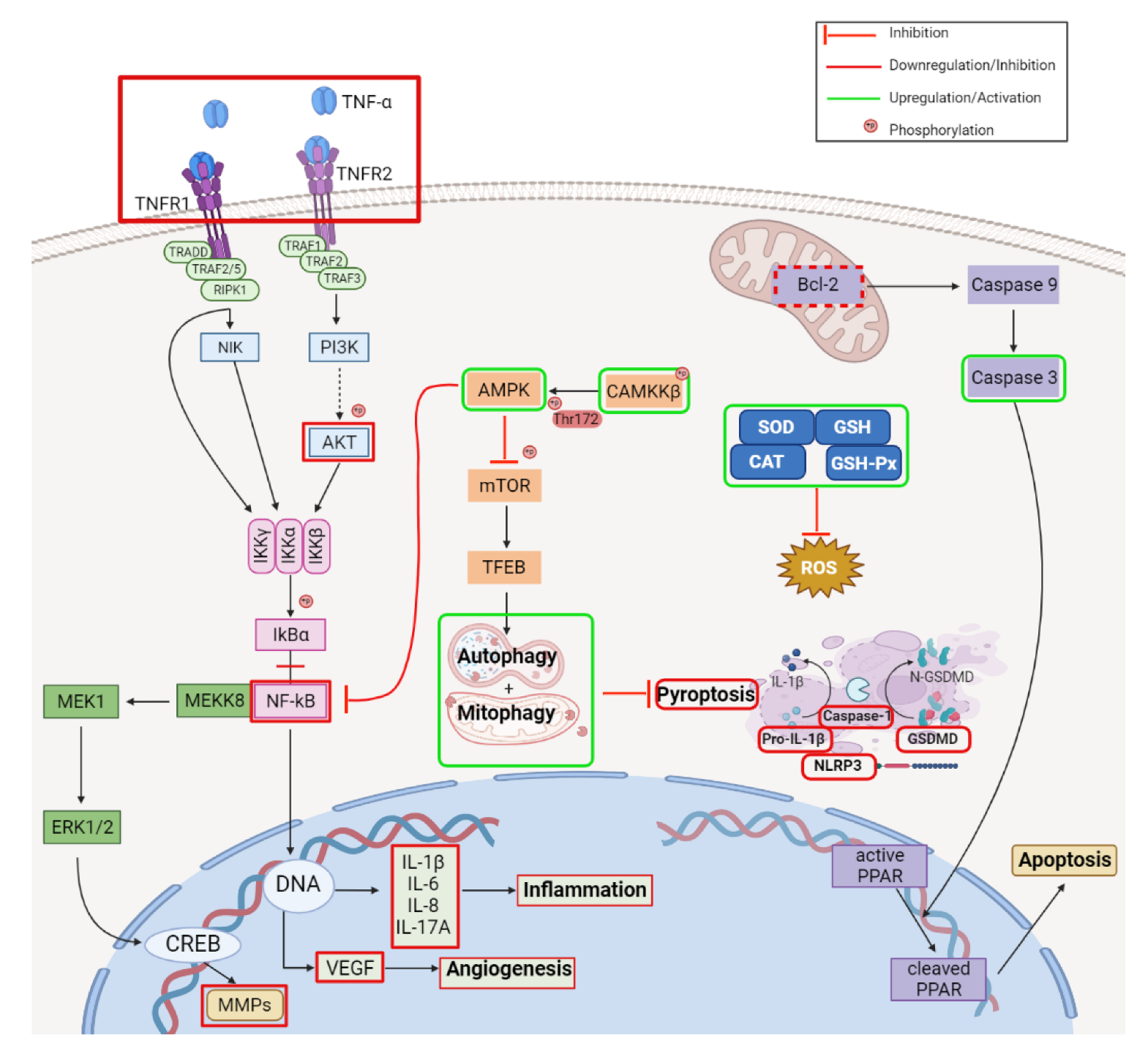



| Type of Cancer | Experimental Conditions | Mechanism | Outcome | Reference |
|---|---|---|---|---|
| Glioblastoma | In vitro—U87MG and A172 cell lines |
|
| [24] |
| Oral squamous carcinoma | In vitro—OSCC-derived cell line KB In vivo—KB injected Balb/c nude mice |
|
| [25] |
| In vitro—CAL-27 and Tca-83 oral squamous carcinoma cell lines |
|
| [26] | |
| Gastric cancer | SGC-7901 drug-resistant cancer cell lines |
|
| [27] |
| Hepatocarcinoma | In vitro—HepG2 and SMMC-7721 hepatocarcinoma cell lines |
|
| [28] |
| In vitro—PLC/PRF/5 and MHCC97L hepatocarcinoma cells |
|
| [29] | |
| Pancreatic cancer | In vitro—SW1990 and PANC-1 pancreatic carcinoma cell lines In vivo—mouse xenograft model |
|
| [30] |
| Acute myeloid leukemia | In vitro—Kasumi-1, HL-60 and THP-1 leukemia cell lines |
|
| [34] |
| In vitro—u937 leukemia cell line |
|
| [35] | |
| Non-small lung cancer | In vitro—A549, H358 and NCI-H1703 cell lines |
|
| [37] |
| Breast cancer | In vitro—MDA-MB-231 cells |
|
| [38] |
| In vitro—MDA-MB-231 cells |
|
| [39] | |
| Ovarian cancer | In vitro—A2780 ovarian cancer cells |
|
| [40] |
| Cervical cancer | In vitro—HeLa cells |
|
| [41] |
| In vitro—HeLA cells |
|
| [42] | |
| Bladder cancer | In vitro—T-24, UMUC-3 and 5637 human bladder cancer cell lines |
|
| [43] |
| In vitro—EJ and T24 human bladder cancer cell lines |
|
| [44] | |
| Colorectal cancer | In vitro—HCT116 cell line |
|
| [45] |
| In vitro—HCT116 cell line In vivo—mouse model |
|
| [46] | |
| In vitro—HCT116 cell line |
|
| [47] |
| Type of Cancer | Experimental Conditions | Mechanism | Outcome | Reference |
|---|---|---|---|---|
| Gastric cancer | In vitro |
|
| [116] |
|
| [117] | ||
| Colorectal cancer | In vitro—HCT116 and SW480 colorectal cancer cell lines In vivo—colon carcinoma xenograft model |
|
| [118] |
| Liver cancer | In vitro—s Bel-7404, HL-7702, Bel-7402, SMMC-7721, SKHep1, and HEK-293T liver cancer cell lines In vivo—xenotransplantation model |
|
| [120] |
| In vitro—Hep3B and HepG2 hepatocellular carcinoma cell lines In vivo—xenotransplantation model |
|
| [121] | |
| Rectal cancer | In vitro—ACHN and A498 rectal cancer cell lines |
|
| [122] |
| Prostate cancer | In vitro —PC-3 and DU145 prostate cancer cell lines In vitro—xenograft tumor model |
|
| [123] |
| Glioblastoma | In vitro—GBM8401, M059K and U-87MG malignant glioma cell lines |
|
| [124] |
| Retinoblastoma | In vitro—Y-79 and ARPE-19 human retinoblastoma cell lines |
|
| [125] |
| Type of Cancer | Experimental Conditions | Mechanism | Outcome | Reference |
|---|---|---|---|---|
| Ovarian cancer | In vitro—Human umbilical vein endothelial cells (HUVECs) and A2780 human ovarian cancer cells Ex vivo and in vivo models: rat aortic ring assay, zebrafish, and mouse Matrigel plug assay |
|
| [145] |
| Hepato-cellular carcinoma (HCC) | In vitro—hepatocellular carcinoma HepG2, SMMC-7721, HLF, HLE, LM3, and Hep3B cell lines In vivo—xenograft tumorigenicity assay |
|
| [147] |
| Gastric cancer | In vitro—human gastric cancer cell line SGC-7901 |
|
| [148] |
| Prostate cancer | In vitro—LNCaP, PC3, DU145, human prostate cancer cells |
|
| [149] |
| Colorectal cancer | In vitro—LoVo, SW480 and SW620 colorectal cancer cells In vivo—xenograft tumor models |
|
| [150] |
| Lung cancer | In vitro—A549 lung cancer cells, IMR-90 human embryonic lung fibroblasts |
|
| [151] |
| Sarcoma | In vitro—HOS human osteosarcoma cells and HT1080 human fibrosarcoma cells In vivo—xenograft osteosarcoma models |
|
| [152] |
| Type of Cancer | Experimental Conditions | Mechanism | Outcome | Reference |
|---|---|---|---|---|
| Breast carcinoma | In vitro—docetaxel-resistant MDA-MB-231 breast carcinoma cells |
|
| [192] |
| Cervical cancer | In vitro—HeLa human cervical cancer cells |
|
| [193] |
| Colorectal cancer | In vitro—HT-29, HCT 116, SW480, SW48, and LS 174T colorectal cancer cells |
|
| [194] |
| Colorectal cancer | In vitro—HCT116 and SW480 colorectal cancer cells In vivo—mice and xenograft tumor model |
|
| [195] |
| Gastric cancer | In vitro—MNK28 human gastric cancer cell |
|
| [196] |
| Neuroblastoma | In vitro—SHSY-5Y Human neuroblastoma cell line |
|
| [197] |
| Pancreatic cancer | In vitro—Panc-28 pancreatic cancer cells |
|
| [198] |
| Pancreatic cancer | In vitro—PANC-1 and Patu-8988 pancreatic cancer cells |
|
| [199] |
| Pheochromocytoma | In vitro—PC12 rat adrenal pheochromocytoma cells |
|
| [200] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mioc, M.; Prodea, A.; Racoviceanu, R.; Mioc, A.; Ghiulai, R.; Milan, A.; Voicu, M.; Mardale, G.; Șoica, C. Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part II). Int. J. Mol. Sci. 2022, 23, 8896. https://doi.org/10.3390/ijms23168896
Mioc M, Prodea A, Racoviceanu R, Mioc A, Ghiulai R, Milan A, Voicu M, Mardale G, Șoica C. Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part II). International Journal of Molecular Sciences. 2022; 23(16):8896. https://doi.org/10.3390/ijms23168896
Chicago/Turabian StyleMioc, Marius, Alexandra Prodea, Roxana Racoviceanu, Alexandra Mioc, Roxana Ghiulai, Andreea Milan, Mirela Voicu, Gabriel Mardale, and Codruța Șoica. 2022. "Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part II)" International Journal of Molecular Sciences 23, no. 16: 8896. https://doi.org/10.3390/ijms23168896
APA StyleMioc, M., Prodea, A., Racoviceanu, R., Mioc, A., Ghiulai, R., Milan, A., Voicu, M., Mardale, G., & Șoica, C. (2022). Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part II). International Journal of Molecular Sciences, 23(16), 8896. https://doi.org/10.3390/ijms23168896





.jpg)


