A Systematic Review of the Effects of High-Fat Diet Exposure on Oocyte and Follicular Quality: A Molecular Point of View
Abstract
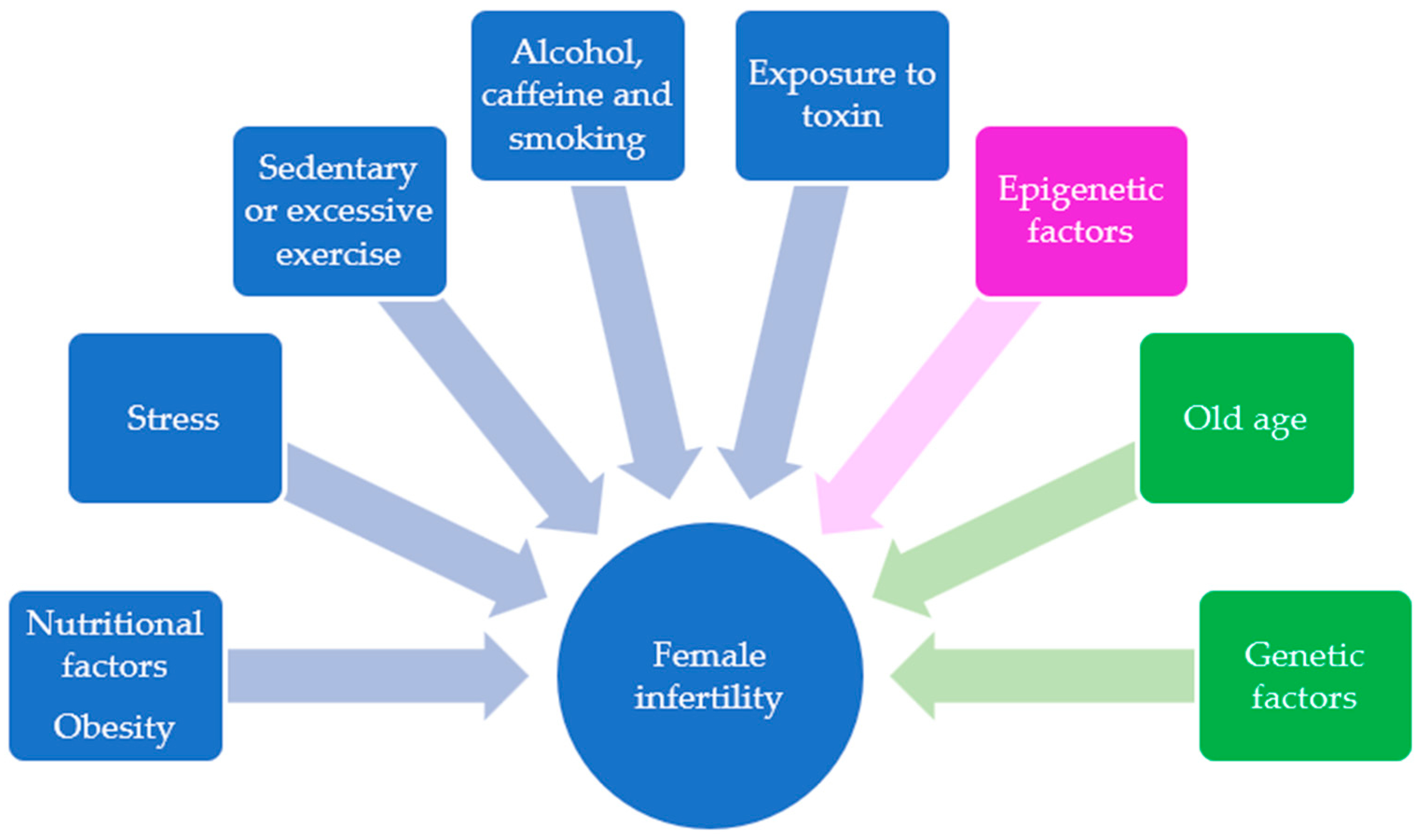
1. Introduction
2. Methods
- Female infertility AND high-fat diet*;
- High fat diet AND folliculogenesis;
- High fat diet* AND-1miRNA AND ovarian follicle AND high-fat diet;
- Epigenetic OR methylation OR miRNA OR gene expression AND oocyte AND high-fat diet;
- Oxidative stress OR inflammation AND oocyte AND high-fat diet.
3. Results and Discussion
3.1. Impact of High-Fat Diet on Gene Expression of Folliculogenesis
3.1.1. Impact of HFD on Inflammation Pathways in Folliculogenesis
3.1.2. Impact of HFD on reactive oxygen species (ROS) Production and Oxidative Stress in Mammal Follicles
3.2. Effects of HFD on Gene Expression of Oogenesis
Inflammation and Oxidative Stress Pathways in Oogenesis
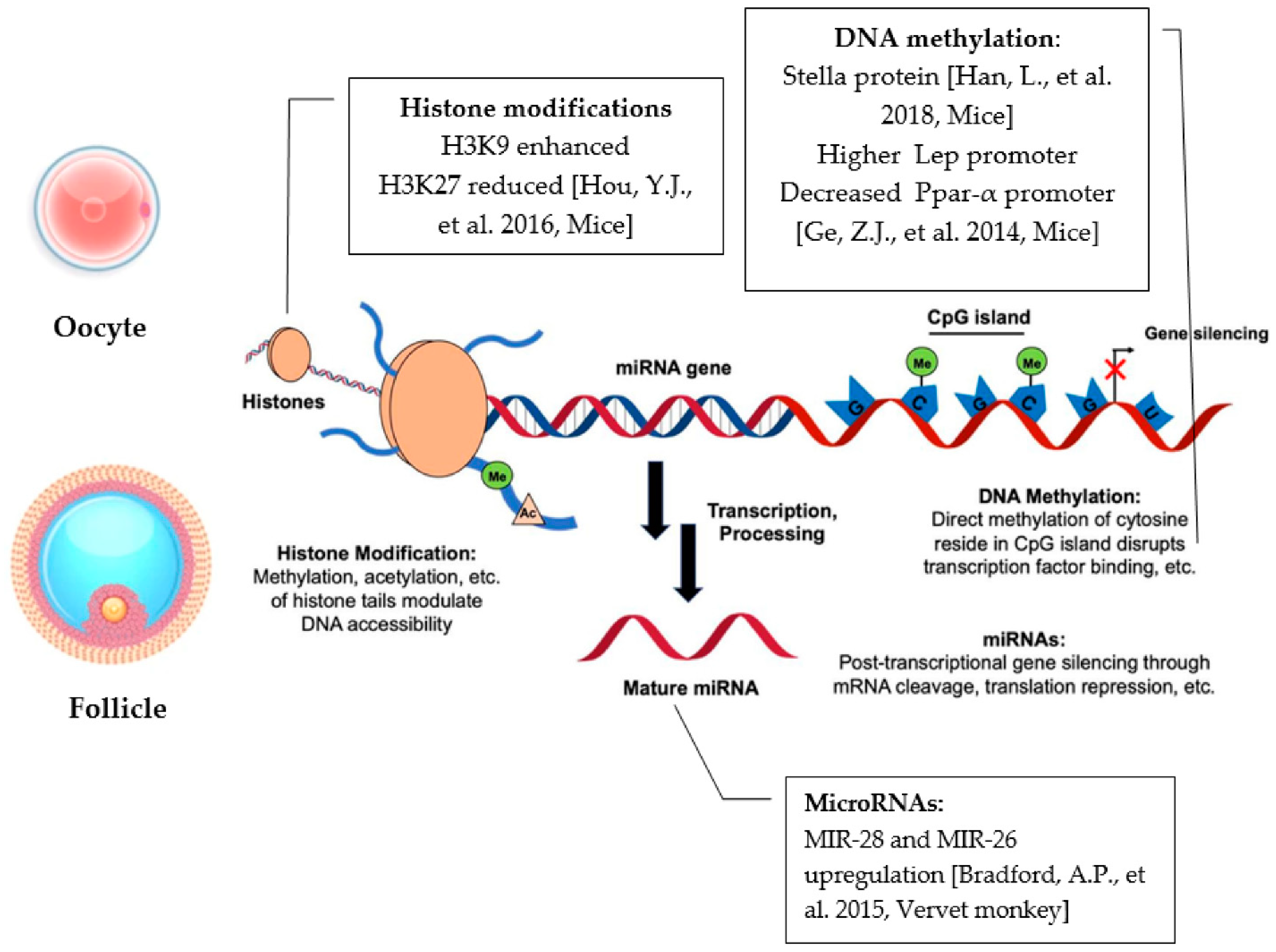
4. HFD-Derived Epigenetic Effects
4.1. Epigenetics Mechanisms
4.1.1. HFD Modifications to Gene Promoter Methylation
4.1.2. HFD Modifications to microRNAs’ Expression
4.1.3. HFD Modifications on Histone Modifications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pisarska, M.D.; Chan, J.L.; Lawrenson, K.; Gonzalez, T.L.; Wang, E.T. Genetics and Epigenetics of Infertility and Treatments on Outcomes. J. Clin. Endocrinol. Metab. 2018, 104, 1871–1886. [Google Scholar] [CrossRef] [PubMed]
- McLernon, D.J.; Maheshwari, A.; Lee, A.J.; Bhattacharya, S.; Nelson, S.M.; Lawlor, D.A. Cumulative live birth rates after one or more complete cycles of IVF: A population-based study of linked cycle data from 178,898 women. Hum. Reprod. 2016, 31, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Skakkebæk, N.E.; Lindahl-Jacobsen, R.; Levine, H.; Andersson, A.M.; Jørgensen, N.; Main, K.M.; Lidegaard, Ø.; Priskorn, L.; Holmboe, S.A.; Bräuner, E.V.; et al. Environmental factors in declining human fertility. Nat. Rev. Endocrinol. 2022, 18, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2013, 99, 63. [Google Scholar] [CrossRef]
- Ombelet, W.; Cooke, I.; Dyer, S.; Serour, G.; Devroey, P. Infertility and the provision of infertility medical services in developing countries. Hum. Reprod. Update 2008, 14, 605–621. [Google Scholar] [CrossRef]
- Hilal, G.; Fatma, T.; Ferruh, Y.; Sabire, G.; Yüksel, A. Effect of high-fat diet on the various morphological parameters of the ovary. Anat. Cell Biol. 2020, 53, 58–67. [Google Scholar] [CrossRef]
- Karbalay-Doust, S.; Noorafshan, A. Stereological estimation of ovarian oocyte volume, surface area and number: Application on mice treated with nandrolone decanoate. Folia Histochem. Cytobiol. 2012, 50, 275–279. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Liao, X.; Wang, Z.; Li, R.; Zou, S.; Jiang, T.; Zheng, B.; Duan, P.; Xiao, J. Diabetes Induces Abnormal Ovarian Function via Triggering Apoptosis of Granulosa Cells and Suppressing Ovarian Angiogenesis. Int. J. Biol. Sci. 2017, 13, 1297–1308. [Google Scholar] [CrossRef]
- Minabe, S.; Iwata, K.; Tsuchida, H.; Tsukamura, H.; Ozawa, H. Effect of diet-induced obesity on kisspeptin-neurokinin B-dynorphin A neurons in the arcuate nucleus and luteinizing hormone secretion in sex hormone-primed male and female rats. Peptides 2021, 142, 170546. [Google Scholar] [CrossRef]
- Konstantinidou, F.; Budani, M.C.; Sarra, A.; Stuppia, L.; Tiboni, G.M.; Gatta, V. Impact of Cigarette Smoking on the Expression of Oxidative Stress-Related Genes in Cumulus Cells Retrieved from Healthy Women Undergoing IVF. Int. J. Mol. Sci. 2021, 22, 13147. [Google Scholar] [CrossRef]
- Konstantinidou, F.; Stuppia, L.; Gatta, V. Looking Inside the World of Granulosa Cells: The Noxious Effects of Cigarette Smoke. Biomedicines 2020, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Stuppia, L.; Franzago, M.; Ballerini, P.; Gatta, V.; Antonucci, I. Epigenetics and male reproduction: The consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin. Epigenetics 2015, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Series Preconception health 1 Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Budani, M.C.; D’Aurora, M.; Stuppia, L.; Gatta, V.; Tiboni, G.M. Whole-body exposure to cigarette smoke alters oocyte miRNAs expression in C57BL/6 mice. Mol. Reprod. Dev. 2019, 86, 1741–1757. [Google Scholar] [CrossRef]
- Hohos, N.M.; Cho, K.J.; Swindle, D.C.; Skaznik-Wikiel, M.E. High-fat diet exposure, regardless of induction of obesity, is associated with altered expression of genes critical to normal ovulatory function. Mol. Cell. Endocrinol. 2018, 470, 199–207. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Z.; Liao, X.; Qi, L.; Liu, Y.; Wang, Z. Effect of high-fat diet-induced obesity on the Akt/FoxO/Smad signaling pathway and the follicular development of the mouse ovary. Mol. Med. Rep. 2016, 14, 3894–3900. [Google Scholar] [CrossRef]
- Wise, L.A.; Wesselink, A.K.; Tucker, K.L.; Saklani, S.; Mikkelsen, E.M.; Cueto, H.; Riis, A.H.; Trolle, E.; McKinnon, C.J.; Hahn, K.A.; et al. Dietary Fat Intake and Fecundability in 2 Preconception Cohort Studies. Am. J. Epidemiol. 2018, 187, 60–74. [Google Scholar] [CrossRef]
- Foucaut, A.M.; Faure, C.; Julia, C.; Czernichow, S.; Levy, R.; Dupont, C.; ALIFERT collaborative group. Sedentary behavior, physical inactivity and body composition in relation to idiopathic infertility among men and women. PLoS ONE 2019, 14, e0210770. [Google Scholar] [CrossRef]
- Noli, S.A.; Ricci, E.; Cipriani, S.; Ferrari, S.; Castiglioni, M.; La Vecchia, I.; Somigliana, E.; Parazzini, F. Dietary Carbohydrate Intake, Dietary Glycemic Load and Outcomes of In Vitro Fertilization: Findings from an Observational Italian Cohort Study. Nutrients 2020, 12, 1568. [Google Scholar] [CrossRef]
- Kazemi, A.; Ramezanzadeh, F.; Nasr-Esfahani, M.H. Relationship between Dietary Fat Intake, Its Major Food Sources and Assisted Reproduction Parameters. J. Reprod. Infertil. 2014, 15, 214–221. [Google Scholar]
- Skaznik-Wikiel, M.E.; Swindle, D.C.; Allshouse, A.A.; Polotsky, A.J.; McManaman, J.L. High-Fat Diet Causes Subfertility and Compromised Ovarian Function Independent of Obesity in Mice. Biol. Reprod. 2016, 94, 108. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Ramezanzadeh, F.; Nasr-Esfahani, M.H.; Saboor Yaraghi, A.A.; Ahmadi, M. Does dietary fat intake influence oocyte competence and embryo quality by inducing oxidative stress in follicular fluid? Iran J. Reprod. Med. 2013, 11, 1005–1012. [Google Scholar] [PubMed]
- Nteeba, J.; Ross, J.W.; Perfield, J.W., II.; Keating, A.F. High fat diet induced obesity alters ovarian phosphatidylinositol-3 kinase signaling gene expression. Reprod. Toxicol. 2013, 42, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.B.; Liu, X.C.; Qin, X.Y.; Chen, J.; Ren, P.G.; Deng, W.F.; Zhang, J. Effect of High-Fat Diet on Immature Female Mice and Messenger and Noncoding RNA Expression Profiling in Ovary and White Adipose Tissue. Reprod. Sci. 2019, 26, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Thornton, K.; Bonney, E.; Cipolla, M.J.; Charron, M.J.; Buyuk, E. Ovarian kisspeptin expression is related to age and to monocyte chemoattractant protein-1. J. Assist. Reprod. Genet. 2016, 33, 535–543. [Google Scholar] [CrossRef]
- Su, W.X.; Li, Q.Z.; Zhang, L.Q.; Fan, G.L.; Wu, C.Y.; Yan, Z.H.; Zuo, Y.C. Gene expression classification using epigenetic features and DNA sequence composition in the human embryonic stem cell line H1. Gene 2016, 592, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wołodko, K.; Walewska, E.; Adamowski, M.; Castillo-Fernandez, J.; Kelsey, G.; Galvão, A. Leptin Resistance in the Ovary of Obese Mice is Associated with Profound Changes in the Transcriptome of Cumulus Cells. Cell. Physiol. Biochem. 2020, 54, 417–437. [Google Scholar] [CrossRef]
- Ma, X.; Hayes, E.; Prizant, H.; Srivastava, R.K.; Hammes, S.R.; Sen, A. Leptin-Induced CART (Cocaine- and Amphetamine-Regulated Transcript) Is a Novel Intraovarian Mediator of Obesity-Related Infertility in Females. Endocrinology 2016, 157, 1248–1257. [Google Scholar] [CrossRef]
- Hohos, N.M.; Elliott, E.M.; Giornazi, A.; Silva, E.; Rice, J.D.; Skaznik-Wikiel, M.E. High-fat diet induces an ovulatory defect associated with dysregulated endothelin-2 in mice. Reproduction 2021, 161, 307–317. [Google Scholar] [CrossRef]
- Long, X.; Yang, Q.; Qian, J.; Yao, H.; Yan, R.; Cheng, X.; Zhang, Q.; Gu, C.; Gao, F.; Wang, H.; et al. Obesity modulates cell-cell interactions during ovarian folliculogenesis. Iscience 2021, 25, 103627. [Google Scholar] [CrossRef]
- Nteeba, J.; Ganesan, S.; Keating, A.F. Progressive obesity alters ovarian folliculogenesis with impacts on pro-inflammatory and steroidogenic signaling in female mice. Biol. Reprod. 2014, 91, 86. [Google Scholar] [CrossRef] [PubMed]
- Nteeba, J.; Ortinau, L.C.; Perfield, J.W., II.; Keating, A.F. Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol. Reprod. Dev. 2013, 80, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Ruebel, M.; Shankar, K.; Gaddy, D.; Lindsey, F.; Badger, T.; Andres, A. Maternal obesity is associated with ovarian inflammation and upregulation of early growth response factor 1. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E269–E277. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.B.; Lane, M.; Knight, E.J.; Robker, R.L. Inflammatory markers in human follicular fluid correlate with lipid levels and Body Mass Index. J. Reprod. Immunol. 2018, 130, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Polotsky, A.J.; Bradford, A.P.; Buyuk, E.; Chosich, J.; Phang, T.; Jindal, S.; Santoro, N. Adiposity Alters Genes Important in Inflammation and Cell Cycle Division in Human Cumulus Granulosa Cell. Reprod. Sci. 2015, 22, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Zhu, C.C.; Duan, X.; Liu, H.L.; Wang, Q.; Sun, S.C. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci. Rep. 2016, 6, 18858. [Google Scholar] [CrossRef]
- Xu, M.; Che, L.; Yang, Z.; Zhang, P.; Shi, J.; Li, J.; Lin, Y.; Fang, Z.; Che, L.; Feng, B.; et al. Effect of High Fat Dietary Intake during Maternal Gestation on Offspring Ovarian Health in a Pig Model. Nutrients 2016, 8, 498. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Carpenter, R.M.; Blythe, S.N.; Toporikova, N. FSH/AMH Ratio and Adipocyte Size are Linked to Ovarian Dysfunction. Endocr. Res. 2020, 45, 174–189. [Google Scholar] [CrossRef]
- Yao, J.; Li, Z.; Fu, Y.; Wu, R.; Wang, Y.; Liu, C.; Yang, L.; Zhang, H. Involvement of obesity-associated upregulation of chemerin/chemokine-like receptor 1 in oxidative stress and apoptosis in ovaries and granulosa cells. Biochem. Biophys. Res. Commun. 2019, 510, 449–455. [Google Scholar] [CrossRef]
- Pohlmeier, W.E.; Xie, F.; Kurz, S.G.; Lu, N.; Wood, J.R. Progressive obesity alters the steroidogenic response to ovulatory stimulation and increases the abundance of mRNAs stored in the ovulated oocyte. Mol. Reprod. Dev. 2014, 81, 735–747. [Google Scholar] [CrossRef]
- Sohrabi, M.; Roushandeh, A.M.; Alizadeh, Z.; Vahidinia, A.; Vahabian, M.; Hosseini, M. Effect of a high fat diet on ovary morphology, in vitro development, in vitro fertilisation rate and oocyte quality in mice. Singap. Med. J. 2015, 56, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, S.; Wu, X.; Liu, Y.; Ge, J.; Wang, Q.; Gu, L. NAMPT reduction-induced NAD+ insufficiency contributes to the compromised oocyte quality from obese mice. Aging Cell 2021, 20, e13496. [Google Scholar] [CrossRef] [PubMed]
- Sutton-McDowall, M.L.; Wu, L.L.; Purdey, M.; Abell, A.D.; Goldys, E.M.; MacMillan, K.L.; Thompson, J.G.; Robker, R.L. Nonesterified Fatty Acid-Induced Endoplasmic Reticulum Stress in Cattle Cumulus Oocyte Complexes Alters Cell Metabolism and Developmental Competence. Biol. Reprod. 2016, 94, 23. [Google Scholar] [CrossRef]
- Wong, S.L.; Wu, L.L.; Robker, R.L.; Thompson, J.G.; McDowall, M.L. Hyperglycaemia and lipid differentially impair mouse oocyte developmental competence. Reprod. Fertil. Dev. 2015, 27, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Ruebel, M.L.; Cotter, M.; Sims, C.R.; Moutos, D.M.; Badger, T.M.; Cleves, M.A.; Shankar, K.; Andres, A. Obesity Modulates Inflammation and Lipid Metabolism Oocyte Gene Expression: A Single-Cell Transcriptome Perspective. J. Clin. Endocrinol. Metab. 2017, 102, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, L.; Ma, R.; Hou, X.; Yu, Y.; Sun, S.; Xu, Y.; Schedl, T.; Moley, K.H.; Wang, Q. Sirt3 prevents maternal obesity-associated oxidative stress and meiotic defects in mouse oocytes. Cell Cycle 2015, 14, 2959–2968. [Google Scholar] [CrossRef]
- Iljas, J.D.; Homer, H.A. Sirt3 is dispensable for oocyte quality and female fertility in lean and obese mice. FASEB J. 2020, 34, 6641–6653. [Google Scholar] [CrossRef]
- Bradley, J.; Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 2019, 63, 93–103. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Q.; Li, X.; Hu, F.; Han, L.; Zhang, H.; Li, L.; Ge, J.; Ying, X.; Guo, X.; et al. Loss of TIGAR Induces Oxidative Stress and Meiotic Defects in Oocytes from Obese Mice. Mol. Cell. Proteom. 2018, 17, 1354–1364. [Google Scholar] [CrossRef]
- Nicoglou, A.; Merlin, F. Epigenetics: A way to bridge the gap between biological fields. Stud. Hist. Philos. Biol. Biomed. Sci. 2017, 66, 73–82. [Google Scholar] [CrossRef]
- Greally, J.M. A user’s guide to the ambiguous word ‘epigenetics’. Nat. Rev. Mol. Cell Biol. 2018, 19, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Monk, D.; Mackay, D.J.G.; Eggermann, T.; Maher, E.R.; Riccio, A. Genomic imprinting disorders: Lessons on how genome, epigenome and environment interact. Nat. Rev. Genet. 2019, 20, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Ibeagha-Awemu, E.M.; Zhao, X. Epigenetic marks: Regulators of livestock phenotypes and conceivable sources of missing variation in livestock improvement programs. Front. Genet. 2015, 6, 302. [Google Scholar] [CrossRef] [PubMed]
- Paiva, J.; Resende, M.; Resende, R.; Oliveira, H.; Silva, H.; Caetano, G.; Lopes, P.; Silva, F. Epigenetics: Mechanisms, inheritance and implications in animal breeding. Arch. Zootec. 2019, 68, 304–311. [Google Scholar] [CrossRef][Green Version]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenetics 2018, 4, dvy016. [Google Scholar] [CrossRef]
- Peral-Sanchez, I.; Hojeij, B.; Ojeda, D.A.; Steegers-Theunissen, R.P.M.; Willaime-Morawek, S. Epigenetics in the Uterine Environment: How Maternal Diet and ART May Influence the Epigenome in the Offspring with Long-Term Health Consequences. Genes 2021, 13, 31. [Google Scholar] [CrossRef]
- Han, L.; Ren, C.; Li, L.; Li, X.; Ge, J.; Wang, H.; Miao, Y.L.; Guo, X.; Moley, K.H.; Shu, W.; et al. Embryonic defects induced by maternal obesity in mice derive from Stella insufficiency in oocytes. Nat. Genet. 2018, 50, 432–442. [Google Scholar] [CrossRef]
- Ge, Z.J.; Luo, S.M.; Lin, F.; Liang, Q.X.; Huang, L.; Wei, Y.C.; Hou, Y.; Han, Z.M.; Schatten, H.; Sun, Q.Y. DNA methylation in oocytes and liver of female mice and their offspring: Effects of high-fat-diet-induced obesity. Environ. Health Perspect. 2014, 122, 159–164. [Google Scholar] [CrossRef]
- Dunning, K.R.; Anastasi, M.R.; Zhang, V.J.; Russell, D.L.; Robker, R.L. Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS ONE 2014, 9, e87327. [Google Scholar] [CrossRef]
- Bradford, A.P.; Jones, K.; Kechris, K.; Chosich, J.; Montague, M.; Warren, W.C.; May, M.C.; Al-Safi, Z.; Kuokkanen, S.; Appt, S.E.; et al. Joint MiRNA/mRNA expression profiling reveals changes consistent with development of dysfunctional corpus luteum after weight gain. PLoS ONE 2015, 10, e0135163. [Google Scholar] [CrossRef]
| References | Species | Main Findings |
|---|---|---|
| [27] | Mice | Upregulation of sets of genes involved in cellular trafficking and impairment in cytoskeleton organization Alterated gene expression related to inflammatory responses, cell morphogenesis and decreased metabolism |
| [28] | Mice | Higher Cart gene expression contributes to lower estradiol synthesis, fewer ovulated oocytes and possible subfertility |
| [29] | Mice | Edn2 down-, Ece1 up- gene expression and ET2 protein dysregulation influence the number of ovulated oocytes and overall ovulatory mechanism |
| [30] | Mice | Inhbb, Stmn1, and Hsd3b1 higher levels modulate metabolic homeostasis and steroidogenesis in ovarian follicles; Marcks and Prkar2b are potential indicators of folliculogenesis abnormalities |
| [31] | Mice | Alteration of steroidogenic genes (Star and Cyp11a1) and E2 receptors (Erα and Erβ) |
| References | Species | Main Findings |
|---|---|---|
| [21] | Mice | Higher levels of proinflammatory cytokines, increased infiltration of ovarian macrophages |
| [32] | Mice | Greater penetration of immune cells and higher gene expression inflammatory genes |
| [33] | Rat | High levels TNF-α, IL-6 and IL-8 and Egr-1 gene |
| [34] | Human | High levels IL-6, IL-8, TNFα and IL-10 |
| [35] | Human | FGF-12 and PPM1L over-regulation, activation of inflammatory pathways |
| References | Species | Main Findings |
|---|---|---|
| [10] | Human | Factors related to individual’s lifestyle—smoking |
| [21] | Mice | Abnormal inflammatory responses, inflammatory and oxidative stress markers |
| [22] | Human | Higher oxidative stress in follicular fluid |
| [37] | Pig | Oxidative stress compromises follicular and ovarian development |
| [38] | Rat | Significant follicular development damage |
| [32] | Mice | Altered ovarian function and female reproductive potential |
| [39] | Mice | Activation of glutathione system and Cat gene inducing antioxidant response; Sod2 downregulation correlated to decreased capacity of eliminating ROS |
| References | Species | Main Findings |
|---|---|---|
| [40] | Mice | Higher expression of Gdf9, negative effects on development beyond the zygote stage Raly upregulation affects negatively the development at the 2-cell stage and/or blastocyst |
| [41] | Mice | Bmp15 upregulation in GV and MII oocytes; developmental failure-related mechanisms |
| [42] | Mice | NAMPT reduction-induced NAD+ insufficiency; compromised quality of oocytes |
| [43] | Cow | COC’s ATF4–HSPA5 significantly increased negative impact on protein-folding pathways, oocyte maturation, metabolic dysfunction and embryonic development |
| [44] | Mice | COC’s Atf4–Hspa5 significantly increased |
| References | Species | Main Findings |
|---|---|---|
| [45] | Human | GAS7 and TNXIP downregulation; Higher expression of CXCL2, CXCL3, IL-34 and CCL20; decreased oocyte quality and development |
| [46] | Mice | Sirt3 significatly lower may contribute to the penetrance of oxidative stress, as well as metabolic and meiotic alteration |
| [36] | Mice | Higher expression of pro-apoptotic genes Bak and Bcl-2; cause-related reduction in oocyte quality |
| [48] | Mice | Increase in ROS and toxic lipid peroxides |
| [49] | Mice | Tigar downregulation further contribution of oxidative stress development |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonnella, F.; Konstantinidou, F.; Di Berardino, C.; Capacchietti, G.; Peserico, A.; Russo, V.; Barboni, B.; Stuppia, L.; Gatta, V. A Systematic Review of the Effects of High-Fat Diet Exposure on Oocyte and Follicular Quality: A Molecular Point of View. Int. J. Mol. Sci. 2022, 23, 8890. https://doi.org/10.3390/ijms23168890
Gonnella F, Konstantinidou F, Di Berardino C, Capacchietti G, Peserico A, Russo V, Barboni B, Stuppia L, Gatta V. A Systematic Review of the Effects of High-Fat Diet Exposure on Oocyte and Follicular Quality: A Molecular Point of View. International Journal of Molecular Sciences. 2022; 23(16):8890. https://doi.org/10.3390/ijms23168890
Chicago/Turabian StyleGonnella, Francesca, Fani Konstantinidou, Chiara Di Berardino, Giulia Capacchietti, Alessia Peserico, Valentina Russo, Barbara Barboni, Liborio Stuppia, and Valentina Gatta. 2022. "A Systematic Review of the Effects of High-Fat Diet Exposure on Oocyte and Follicular Quality: A Molecular Point of View" International Journal of Molecular Sciences 23, no. 16: 8890. https://doi.org/10.3390/ijms23168890
APA StyleGonnella, F., Konstantinidou, F., Di Berardino, C., Capacchietti, G., Peserico, A., Russo, V., Barboni, B., Stuppia, L., & Gatta, V. (2022). A Systematic Review of the Effects of High-Fat Diet Exposure on Oocyte and Follicular Quality: A Molecular Point of View. International Journal of Molecular Sciences, 23(16), 8890. https://doi.org/10.3390/ijms23168890










