Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli
Abstract
:1. Introduction
2. Results
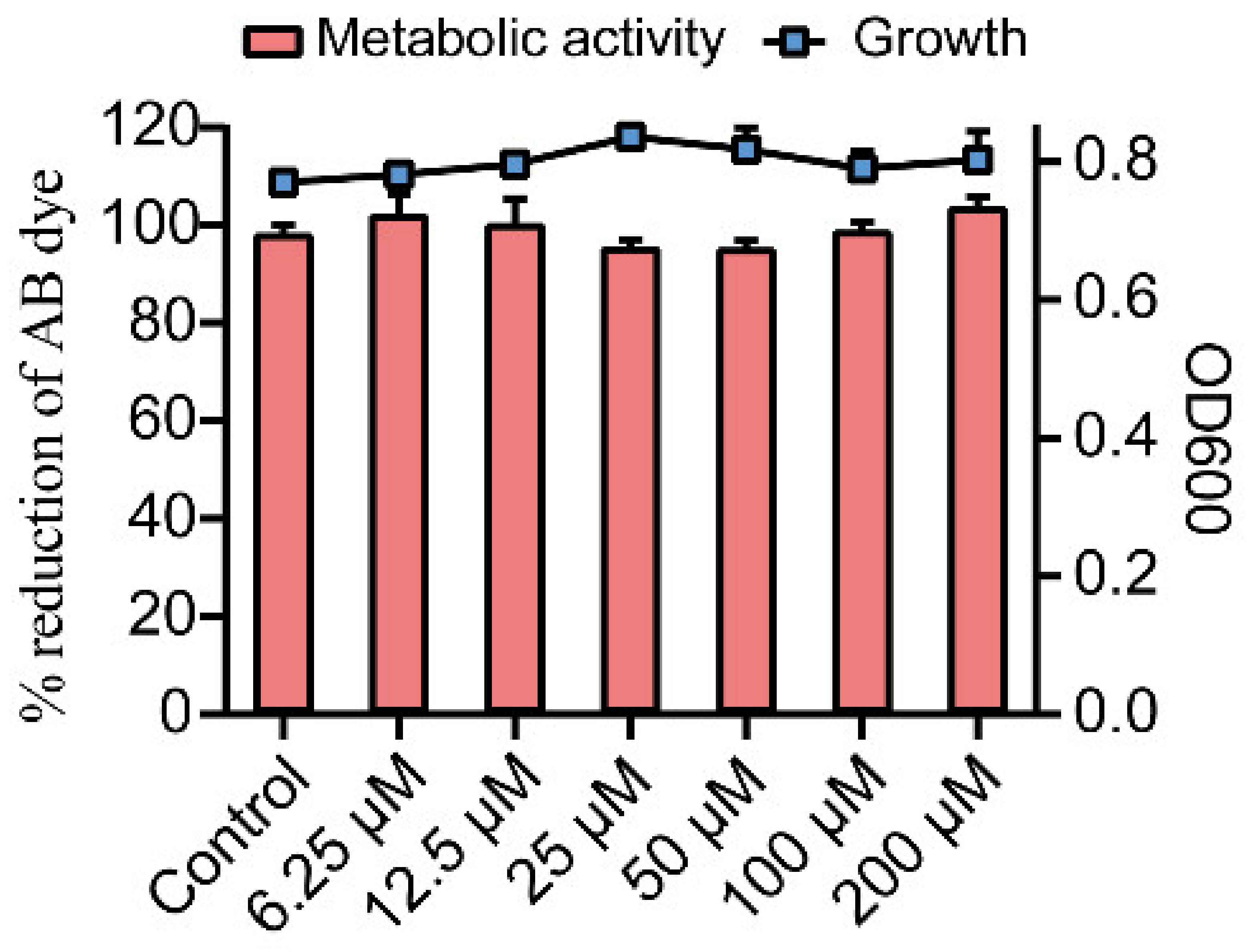
2.1. Effects of Gin on Growth and Metabolic Activity of E. coli
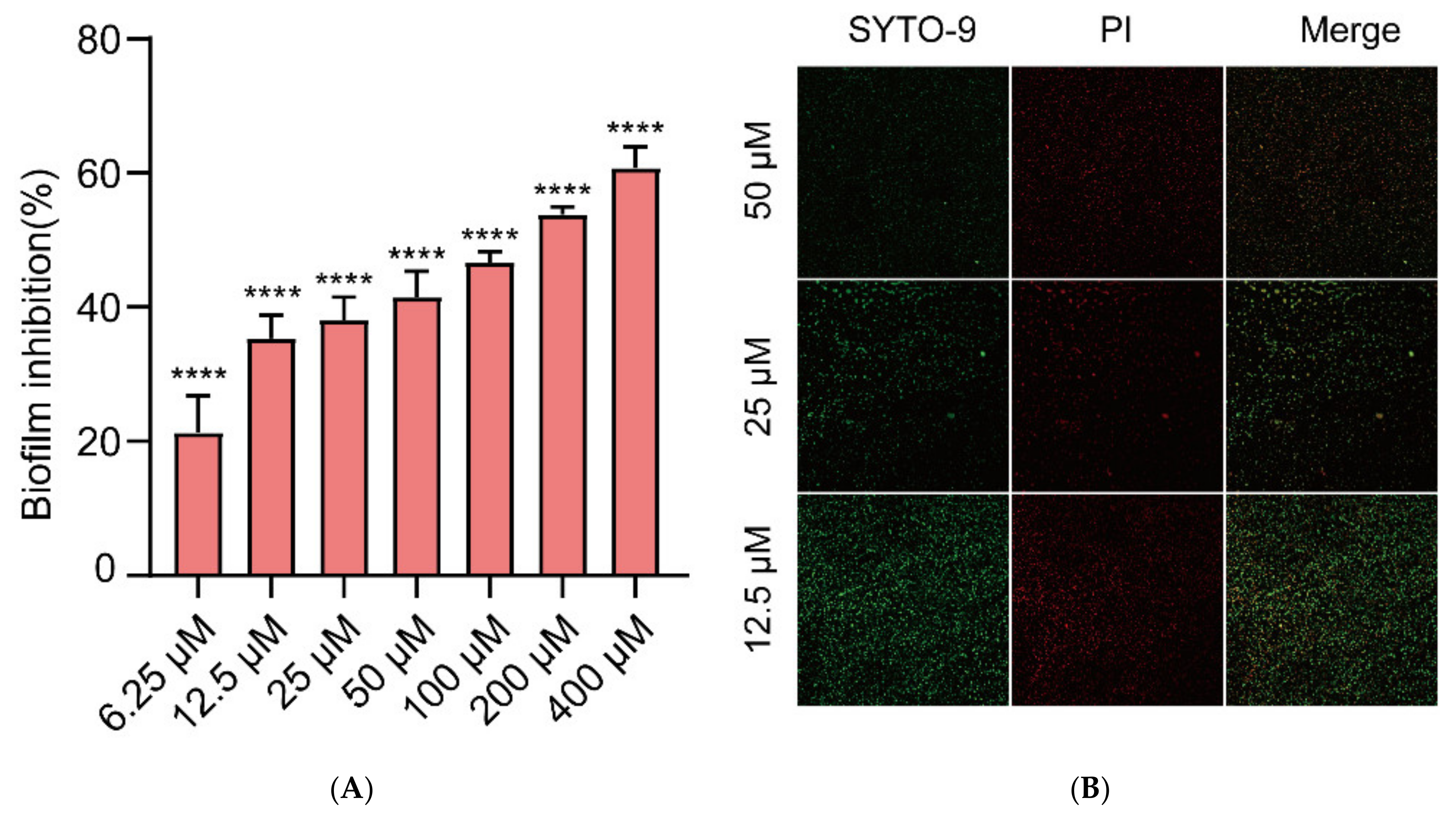
2.2. Inhibition of Biofilm Formation in E. coli by Gin
2.3. Cytotoxicity of Gin on Caco-2 and IPEC-J2 Cells
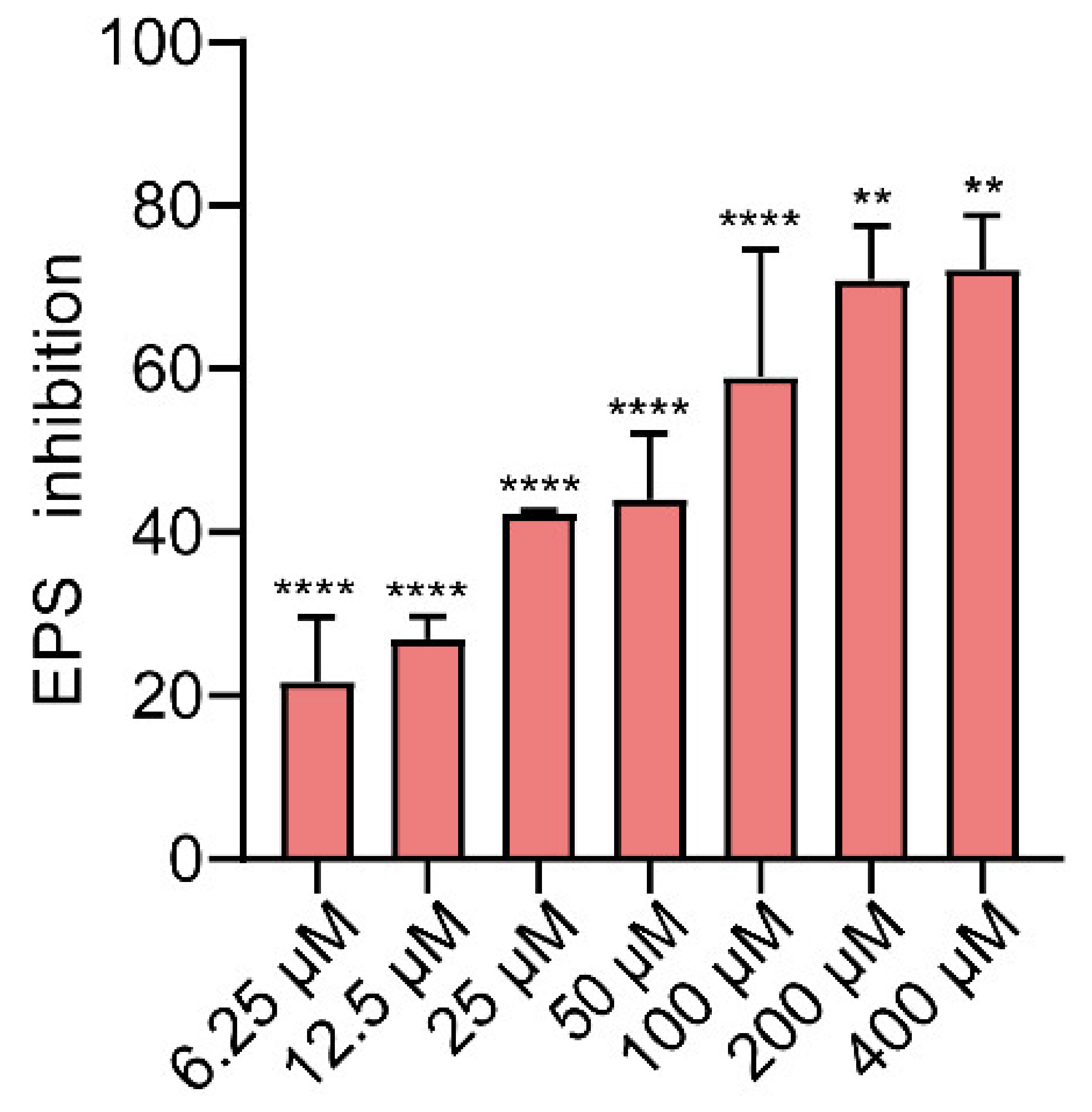
2.4. Effects of Gin on EPS Production of E. coli
2.5. Effects of Gin on the Motility of E. coli
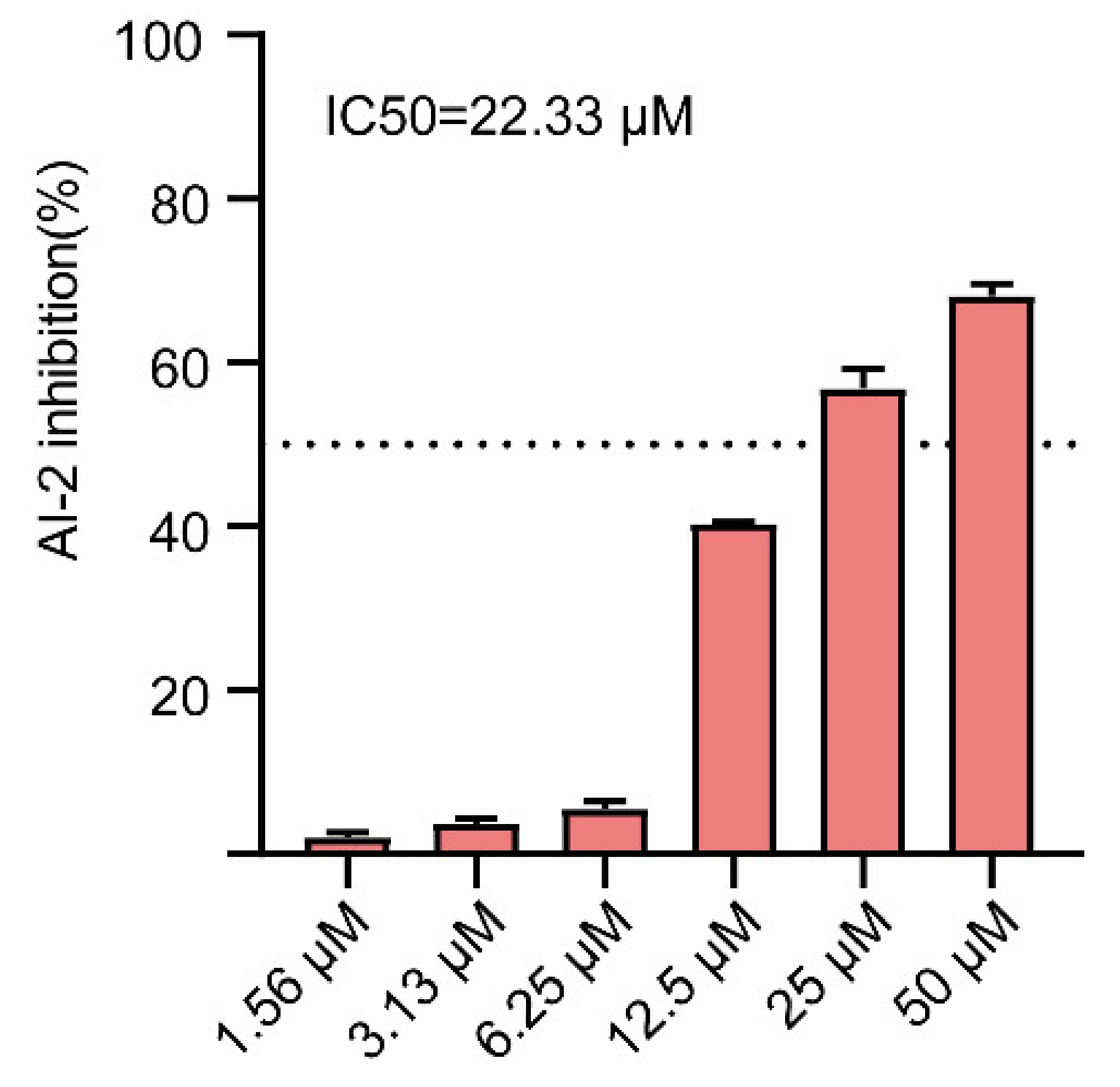
2.6. Effect of Gin on AI-2 Production in E. coli
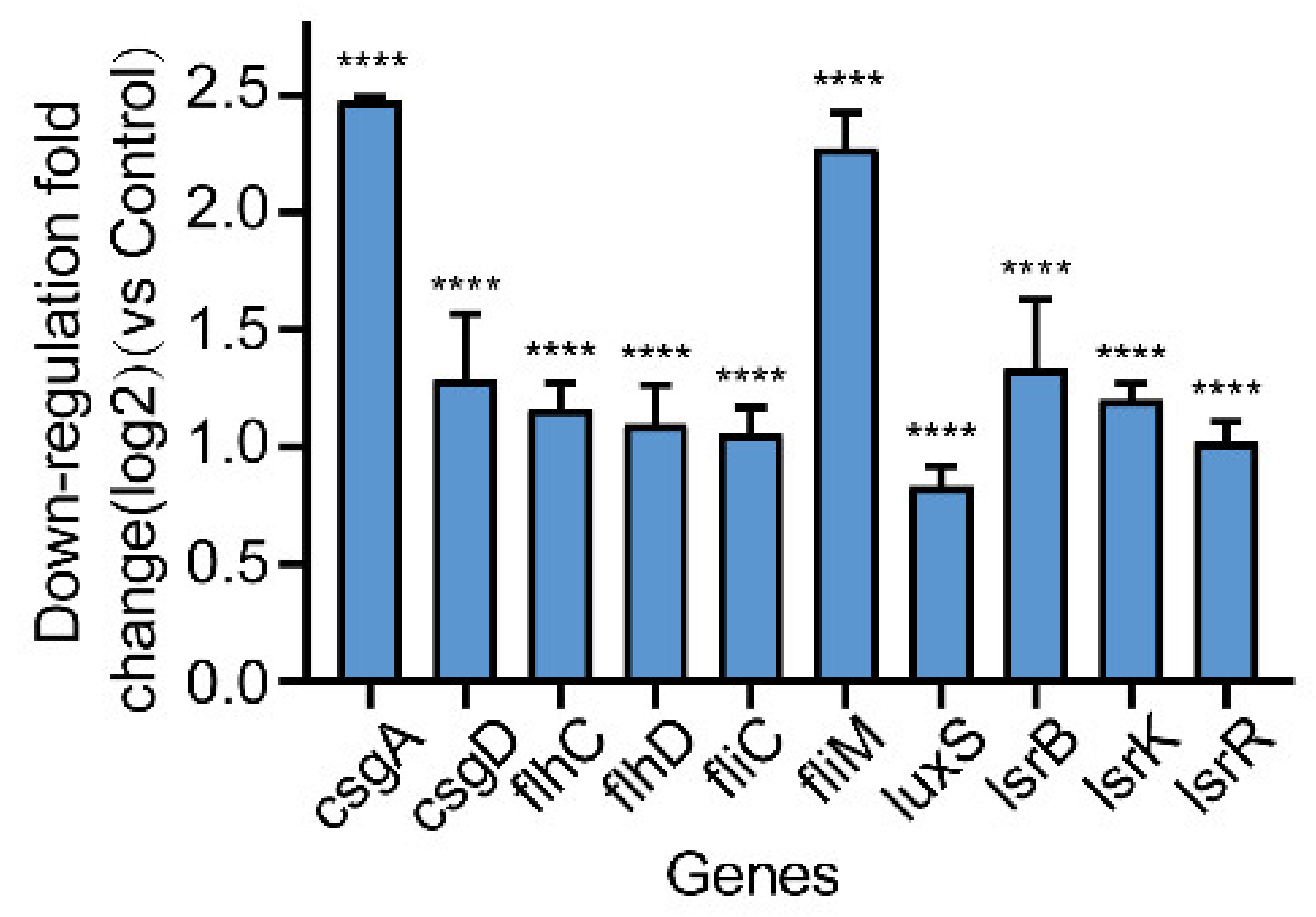
2.7. Effect of Gin on the Transcription of Biofilm-Regulated Genes of E. coli
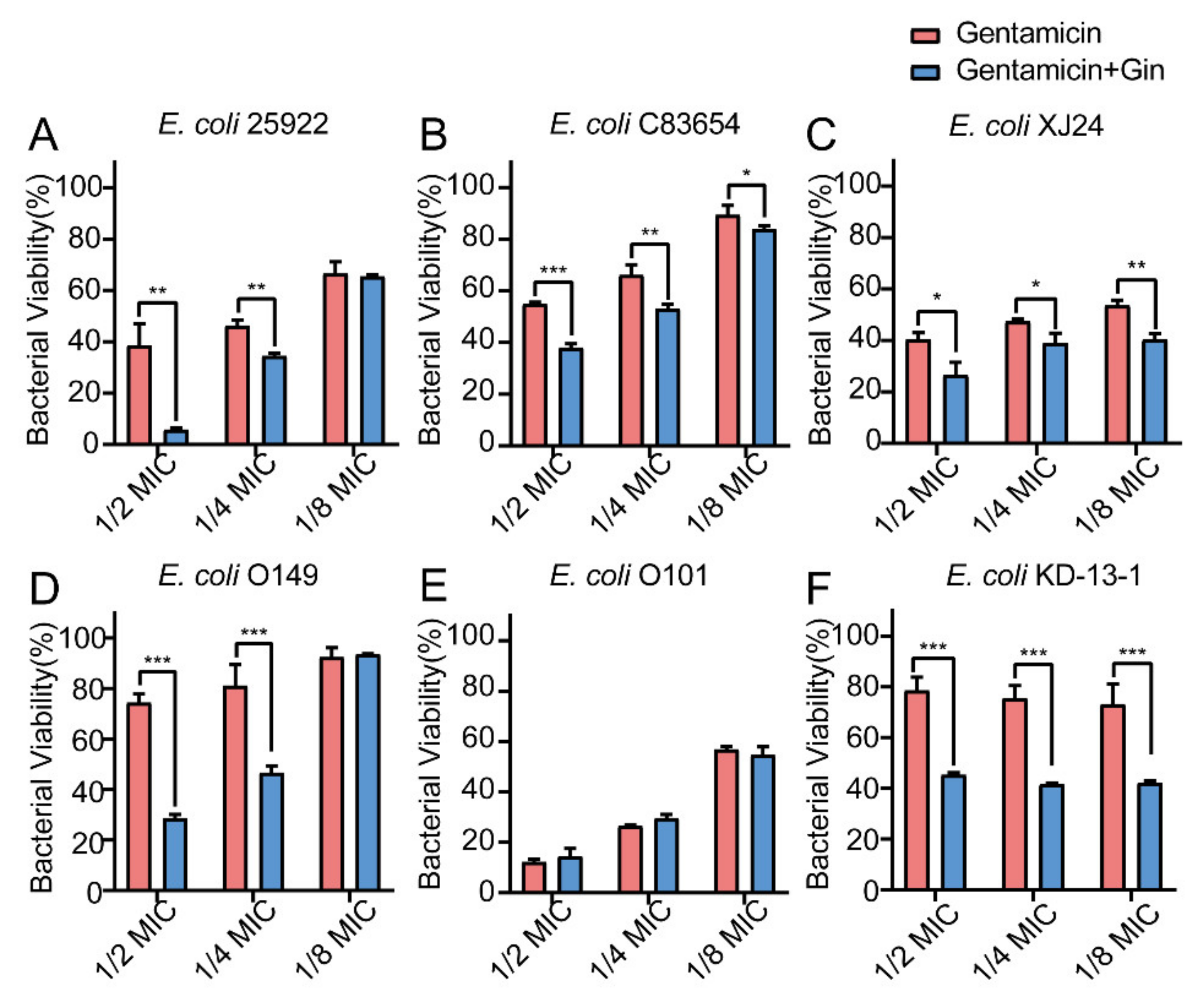
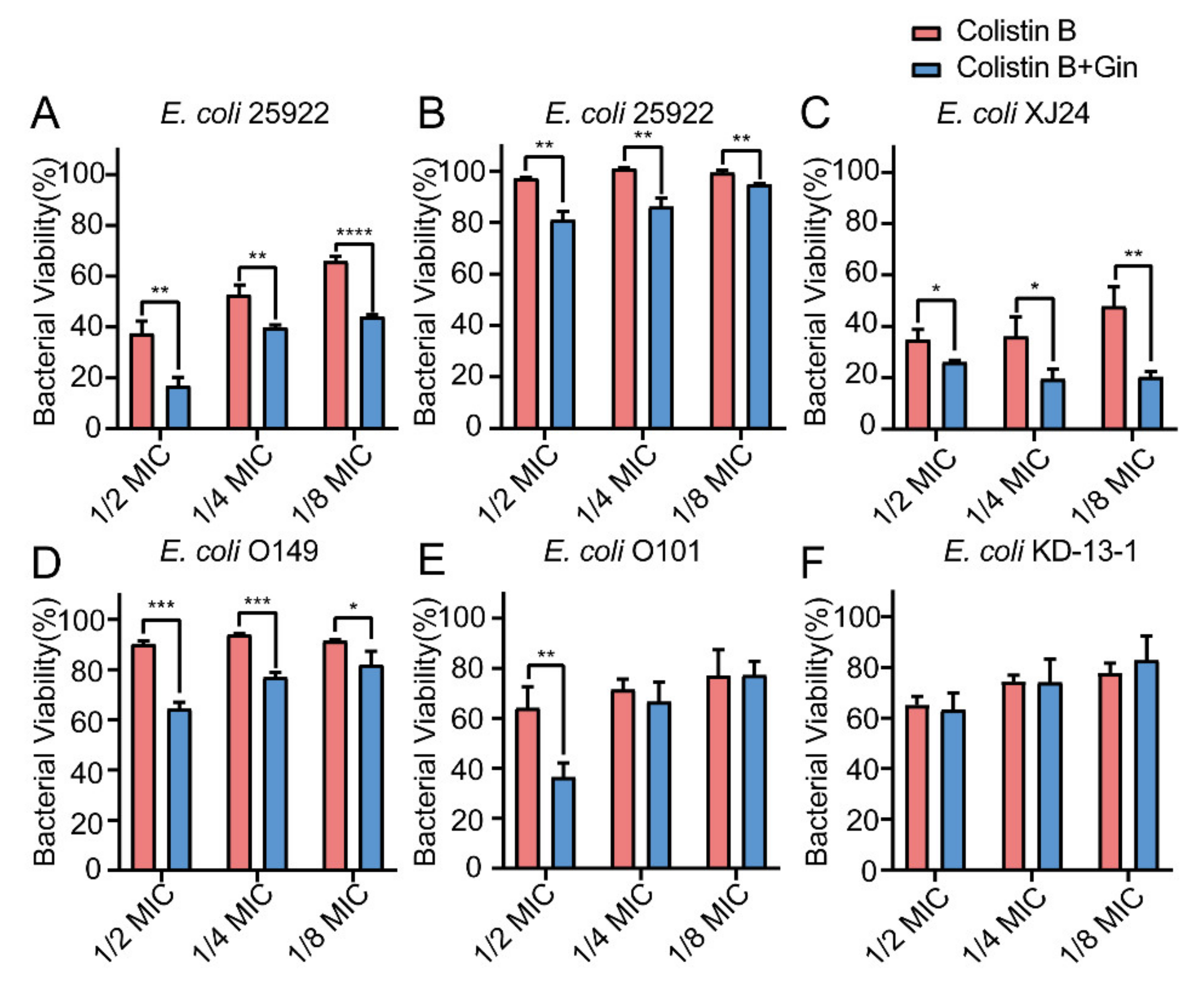
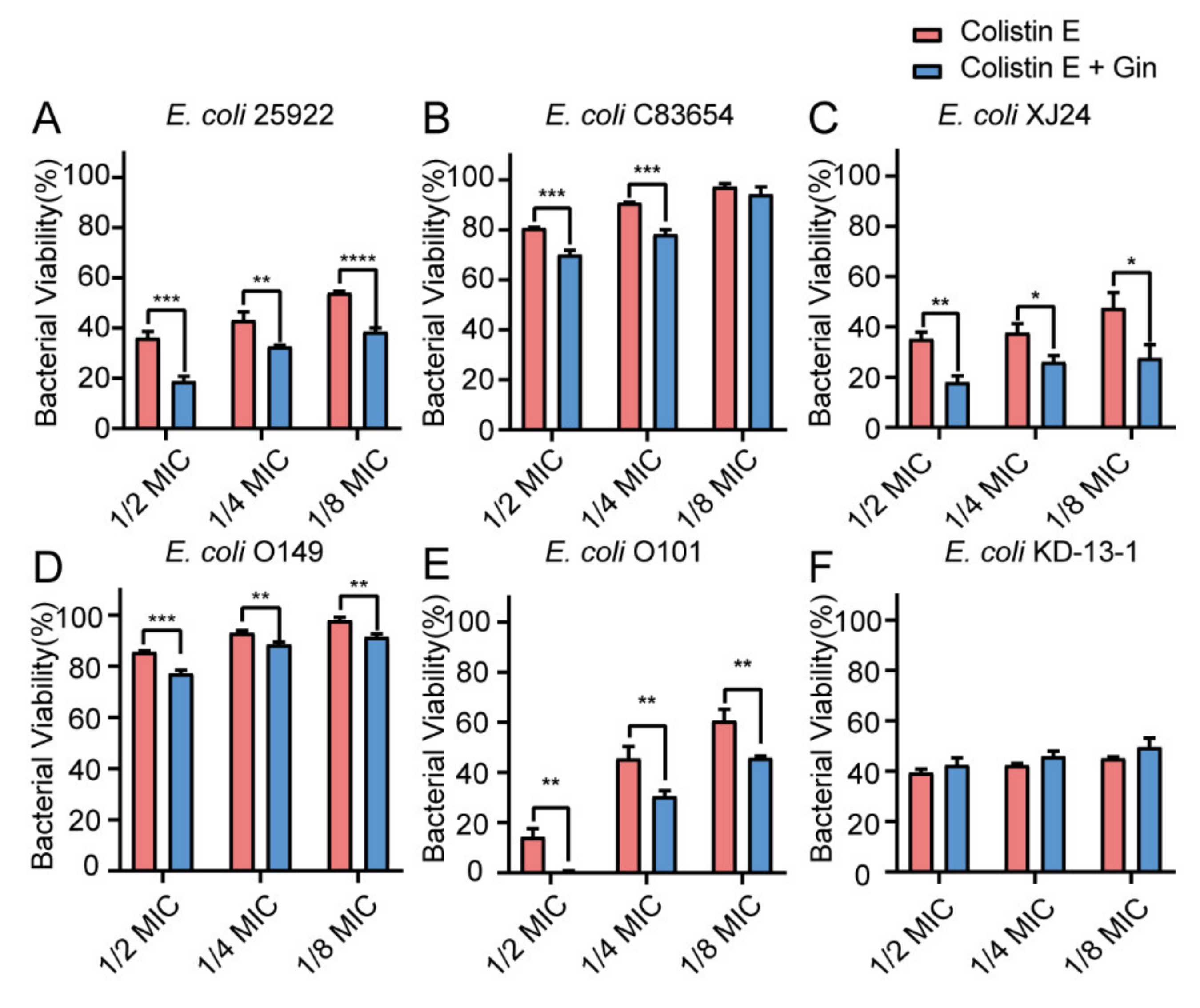
2.8. Synergistic Effects of Gin in Combination with Antibiotics against Six E. coli Strains
3. Discussion
4. Materials and Methods
4.1. Materials, Bacterial Strains, and Cells
4.2. Growth and Metabolic Activity
- Eoxi (OD570)—extinction coefficient of AB in its oxidized form at 570 nm = 80,586;
- Ered (OD570)—extinction coefficient of AB in its reduced form at 570 nm = 155,677;
- Eoxi (OD600)—extinction coefficient of AB in its oxidized form at 600 nm = 117,216;
- Ered (OD600)—extinction coefficient of AB in its reduced form at 570 nm = 14,652;
- B—blank; T—samples.
4.3. Biofilm Assay
4.3.1. Crystal Violet (CV) Staining
4.3.2. Confocal Laser Scanning Microscopy
4.4. Cytotoxicity
4.5. EPS Production
- AB = absorbance of blank
- AS = absorbance of sample
- AP = absorbance of positive control
4.6. Motility Assay
4.7. AI-2 Bioluminescence Assay
4.8. qRT-PCR
4.9. Antibacterial Activity
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Hancock, R.E. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Yuan, M.; Jakobsen, T.H.; Mortensen, K.T.; Delos Santos, M.M.; Chua, S.L.; Yang, L.; Tan, C.H.; Nielsen, T.E.; Givskov, M. Disulfide bond-containing ajoene analogues as novel quorum sensing inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 2017, 60, 215–227. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Hobley, L.; Harkins, C.; MacPhee, C.E.; Stanley-Wall, N.R. Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 35, 322–332. [Google Scholar] [CrossRef]
- Cohen, N.R.; Lobritz, M.A.; Collins, J.J. Microbial persistence and the road to drug resistance. Cell Host Microbe 2013, 13, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [Green Version]
- Bakkeren, E.; Huisman, J.S.; Fattinger, S.A.; Hausmann, A.; Furter, M.; Egli, A.; Slack, E.; Sellin, M.E.; Bonhoeffer, S.; Regoes, R.R.; et al. Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut. Nature 2019, 573, 276–280. [Google Scholar] [CrossRef]
- Adnan, M.; Morton, G.; Singh, J.; Hadi, S. Contribution of rpoS and bolA genes in biofilm formation in Escherichia coli K-12 MG1655. Mol. Cell Biochem. 2010, 342, 207–213. [Google Scholar] [CrossRef]
- Adnan, M.; Sousa, A.M.; Machado, I.; Pereira, M.O.; Khan, S.; Morton, G.; Hadi, S. Role of bolA and rpoS genes in biofilm formation and adherence pattern by Escherichia coli K-12 MG1655 on polypropylene, stainless steel, and silicone surfaces. Acta Microbiol. Immunol. Hung. 2017, 64, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Meesilp, N.; Mesil, N. Effect of microbial sanitizers for reducing biofilm formation of Staphylococcus aureus and Pseudomonas aeruginosa on stainless steel by cultivation with UHT milk. Food Sci. Biotechnol. 2019, 28, 289–296. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob Resist. Infect Control. 2019, 8, 76. [Google Scholar] [CrossRef]
- Vuotto, C.; Donelli, G. Novel treatment strategies for biofilm-based infections. Drugs 2019, 79, 1635–1655. [Google Scholar] [CrossRef]
- Jiang, Y.; Geng, M.; Bai, L. Targeting biofilms therapy: Current research strategies and development hurdles. Microorganisms 2020, 8, 1222. [Google Scholar] [CrossRef]
- Grandclement, C.; Tannieres, M.; Morera, S.; Dessaux, Y.; Faure, D. Quorum quenching: Role in nature and applied developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef]
- Beloin, C.; Renard, S.; Ghigo, J.M.; Lebeaux, D. Novel approaches to combat bacterial biofilms. Curr. Opin. Pharmacol. 2014, 18, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, M.; Zhou, C.; Kallenbach, N.R.; Ren, D. Control of bacterial persister cells by Trp/Arg-containing antimicrobial peptides. Appl. Environ. Microbiol. 2011, 77, 4878–4885. [Google Scholar] [CrossRef] [Green Version]
- Lebeaux, D.; Chauhan, A.; Letoffe, S.; Fischer, F.; de Reuse, H.; Beloin, C.; Ghigo, J.M. PH-mediated potentiation of aminoglycosides kills bacterial persisters and eradicates in vivo biofilms. J. Infect. Dis. 2014, 210, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.S.L.; Tan, L.T.H.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Chuah, L.H.; Ming, L.C.; Khan, T.M.; Lee, L.H.; Goh, B.H. Resveratrol-potential antibacterial agent against foodborne pathogens. Front Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.W.; Chen, T.T.; Tan, X.J.; Sheng, J.Y.; Jia, A.Q. Can the quorum sensing inhibitor resveratrol function as an aminoglycoside antibiotic accelerant against Pseudomonas aeruginosa? Int. J. Antimicrob Agents 2018, 52, 35–41. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Huang, X.; Yang, H.; Niu, X.; Li, D.; Yang, C.; Li, L.; Zou, L.; Qiu, Z.; Wu, S.; et al. Antibacterial activity and anti-quorum sensing mediated phenotype in response to essential oil from Melaleuca bracteata leaves. Int. J. Mol. Sci. 2019, 20, 5696. [Google Scholar] [CrossRef] [Green Version]
- Minich, A.; Levarski, Z.; Mikulášová, M.; Straka, M.; Liptáková, A.; Stuchlík, S. Complex analysis of vanillin and syringic acid as natural antimicrobial agents against Staphylococcus epidermidis biofilms. Int. J. Mol. Sci. 2022, 23, 1816. [Google Scholar] [CrossRef]
- Manner, S.; Fallarero, A. Screening of natural product derivatives identifies two structurally related flavonoids as potent quorum sensing inhibitors against Gram-negative bacteria. Int. J. Mol. Sci. 2018, 19, 1346. [Google Scholar] [CrossRef] [Green Version]
- Bassler, B.L. Small talk. cell-to-cell communication in bacteria. Cell 2002, 109, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Schmidt-Dannert, C. Applications of quorum sensing in biotechnology. Appl. Microbiol. Biotechnol. 2010, 86, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Reading, N.C.; Sperandio, V. Quorum sensing: The many languages of bacteria. FEMS Microbiol. Lett. 2006, 254, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sturme, M.H.; Kleerebezem, M.; Nakayama, J.; Akkermans, A.D.; Vaugha, E.E.; de Vos, W.M. Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van Leeuwenhoek 2002, 81, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.Y.; Yuan, M.; Wu, Z.M.; Song, K.; Zhang, C.L.; An, Q.; Xia, F.; Yu, J.L.; Yi, P.F.; Fu, B.D.; et al. Anti-bacterial activity of baicalin against APEC through inhibition of quorum sensing and inflammatory responses. Sci. Rep. 2019, 9, 4063. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Fernandes, R.; Tsao, C.Y.; Bentley, W.E. Cross species quorum quenching using a native AI-2 processing enzyme. ACS Chem. Biol. 2010, 5, 223–232. [Google Scholar] [CrossRef]
- Römling, U.; Rohde, M.; Olsén, A.; Normark, S.; Reinköster, J. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 2000, 36, 10–23. [Google Scholar] [CrossRef]
- Ogasawara, H.; Yamamoto, K.; Ishihama, A. Role of the biofilm master regulator csgD in cross-regulation between biofilm formation and flagellar synthesis. J. Bacteriol. 2011, 193, 2587–2597. [Google Scholar] [CrossRef] [Green Version]
- Barnhart, M.M.; Chapman, M.R. Curli biogenesis and function. Ann. Rev. Microbiol. 2006, 60, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Blanco, L.P.; Evans, M.L.; Smith, D.R.; Badtke, M.P.; Chapman, M.R. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 2012, 20, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Arita-Morioka, K.I.; Yamanaka, K.; Mizunoe, Y.; Tanaka, Y.; Ogura, T.; Sugimoto, S. Inhibitory effects of myricetin derivatives on curli-dependent biofilm formation in Escherichia coli. Sci. Rep. 2018, 8, 8452. [Google Scholar] [CrossRef] [Green Version]
- Guttenplan, S.B.; Kearns, D.B. Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 2013, 37, 849–871. [Google Scholar] [CrossRef] [Green Version]
- Wood, T.K.; González Barrios, A.F.; Herzberg, M.; Lee, J. Motility influences biofilm architecture in Escherichia coli. Appl. Microbiol. Biotechnol. 2006, 72, 361–367. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, M.; Burgess, R.R. Adaptation in bacterial flagellar and motility systems: From regulon members to ‘foraging’-like behavior in E. coli. Nucleic Acids Res. 2007, 35, 4441–4452. [Google Scholar] [CrossRef] [Green Version]
- Guan, G.H.; Wang, Y.; Chen, W.F.; Li, Y. Microbial physiology teaching exploration—Taking the teaching of the structure and function of bacterial flagella as an example. Microbiol. China 2016, 43, 756–761. [Google Scholar]
- Chakraborty, S.; Randall, A.; Vickers, T.J.; Molina, D.; Harro, C.D.; DeNearing, B.; Brubaker, J.; Sack, D.A.; Bourgeois, A.L.; Felgner, P.L.; et al. Human experimental challenge with enterotoxigenic Escherichia coli elicits immune responses to canonical and novel antigens relevant to vaccine development. J. Infect. Dis. 2018, 218, 1436–1446. [Google Scholar] [CrossRef]
- Henderson, L.D.; Matthews-Palmer, T.R.S.; Gulbronson, C.J.; Ribardo, D.A.; Beeby, M.; Hendrixson, D.R. Diversification of Campylobacter jejuni flagellar C-ring composition impacts its structure and function in motility, flagellar assembly, and cellular processes. mBio 2020, 11, e02286-19. [Google Scholar] [CrossRef] [Green Version]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [Green Version]
- Braz, V.S.; Melchior, K.; Moreira, C.G. Escherichia coli as a multifaceted pathogenic and versatile bacterium. Front Cell Infect. Microbiol. 2020, 10, 548492. [Google Scholar] [CrossRef]
- Anderson, G.G.; Palermo, J.J.; Schilling, J.D.; Roth, R.; Heuser, J.; Hultgren, S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 2003, 301, 105–107. [Google Scholar] [CrossRef] [Green Version]
- Ageorges, V.; Monteiro, R.; Leroy, S.; Burgess, C.M.; Pizza, M.; Chaucheyras-Durand, F.; Desvaux, M. Molecular determinants of surface colonisation in diarrhoeagenic Escherichia coli (DEC): From bacterial adhesion to biofilm formation. FEMS Microbiol. Rev. 2020, 44, 314–350. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant natural products targeting bacterial virulence factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Escaich, S. Novel agents to inhibit microbial virulence and pathogenicity. Expert Opin. Ther. Pat. 2010, 20, 1401–1418. [Google Scholar] [CrossRef] [PubMed]
- Cegelski, L.; Marshall, G.R.; Eldridge, G.R.; Hultgren, S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008, 6, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Buroni, S.; Chiarelli, L.R. Antivirulence compounds: A future direction to overcome antibiotic resistance? Future Microbiol. 2020, 15, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Patsilinakos, A.; Artini, M.; Papa, R.; Sabatino, M.; Božović, M.; Garzoli, S.; Vrenna, G.; Buzzi, R.; Manfredini, S.; Selan, L.; et al. Machine learning analyses on data including essential oil chemical composition and in vitro experimental antibiofilm activities against Staphylococcus species. Molecules 2019, 24, 890. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, A.; Asghar, A.; Algburi, A.; Huang, Q.; Ahmad, T.; Zhong, H.; Javed, H.U.; Ermakov, A.M.; Chikindas, M.L. Anti-biofilm potential of Elletaria cardamomum essential oil against Escherichia coli O157:H7 and Salmonella Typhimurium JSG 1748. Front Microbiol. 2021, 12, 620227. [Google Scholar] [CrossRef]
- Townsley, L.; Shank, E.A. Natural-product antibiotics: Cues for modulating bacterial biofilm formation. Trends Microbiol. 2017, 25, 1016–1026. [Google Scholar] [CrossRef]
- Ruan, X.; Deng, X.; Tan, M.; Wang, Y.; Hu, J.; Sun, Y.; Yu, C.; Zhang, M.; Jiang, N.; Jiang, R. Effect of resveratrol on the biofilm formation and physiological properties of avian pathogenic Escherichia coli. J. Proteom. 2021, 249, 104357. [Google Scholar] [CrossRef]
- Kim, U.; Kim, J.H.; Oh, S.W. Review of multi-species biofilm formation from foodborne pathogens: Multi-species biofilms and removal methodology. Crit. Rev. Food Sci. Nutr. 2021, 62, 5783–5793. [Google Scholar] [CrossRef]
- Adnan, M.; Rasul, A.; Hussain, G.; Shah, M.A.; Zahoor, M.K.; Anwar, H.; Sarfraz, I.; Riaz, A.; Manzoor, M.; Adem, Ş.; et al. Ginkgetin: A natural biflavone with versatile pharmacological activities. Food Chem. Toxicol. 2020, 145, 111642. [Google Scholar] [CrossRef]
- Jayathilake, P.G.; Jana, S.; Rushton, S.; Swailes, D.; Bridgens, B.; Curtis, T.; Chen, J. Extracellular polymeric substance production and aggregated bacteria colonization influence the competition of microbes in biofilms. Front. Microbiol. 2017, 8, 1865. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Ann. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Spellberg, B.; Bartlett, J.G.; Gilbert, D.N. The future of antibiotics and resistance. N. Engl. J. Med. 2013, 368, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Christner, M.; Franke, G.C.; Schommer, N.N.; Wendt, U.; Wegert, K.; Pehle, P.; Kroll, G.; Schulze, C.; Buck, F.; Mack, D.; et al. The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin. Mol. Microbiol. 2010, 75, 187–207. [Google Scholar] [CrossRef]
- Hussain, M.; Herrmann, M.; von Eiff, C.; Perdreau-Remington, F.; Peters, G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997, 65, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.L.; Bessho, S.; Grando, K.; Tükel, Ç. Microbiome or infections: Amyloid-containing biofilms as a trigger for complex human diseases. Front Immunol. 2021, 12, 638867. [Google Scholar] [CrossRef]
- Gonzalez Barrios, A.F.; Zuo, R.; Hashimoto, Y.; Yang, L.; Bentley, W.E.; Wood, T.K. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (mqsR, B3022). J. Bacteriol. 2006, 188, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Bansal, T.; Jesudhasan, P.; Pillai, S.; Wood, T.K.; Jayaraman, A. Temporal regulation of enterohemorrhagic Escherichia coli virulence mediated by autoinducer-2. Appl. Microbiol. Biotechnol. 2008, 78, 811–819. [Google Scholar] [CrossRef]
- Hardie, K.R.; Cooksley, C.; Green, A.D.; Winzer, K. Autoinducer 2 activity in Escherichia coli culture supernatants can be actively reduced despite maintenance of an active synthase, luxS. Microbiology 2003, 149 Pt 3, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Drees, S.L.; Li, C.; Prasetya, F.; Saleem, M.; Dreveny, I.; Williams, P.; Hennecke, U.; Emsley, J.; Fetzner, S. PqsBC, a condensing enzyme in the biosynthesis of the Pseudomonas aeruginosa quinolone signal: Crystal structure, inhibition, and reaction mechanism. J. Biol. Chem. 2016, 291, 6610–6624. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Qiu, S.; Jiang, Q.; Sun, H.; Xue, T.; Cai, G.; Sun, B. AI-2 quorum sensing negatively regulates rbf expression and biofilm formation in Staphylococcus aureus. Int. J. Med. Microbiol. 2017, 307, 257–267. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Swetha, T.K.; Pooranachithra, M.; Subramenium, G.A.; Divya, V.; Balamurugan, K.; Pandian, S.K. Umbelliferone impedes biofilm formation and virulence of methicillin-resistant Staphylococcus epidermidis via impairment of initial attachment and intercellular adhesion. Front. Cell Infect Microbiol. 2019, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Yin, H.; Hu, J.; Miao, J.; Chen, Z.; Qi, K.; Wang, Z.; Gong, J.; Phouthapane, V.; Jiang, W.; et al. Lsr operon is associated with AI-2 transfer and pathogenicity in avian pathogenic Escherichia coli. Vet. Res. 2019, 50, 109. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Xu, C.; Zhou, X.; Qi, R.; Liu, L.; Lv, F.; Li, Z.; Wang, S. Cationic conjugated polymers for enhancing beneficial bacteria adhesion and biofilm formation in gut microbiota. Colloids Surf. B Biointerfaces 2020, 188, 110815. [Google Scholar] [CrossRef]
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; De Feo, V. Effect of adiantum philippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens, and characterization of bioactive metabolites: An in vitro-in silico approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jayaprakasha, G.K.; Uckoo, R.M.; Patil, B.S. Inhibition of Escherichia coli O157:H7 motility and biofilm by β-sitosterol glucoside. Biochim. Biophys. Acta 2013, 1830, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.E.; Xavier, K.B. Methods for analysis of bacterial autoinducer-2 production. Curr. Protoc. Microbiol. 2011, 23, 1C-1. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L.; Wright, M.; Silverman, M.R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: Sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 1994, 13, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Surette, M.G.; Miller, M.B.; Bassler, B.L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: A new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 1999, 96, 1639–1644. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wang, B.; Fang, X.; Means, W.J.; McCormick, R.J.; Gomelsky, M.; Zhu, M.J. C-di-GMP signaling regulates E. coli O157:H7 adhesion to colonic epithelium. Vet. Microbiol. 2013, 164, 344–351. [Google Scholar] [CrossRef]
- Liu, J.; Hou, J.S.; Chang, Y.Q.; Peng, L.J.; Zhang, X.Y.; Miao, Z.Y.; Sun, P.H.; Lin, J.; Chen, W.M. New pqs quorum sensing system inhibitor as an antibacterial synergist against multidrug-resistant Pseudomonas aeruginosa. J. Med. Chem. 2021, 65, 688–709. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Wang, W.; Shi, M.; Wei, X.; Zhou, X.; Li, B.; Zhang, J. Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli. Int. J. Mol. Sci. 2022, 23, 8809. https://doi.org/10.3390/ijms23158809
Bai Y, Wang W, Shi M, Wei X, Zhou X, Li B, Zhang J. Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli. International Journal of Molecular Sciences. 2022; 23(15):8809. https://doi.org/10.3390/ijms23158809
Chicago/Turabian StyleBai, Yubin, Weiwei Wang, Mengyan Shi, Xiaojuan Wei, Xuzheng Zhou, Bing Li, and Jiyu Zhang. 2022. "Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli" International Journal of Molecular Sciences 23, no. 15: 8809. https://doi.org/10.3390/ijms23158809
APA StyleBai, Y., Wang, W., Shi, M., Wei, X., Zhou, X., Li, B., & Zhang, J. (2022). Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli. International Journal of Molecular Sciences, 23(15), 8809. https://doi.org/10.3390/ijms23158809




