Inborn Errors of Nucleoside Transporter (NT)-Encoding Genes (SLC28 and SLC29)
Abstract
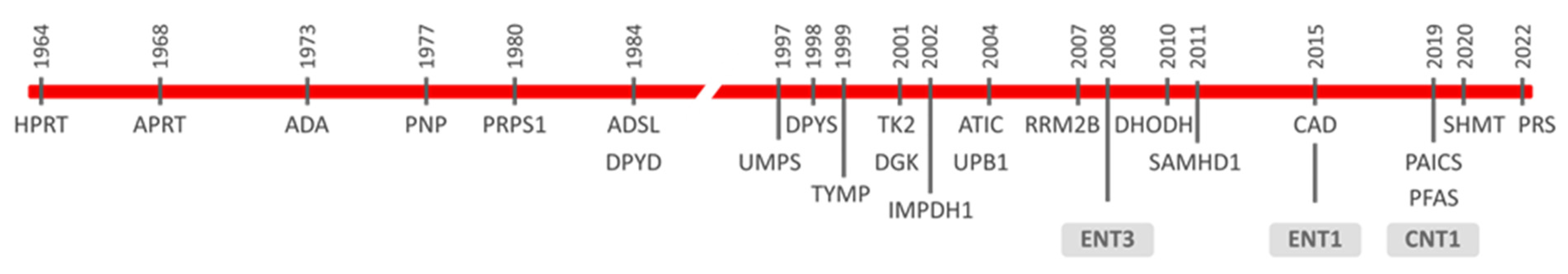
1. Introductory Remarks on Inborn Errors Impacting on Nucleotide Metabolism
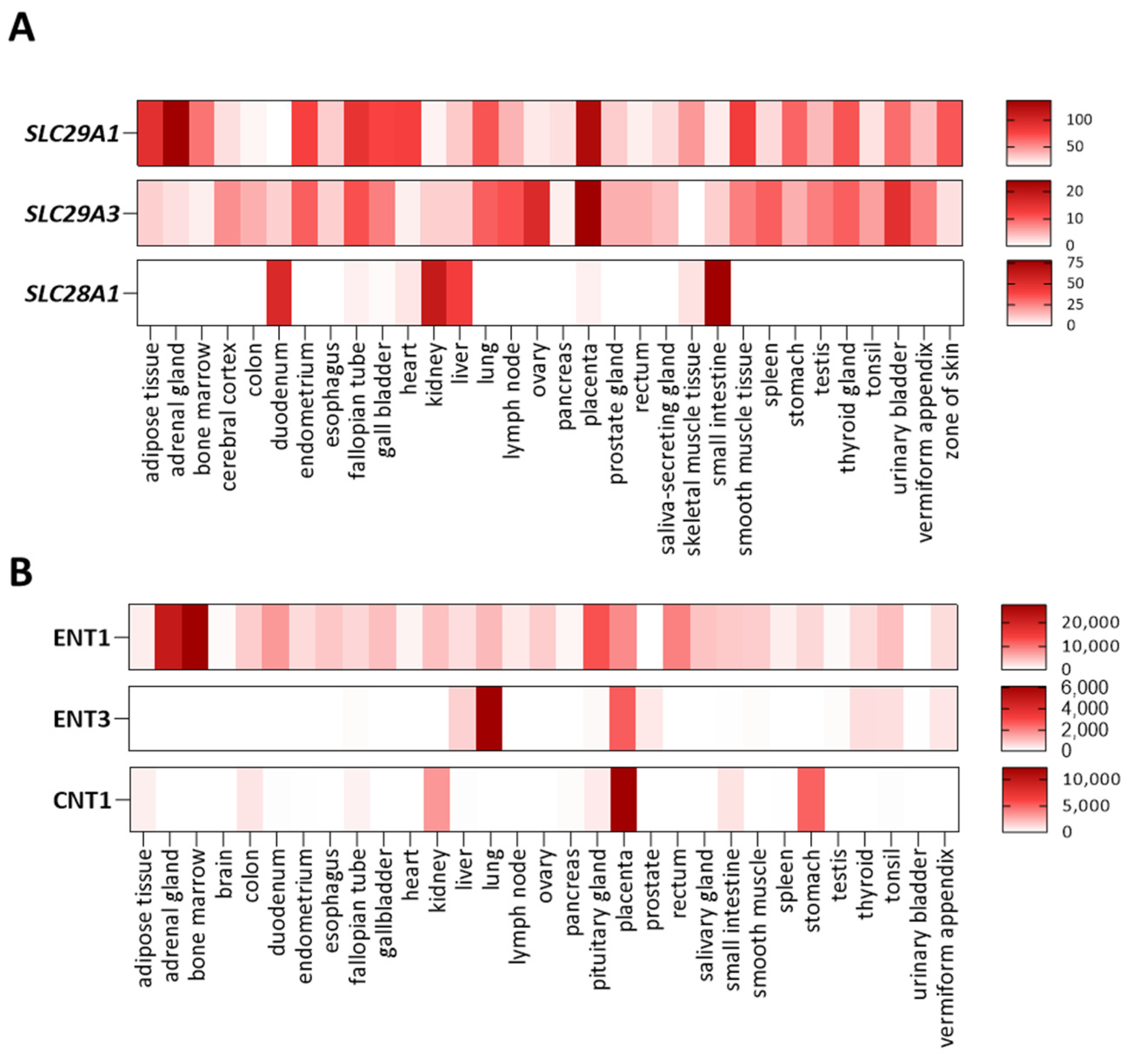
2. Nucleoside and Nucleobase Transporter Proteins
3. Nucleoside and Nucleobase Transporter Protein Variants and Disease
3.1. Genetic Alterations of ENT1. Augustine-Null Blood Type
3.2. Genetic Alterations of ENT3. Histiocytosis-Lymphoadenopathy Plus Syndrome. OMIM#602782
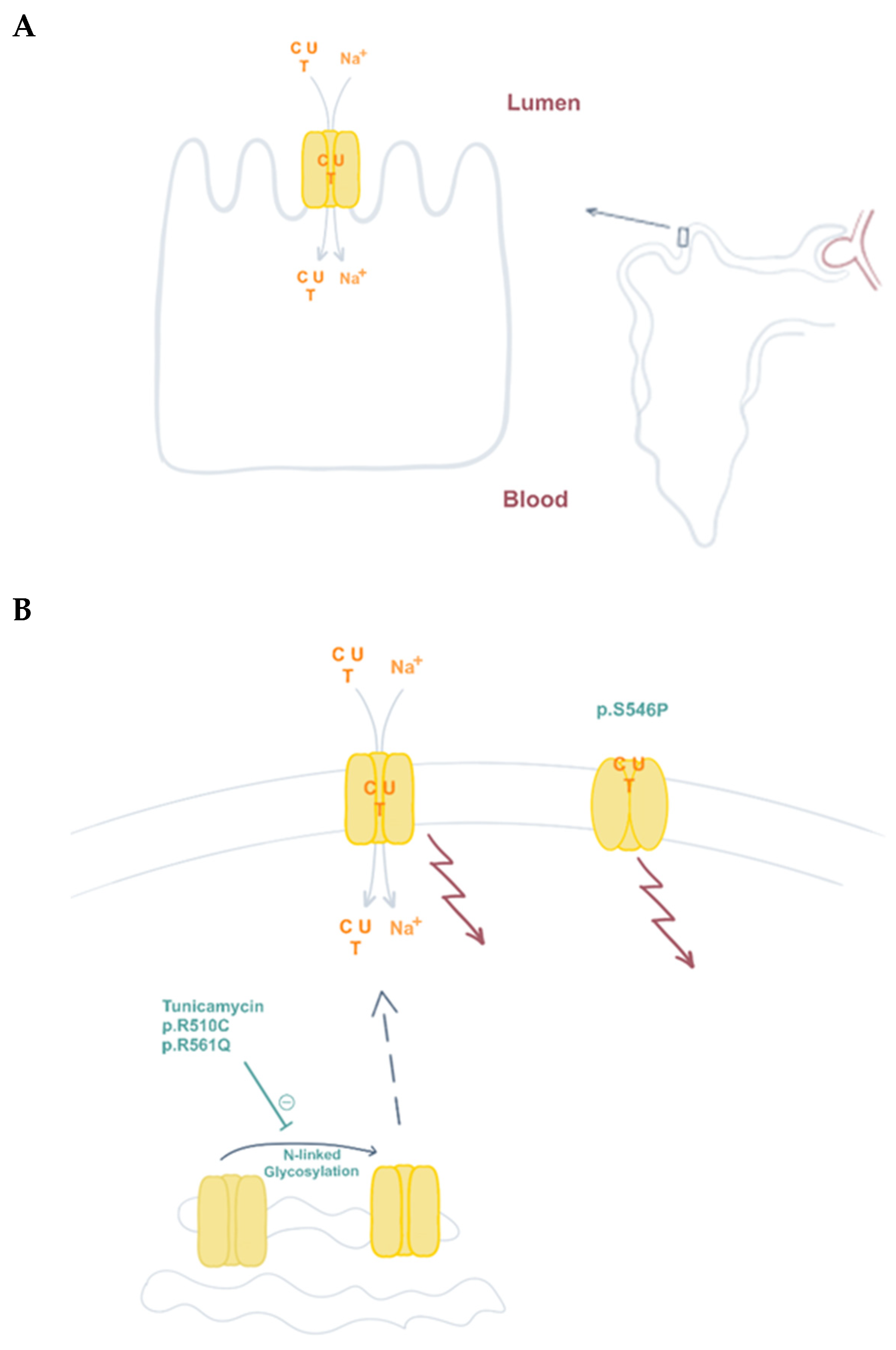
3.3. Genetic Alterations of CNT1. Uridine-Cytidineuria (URCTU). OMIM#618477
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jurecka, A.; Tylki-Szymanska, A. Inborn errors of purine and pyrimidine metabolism: A guide to diagnosis. Mol. Genet. Metab. 2022, 136, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, J.P.; Marie, S.; Nassogne, M.-C. Disorders of purine biosynthesis metabolism. Mol. Genet. Metab. 2021, 136, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Jurecka, A. Inborn errors of purine and pyrimidine metabolism. J. Inherit. Metab. Dis. 2009, 32, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Lesch, M.; Nyhan, W.L. A familial disorder of uric acid metabolism and central nervous system function. Am. J. Med. 1964, 36, 561–570. [Google Scholar] [CrossRef]
- Seegmiller, J.E.; Rosenbloom, F.M.; Kelley, W.N. Enzyme Defect Associated with a Sex-Linked Human Neurological Disorder and Excessive Purine Synthesis. Science 1967, 155, 1682–1684. [Google Scholar] [CrossRef] [PubMed]
- Ochs, H.; Yount, J.; Giblett, E.; Chen, S.; Scott, C.; Wedgwood, R. Adenosine-deaminase deficiency and severe combined immunodeficiency syndrome. Lancet 1973, 301, 1393–1394. [Google Scholar] [CrossRef]
- Wadman, S.K.; de Bree, P.K.; van Gennip, A.H.; Stoop, J.W.; Zegers, B.J.M.; Staal, G.E.J.; van Heukelom, L.H.S. Urinary Purines in a Patient with a Severely Defective T Cell Immunity and a Purine Nucleoside Phosphorylase Deficiency. In Metabolism in Man—II. Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1977; pp. 471–476. [Google Scholar] [CrossRef]
- Cámara, Y.; González-Vioque, E.; Scarpelli, M.; Torres-Torronteras, J.; Martí, R. Feeding the deoxyribonucleoside salvage pathway to rescue mitochondrial DNA. Drug Discov. Today 2013, 18, 950–957. [Google Scholar] [CrossRef]
- Ramón, J.; Vila-Julià, F.; Molina-Granada, D.; Molina-Berenguer, M.; Melià, M.; García-Arumí, E.; Torres-Torronteras, J.; Cámara, Y.; Martí, R. Therapy Prospects for Mitochondrial DNA Maintenance Disorders. Int. J. Mol. Sci. 2021, 22, 6447. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, C.; Miazzi, C.; Franzolin, E.; Pontarin, G.; Ferraro, P.; Frangini, M.; Reichard, P.; Bianchi, V. Regulation by degradation, a cellular defense against deoxyribonucleotide pool imbalances. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 703, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.B.; Buckingham, K.J.; Lee, C.; Bigham, A.W.; Tabor, H.K.; Dent, K.M.; Huff, C.D.; Shannon, P.T.; Jabs, E.W.; Nickerson, D.A.; et al. Exome sequencing identifies the cause of a mendelian disorder. Nat. Genet. 2009, 42, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Löffler, M.; Carrey, E.A.; Zameitat, E. Orotic Acid, More Than Just an Intermediate of Pyrimidine de novo Synthesis. J. Genet. Genom. 2015, 42, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Caño-Ochoa, F.; Ramón-Maiques, S. Deciphering CAD: Structure and function of a mega-enzymatic pyrimidine factory in health and disease. Protein Sci. 2021, 30, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.G.; Wolfe, L.A.; Ichikawa, M.; Markello, T.; He, M.; Tifft, C.J.; Gahl, W.A.; Freeze, H.H. Biallelic mutations in CAD, impair de novo pyrimidine biosynthesis and decrease glycosylation precursors. Hum. Mol. Genet. 2015, 24, 3050–3057. [Google Scholar] [CrossRef] [PubMed]
- Jaeken, J.; van den Berghe, G. An infantile autistic syndrome characterised by the presence of succinylpurines in body fluids. Lancet 1984, 2, 1058–1061. [Google Scholar]
- Stone, R.L.; Aimi, J.; Barshop, B.A.; Jaeken, J.; Berghe, G.V.D.; Zalkin, H.; Dixon, J.E. A mutation in adenylosuccinate lyase associated with mental retardation and autistic features. Nat. Genet. 1992, 1, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiorgio, G.; Macchiaiolo, M.; Buonuomo, P.S.; Bellacchio, E.; Bordi, M.; Vecchio, D.; Brown, K.P.; Watson, N.K.; Contardi, B.; Cecconi, F.; et al. Clinical and molecular characterization of patients with adenylosuccinate lyase deficiency. Orphanet J. Rare Dis. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Marie, S.; Heron, B.; Bitoun, P.; Timmerman, T.; Berghe, G.V.D.; Vincent, M.-F. AICA-Ribosiduria: A Novel, Neurologically Devastating Inborn Error of Purine Biosynthesis Caused by Mutation of ATIC. Am. J. Hum. Genet. 2004, 74, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Pelet, A.; Skopova, V.; Steuerwald, U.; Baresova, V.; Zarhrate, M.; Plaza, J.-M.; Hnizda, A.; Krijt, M.; Souckova, O.; Wibrand, F.; et al. PAICS deficiency, a new defect of de novo purine synthesis resulting in multiple congenital anomalies and fatal outcome. Hum. Mol. Genet. 2019, 28, 3805–3814. [Google Scholar] [CrossRef] [PubMed]
- Ramond, F.; Rio, M.; Héron, B.; Imbard, A.; Marie, S.; Billiemaz, K.; Denommé-Pichon, A.; Kuentz, P.; Ceballos, I.; Piraud, M.; et al. AICA-ribosiduria due to ATIC deficiency: Delineation of the phenotype with three novel cases, and long-term update on the first case. J. Inherit. Metab. Dis. 2020, 43, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Krijt, M.; Souckova, O.; Baresova, V.; Skopova, V.; Zikanova, M. Metabolic Tools for Identification of New Mutations of Enzymes Engaged in Purine Synthesis Leading to Neurological Impairment. Folia Biologica 2019, 65, 152–157. [Google Scholar]
- Jing, X.; Wang, X.-J.; Zhang, T.; Zhu, W.; Fang, Y.; Wu, H.; Liu, X.; Ma, D.; Ji, X.; Jiang, Y.; et al. Cell-Cycle–Dependent Phosphorylation of PRPS1 Fuels Nucleotide Synthesis and Promotes Tumorigenesis. Cancer Res. 2019, 79, 4650–4664. [Google Scholar] [CrossRef] [PubMed]
- Molina-Arcas, M.; Javier Casado, F.; Pastor-Anglada, M. Nucleoside Transporter Proteins. Curr. Vasc. Pharmacol. 2009, 7, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Young, J.D.; Yao, S.Y.; Baldwin, J.M.; Cass, C.E.; Baldwin, S.A. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol. Asp. Med. 2013, 34, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Anglada, M.; Pérez-Torras, S. Who Is Who in Adenosine Transport. Front. Pharmacol. 2018, 9, 627. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Urtasun, N.; Pérez-Torras, S. Intestinal Nucleoside Transporters: Function, Expression, and Regulation. Compr. Physiol. 2011, 8, 1003–1017. [Google Scholar] [CrossRef]
- Yao, S.Y.; Ng, A.M.; Cass, C.E.; Baldwin, S.A.; Young, J.D. Nucleobase Transport by Human Equilibrative Nucleoside Transporter 1 (hENT1). J. Biol. Chem. 2011, 286, 32552–32562. [Google Scholar] [CrossRef]
- Lee, E.-W.; Lai, Y.; Zhang, H.; Unadkat, J.D. Identification of the Mitochondrial Targeting Signal of the Human Equilibrative Nucleoside Transporter 1 (hENT1). J. Biol. Chem. 2006, 281, 16700–16706. [Google Scholar] [CrossRef] [PubMed]
- Grañé-Boladeras, N.; Spring, C.M.; Hanna, W.J.B.; Pastor-Anglada, M.; Coe, I.R. Novel nuclear hENT2 isoforms regulate cell cycle progression via controlling nucleoside transport and nuclear reservoir. Experientia 2016, 73, 4559–4575. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.A.; Yao, S.Y.M.; Hyde, R.J.; Ng, A.M.L.; Foppolo, S.; Barnes, K.; Ritzel, M.W.L.; Cass, C.E.; Young, J.D. Functional Characterization of Novel Human and Mouse Equilibrative Nucleoside Transporters (hENT3 and mENT3) Located in Intracellular Membranes. J. Biol. Chem. 2005, 280, 15880–15887. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Govindarajan, R. ENT3 utilizes a pH Sensing Mechanism for Transport. Channels 2017, 12, 78–80. [Google Scholar] [CrossRef]
- Govindarajan, R.; Leung, G.P.H.; Zhou, M.; Tse, C.-M.; Wang, J.; Unadkat, J.D. Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am. J. Physiol. Liver Physiol. 2009, 296, G910–G922. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.; Wang, J. Interaction of Organic Cations with a Newly Identified Plasma Membrane Monoamine Transporter. Mol. Pharmacol. 2005, 68, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.; Dobrzynski, H.; Foppolo, S.; Beal, P.R.; Ismat, F.; Scullion, E.R.; Sun, L.; Tellez, J.; Ritzel, M.W.; Claycomb, W.C.; et al. Distribution and Functional Characterization of Equilibrative Nucleoside Transporter-4, a Novel Cardiac Adenosine Transporter Activated at Acidic pH. Circ. Res. 2006, 99, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, J.; Inoue, K.; Maeda, J.; Yasujima, T.; Ohta, K.; Kanai, Y.; Takada, T.; Matsuo, H.; Yuasa, H. Functional identification of SLC43A3 as an equilibrative nucleobase transporter involved in purine salvage in mammals. Sci. Rep. 2015, 5, 15057. [Google Scholar] [CrossRef]
- Suzuki, S.; Inoue, K.; Tamai, I.; Shirasaka, Y. Model Analysis of the Apparent Saturation Kinetics of Purine Nucleobase Uptake in Cells co-Expressing Transporter and Metabolic Enzyme. Pharm. Res. 2021, 38, 1585–1592. [Google Scholar] [CrossRef]
- Errasti-Murugarren, E.; Pastor-Anglada, M.; Casado, F.J. Role of CNT3 in the transepithelial flux of nucleosides and nucleoside-derived drugs. J. Physiol. 2007, 582, 1249–1260. [Google Scholar] [CrossRef]
- Rodraguez-Mulero, S.; Errasti-Murugarren, E.; Ballaran, J.; Felipe, A.; Doucet, A.; Casado, F.; Pastor-Anglada, M. Expression of concentrative nucleoside transporters SLC28 (CNT1, CNT2, and CNT3) along the rat nephron: Effect of diabetes. Kidney Int. 2005, 68, 665–672. [Google Scholar] [CrossRef]
- Griffiths, M.; Beaumont, N.; Yao, S.Y.M.; Sundaram, M.; Boumah, C.E.; Davies, A.; Kwong, F.Y.P.; Coe, I.; Cass, C.E.; Young, J.D.; et al. Cloning of a human nucleoside transporter implicated in the Cellular uptake of adenosine and chemotherapeutic drugs. Nat. Med. 1997, 3, 89–93. [Google Scholar] [CrossRef]
- Wright, N.J.; Lee, S.-Y. Structures of human ENT1 in complex with adenosine reuptake inhibitors. Nat. Struct. Mol. Biol. 2019, 26, 599–606. [Google Scholar] [CrossRef]
- Dos Santos-Rodrigues, A.; Grañé-Boladeras, N.; Bicket, A.; Coe, I.R. Nucleoside transporters in the purinome. Neurochem. Int. 2014, 73, 229–237. [Google Scholar] [CrossRef]
- Choi, O.-S.; Cascini, M.-G.; Mailliard, W.; Young, H.; Paredes, P.; McMahon, T.; Diamond, I.; Bonci, A.; O Messing, R. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat. Neurosci. 2004, 7, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Daniels, G.; Ballif, B.A.; Helias, V.; Saison, C.; Grimsley, S.; Mannessier, L.; Hustinx, H.; Lee, E.; Cartron, J.-P.; Peyrard, T.; et al. Lack of the nucleoside transporter ENT1 results in the Augustine-null blood type and ectopic mineralization. Blood 2015, 125, 3651–3654. [Google Scholar] [CrossRef] [PubMed]
- Zwifelhofer, N.M.; Cai, X.; Liao, R.; Mao, B.; Conn, D.J.; Mehta, C.; Keles, S.; Xia, Y.; Bresnick, E.H. GATA factor-regulated solute carrier ensemble reveals a nucleoside transporter-dependent differentiation mechanism. PLoS Genet. 2020, 16, e1009286. [Google Scholar] [CrossRef] [PubMed]
- Mikdar, M.; González-Menéndez, P.; Cai, X.; Zhang, Y.; Serra, M.; Dembélé, A.K.; Boschat, A.-C.; Sanquer, S.; Chhuon, C.; Guerrera, I.C.; et al. The equilibrative nucleoside transporter ENT1 is critical for nucleotide homeostasis and optimal erythropoiesis. Blood 2021, 137, 3548–3562. [Google Scholar] [CrossRef]
- Warraich, S.; Bone, D.; Quinonez, D.; Ii, H.; Choi, D.-S.; Holdsworth, D.; Drangova, M.; Dixon, S.J.; Séguin, C.A.; Hammond, J. Loss of equilibrative nucleoside transporter 1 in mice leads to progressive ectopic mineralization of spinal tissues resembling diffuse idiopathic skeletal hyperostosis in humans. J. Bone Miner. Res. 2012, 28, 1135–1149. [Google Scholar] [CrossRef]
- Fournier, D.E.; Beaucage, K.L.; Beach, R.J.; Kiser, P.K.; Séguin, C.A.; Dixon, S.J. Ectopic mineralisation of the mandibular symphysis in ENT1 knockout mice: A model of dystrophic calcification. Bone Rep. 2021, 15, 101100. [Google Scholar] [CrossRef]
- Carluccio, M.; Ziberi, S.; Zuccarini, M.; Giuliani, P.; Caciagli, F.; Di Iorio, P.; Ciccarelli, R. Adult mesenchymal stem cells: Is there a role for purine receptors in their osteogenic differentiation? Purinergic Signal. 2020, 16, 263–287. [Google Scholar] [CrossRef]
- Altaweraqi, R.A.; Yao, S.Y.; Smith, K.M.; Cass, C.E.; Young, J.D. HPLC reveals novel features of nucleoside and nucleobase homeostasis, nucleoside metabolism and nucleoside transport. Biochim. Biophys. Acta (BBA) Biomembr. 2020, 1862, 183247. [Google Scholar] [CrossRef]
- Chen, Z.-S.; Lee, K.; Kruh, G.D. Transport of Cyclic Nucleotides and Estradiol 17-β-d-Glucuronide by Multidrug Resistance Protein 4. J. Biol. Chem. 2001, 276, 33747–33754. [Google Scholar] [CrossRef]
- Grañe-Boladeras, N.; Williams, D.; Tarmakova, Z.; Stevanovic, K.; Villani, L.A.; Mehrabi, P.; Siu, K.W.M.; Pastor-Anglada, M.; Coe, I.R. Oligomerization of equilibrative nucleoside transporters: A novel regulatory and functional mechanism involving PKC and PP1. FASEB J. 2018, 33, 3841–3850. [Google Scholar] [CrossRef]
- Molho-Pessach, V.; Lerer, I.; Abeliovich, D.; Agha, Z.; Abu Libdeh, A.; Broshtilova, V.; Elpeleg, O.; Zlotogorski, A. The H Syndrome Is Caused by Mutations in the Nucleoside Transporter hENT3. Am. J. Hum. Genet. 2008, 83, 529–534. [Google Scholar] [CrossRef]
- Cliffe, S.T.; Kramer, J.M.; Hussain, K.; Robben, J.H.; de Jong, E.K.; de Brouwer, A.P.; Nibbeling, E.; Kamsteeg, E.-J.; Wong, M.; Prendiville, J.; et al. SLC29A3 gene is mutated in pigmented hypertrichosis with insulin-dependent diabetes mellitus syndrome and interacts with the insulin signaling pathway. Hum. Mol. Genet. 2009, 18, 2257–2265. [Google Scholar] [CrossRef]
- Morgan, N.V.; Morris, M.R.; Cangul, H.; Gleeson, D.; Straatman-Iwanowska, A.; Davies, N.; Keenan, S.; Pasha, S.; Rahman, F.; Gentle, D.; et al. Mutations in SLC29A3, Encoding an Equilibrative Nucleoside Transporter ENT3, Cause a Familial Histiocytosis Syndrome (Faisalabad Histiocytosis) and Familial Rosai-Dorfman Disease. PLoS Genet. 2010, 6, e1000833. [Google Scholar] [CrossRef]
- Campeau, P.; Lu, J.T.; Sule, G.; Jiang, M.-M.; Bae, Y.; Madan, S.; Hoegler, W.; Shaw, N.J.; Mumm, S.; Gibbs, R.A.; et al. Whole-exome sequencing identifies mutations in the nucleoside transporter gene SLC29A3 in dysosteosclerosis, a form of osteopetrosis. Hum. Mol. Genet. 2012, 21, 4904–4909. [Google Scholar] [CrossRef]
- Kang, N.; Jun, A.H.; Bhutia, Y.D.; Kannan, N.; Unadkat, J.D.; Govindarajan, R. Human Equilibrative Nucleoside Transporter-3 (hENT3) Spectrum Disorder Mutations Impair Nucleoside Transport, Protein Localization, and Stability. J. Biol. Chem. 2010, 285, 28343–28352. [Google Scholar] [CrossRef]
- Molho-Pessach, V.; Ramot, Y.; Camille, F.; Doviner, V.; Babay, S.; Luis, S.J.; Broshtilova, V.; Zlotogorski, A. H syndrome: The first 79 patients. J. Am. Acad. Dermatol. 2014, 70, 80–88. [Google Scholar] [CrossRef]
- Wright, N.J.; Lee, S.-Y. Toward a Molecular Basis of Cellular Nucleoside Transport in Humans. Chem. Rev. 2020, 121, 5336–5358. [Google Scholar] [CrossRef]
- de Jesus, J.; Imane, Z.; Senee, V.; Romero, S.; Guillausseau, P.-J.; Balafrej, A.; Julier, C. SLC29A3 mutation in a patient with syndromic diabetes with features of pigmented hypertrichotic dermatosis with insulin-dependent diabetes, H syndrome and Faisalabad histiocytosis. Diabetes Metab. 2013, 39, 281–285. [Google Scholar] [CrossRef]
- Huber-Ruano, I.; Errasti-Murugarren, E.; Godoy, V.; Vera, Á.; Andreu, A.L.; Garcia-Arumi, E.; Martí, R.; Pastor-Anglada, M. Functional outcome of a novel SLC29A3 mutation identified in a patient with H syndrome. Biochem. Biophys. Res. Commun. 2012, 428, 532–537. [Google Scholar] [CrossRef]
- Bolze, A.; Abhyankar, A.; Grant, A.V.; Patel, B.; Yadav, R.; Byun, M.; Caillez, D.; Emile, J.-F.; Anglada, M.P.; Abel, L.; et al. A Mild Form of SLC29A3 Disorder: A Frameshift Deletion Leads to the Paradoxical Translation of an Otherwise Noncoding mRNA Splice Variant. PLoS ONE 2012, 7, e29708. [Google Scholar] [CrossRef]
- Kismet, E.; Köseoglu, V.; Atay, A.A.; Deveci, S.; Demirkaya, E.; Tuncer, K. Sinus histiocytosis with massive lymphadenopathy in three brothers. Pediatr. Int. 2005, 47, 473–476. [Google Scholar] [CrossRef]
- Pérez-Torras, S.; Mata-Ventosa, A.; Drögemöller, B.; Tarailo-Graovac, M.; Meijer, J.; Meinsma, R.; van Cruchten, A.G.; Kulik, W.; Viel-Oliva, A.; Bidon-Chanal, A.; et al. Deficiency of perforin and hCNT1, a novel inborn error of pyrimidine metabolism, associated with a rapidly developing lethal phenotype due to multi-organ failure. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 1182–1191. [Google Scholar] [CrossRef]
- Wevers, R.A.; Christensen, M.; Engelke, U.F.H.; Geuer, S.; Coene, K.; Kwast, J.T.; Lund, A.M.; Vissers, L.E.L.M. Functional disruption of pyrimidine nucleoside transporter CNT1 results in a novel inborn error of metabolism with high excretion of uridine and cytidine. J. Inherit. Metab. Dis. 2019, 42, 494–500. [Google Scholar] [CrossRef]
- Liu, B.; Czajka, A.; Malik, A.; Hussain, K.; Jones, P.; Persaud, S. Equilibrative nucleoside transporter 3 depletion in β-cells impairs mitochondrial function and promotes apoptosis: Relationship to pigmented hypertrichotic dermatosis with insulin-dependent diabetes. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 2086–2095. [Google Scholar] [CrossRef][Green Version]
- Akinci, E.; Cha, M.; Lin, L.; Yeo, G.; Hamilton, M.C.; Donahue, C.J.; Bermudez-Cabrera, H.C.; Zanetti, L.C.; Chen, M.; Barkal, S.A.; et al. Elucidation of remdesivir cytotoxicity pathways through genome-wide CRISPR-Cas9 screening and tran-scriptomics. bioRxiv 2020. [CrossRef]
- Miller, S.R.; McGrath, M.E.; Zorn, K.M.; Ekins, S.; Wright, S.H.; Cherrington, N.J. Remdesivir and EIDD-1931 Interact with Human Equilibrative Nucleoside Transporters 1 and 2: Implications for Reaching SARS-CoV-2 Viral Sanctuary Sites. Mol. Pharmacol. 2021, 100, 548–557. [Google Scholar] [CrossRef]
- Bissa, B.; Beedle, A.M.; Govindarajan, R. Lysosomal solute carrier transporters gain momentum in research. Clin. Pharmacol. Ther. 2016, 100, 431–436. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Lin, W.; Seshasayee, D.; Chen, Y.-H.; Ding, X.; Lin, Z.; Suto, E.; Huang, Z.; Lee, W.P.; Park, H.; et al. Equilibrative Nucleoside Transporter 3 Deficiency Perturbs Lysosome Function and Macrophage Homeostasis. Science 2012, 335, 89–92. [Google Scholar] [CrossRef]
- Nair, S.; Strohecker, A.M.; Persaud, A.K.; Bissa, B.; Muruganandan, S.; McElroy, C.; Pathak, R.; Williams, M.; Raj, R.; Kaddoumi, A.; et al. Adult stem cell deficits drive Slc29a3 disorders in mice. Nat. Commun. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- Wei, C.-W.; Lee, C.-Y.; Lee, D.-J.; Chu, C.-F.; Wang, J.-C.; Wang, T.-C.; Jane, W.-N.; Chang, Z.-F.; Leu, C.-M.; Dzhagalov, I.L.; et al. Equilibrative Nucleoside Transporter 3 Regulates T Cell Homeostasis by Coordinating Lysosomal Function with Nucleoside Availability. Cell Rep. 2018, 23, 2330–2341. [Google Scholar] [CrossRef]
- Persaud, A.K.; Nair, S.; Rahman, F.; Raj, R.; Weadick, B.; Nayak, D.; McElroy, C.; Shanmugam, M.; Knoblaugh, S.; Cheng, X.; et al. Facilitative lysosomal transport of bile acids alleviates ER stress in mouse hematopoietic precursors. Nat. Commun. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Sigurdsson, V.; Takei, H.; Soboleva, S.; Radulovic, V.; Galeev, R.; Siva, K.; Leeb-Lundberg, L.F.; Iida, T.; Nittono, H.; Miharada, K. Bile Acids Protect Expanding Hematopoietic Stem Cells from Unfolded Protein Stress in Fetal Liver. Cell Stem Cell 2016, 18, 522–532. [Google Scholar] [CrossRef]
- Neste, E.V.D.; Berghe, G.V.D.; Bontemps, F. AICA-riboside (acadesine), an activator of AMP-activated protein kinase with potential for application in hematologic malignancies. Expert Opin. Investig. Drugs 2010, 19, 571–578. [Google Scholar] [CrossRef]
- Lara-Reyna, S.; Poulter, J.A.; Vasconcelos, E.J.; Kacar, M.; McDermott, M.F.; Tooze, R.; Doffinger, R.; Savic, S. Identification of Critical Transcriptomic Signaling Pathways in Patients with H Syndrome and Rosai-Dorfman Disease. J. Clin. Immunol. 2020, 41, 441–457. [Google Scholar] [CrossRef]
- Rafiq, N.K.; Hussain, K.; Brogan, P.A. Tocilizumab for the Treatment of SLC29A3 Mutation Positive PHID Syndrome. Pediatrics 2017, 140. [Google Scholar] [CrossRef]
- Behrangi, E.; Sadeghzadeh-Bazargan, A.; Khosravi, S.; Shemshadi, M.; Youssefian, L.; Vahidnezhad, H.; Goodarzi, A.; Uitto, J. Mycophenolate mofetil treatment of an H syndrome patient with a SLC29A3 mutation. Dermatol. Ther. 2020, 33, e14375. [Google Scholar] [CrossRef]
- Nofal, H.; AlAkad, R.; Nofal, A.; Rabie, E.; Chaikul, T.; Chiu, F.P.; Pramanik, R.; Alabdulkareem, A.; Onoufriadis, A. H syndrome: A review of treatment options and a hypothesis of phenotypic variability. Dermatol. Ther. 2021, 34, e15082. [Google Scholar] [CrossRef] [PubMed]
- Arimany-Nardi, C.; Claudio-Montero, A.; Viel-Oliva, A.; Schmidtke, P.; Estarellas, C.; Barril, X.; Bidon-Chanal, A.; Pastor-Anglada, M. Identification and Characterization of a Secondary Sodium-Binding Site and the Main Selectivity Determinants in the Human Concentrative Nucleoside Transporter 3. Mol. Pharm. 2017, 14, 1980–1987. [Google Scholar] [CrossRef]
- Naneh, O.; Avčin, T.; Zavec, A.B. Perforin and Human Diseases. In MACPF/CDC Proteins—Agents of Defence, Attack and Invasion; Springer: Dordrecht, The Netherlands, 2014; Volume 80, pp. 221–239. [Google Scholar] [CrossRef]
- Leabman, M.K.; Huang, C.C.; DeYoung, J.; Carlson, E.J.; Taylor, T.R.; de la Cruz, M.; Johns, S.J.; Stryke, D.; Kawamoto, M.; Urban, T.J.; et al. Natural variation in human membrane transporter genes reveals evolutionary and functional constraints. Proc. Natl. Acad. Sci. USA 2003, 100, 5896–5901. [Google Scholar] [CrossRef]
- Gray, J.H.; Mangravite, L.M.; Owen, R.P.; Urban, T.J.; Chan, W.; Carlson, E.J.; Huang, C.C.; Kawamoto, M.; Johns, S.J.; Stryke, D.; et al. Functional and Genetic Diversity in the Concentrative Nucleoside Transporter, CNT1, in Human Populations. Mol. Pharmacol. 2004, 65, 512–519. [Google Scholar] [CrossRef]
- Cano-Soldado, P.; Gorraitz, E.; Errasti-Murugarren, E.; Casado, F.J.; Lostao, M.P.; Pastor-Anglada, M. Functional analysis of the human concentrative nucleoside transporter-1 variant hCNT1S546P provides insight into the sodium-binding pocket. Am. J. Physiol. Physiol. 2012, 302, C257–C266. [Google Scholar] [CrossRef] [PubMed]
- Farré, X.; Guillén-Gómez, E.; Sánchez, L.; Hardisson, D.; Plaza, Y.; Lloberas, J.; Casado, F.J.; Palacios, J.; Pastor-Anglada, M. Expression of the nucleoside-derived drug transporters hCNT1, hENT1 and hENT2 in gynecologic tumors. Int. J. Cancer 2004, 112, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Urtasun, N.; Boces-Pascual, C.; Boix, L.; Bruix, J.; Pastor-Anglada, M.; Pérez-Torras, S. Role of drug-dependent transporter modulation on the chemosensitivity of cholangiocarcinoma. Oncotarget 2017, 8, 90185–90196. [Google Scholar] [CrossRef] [PubMed]
- Boces-Pascual, C.; Mata-Ventosa, A.; Martín-Satué, M.; Boix, L.; Gironella, M.; Pastor-Anglada, M.; Pérez-Torras, S. OncomiRs miR-106a and miR-17 negatively regulate the nucleoside-derived drug transporter hCNT1. Experientia 2021, 78, 7505–7518. [Google Scholar] [CrossRef]
- Pérez-Torras, S.; Vidal-Pla, A.; Cano-Soldado, P.; Huber-Ruano, I.; Mazo, A.; Pastor-Anglada, M. Concentrative nucleoside transporter 1 (hCNT1) promotes phenotypic changes relevant to tumor biology in a translocation-independent manner. Cell Death Dis. 2013, 4, e648. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Emerging Roles of Nucleoside Transporters. Front. Pharmacol. 2018, 9, 606. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, L.; Wang, C.; Li, J.; Chi, P.; Xiao, Q.; Liu, Q.; Guo, L.; Sun, L.; Deng, D. Cryo-EM structure of the human concentrative nucleoside transporter CNT3. PLoS Biol. 2020, 18, e3000790. [Google Scholar] [CrossRef]


| Syndrome | Gene (Location) | Protein | Variant | Heredity | Clinical Features | Reference |
|---|---|---|---|---|---|---|
| Augustine-null blood type | SLC29A1 (6p21.1) | hENT1 | c.1171G>A (p.E391K) | - | Rare blood type (Ata) | [43] |
| c.589+1G>C | AR | Atnull—progressive ectopic calcification of bone joints | [43,45] | |||
| Histiocytosis- lymphadenopathy plus syndrome (OMIM #602782) entry 3 | SLC29A3 (10q22.1) | hENT3 | p.G427S p.G437R c.1045delC | AR | H syndrome: hyperpigmentation and hypertrichosis, hepatosplenomegaly, heart anomalies, hearing loss, hypogonadism, low height and hyperglycemia | [57] |
| p.M116R c.940delT p.G437R p.E444X p.T449R | AR | Pigmented hypertrichotic dermatosis insulin-dependent diabetes (PHID) | [53] | |||
| c.300+1G>A | AR | Faisalabad histiocytosis (FH) | [54] | |||
| p.G437R | AR | Familial Rosai-Dorfman disease (RDD) | [54] | |||
| p.G437R p.F103X | AR | Familial sinus histiocytosis with massive lymphadenopathy (SHML) | [62] | |||
| p.S203P;R386Q p.T449R | AR | Dysosteosclerosis (osteoporosis) | [54] | |||
| Uridine-cytidineuria, URCTU (OMIM #618477) | SLC28A1 (15q25.3) | hCNT1 | p.R510C;R561Q | CH | Uridine-cytidineuria | [63] |
| p.S564P | AR | Elevated urinary excretion of uridine and cytidine | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor-Anglada, M.; Mata-Ventosa, A.; Pérez-Torras, S. Inborn Errors of Nucleoside Transporter (NT)-Encoding Genes (SLC28 and SLC29). Int. J. Mol. Sci. 2022, 23, 8770. https://doi.org/10.3390/ijms23158770
Pastor-Anglada M, Mata-Ventosa A, Pérez-Torras S. Inborn Errors of Nucleoside Transporter (NT)-Encoding Genes (SLC28 and SLC29). International Journal of Molecular Sciences. 2022; 23(15):8770. https://doi.org/10.3390/ijms23158770
Chicago/Turabian StylePastor-Anglada, Marçal, Aida Mata-Ventosa, and Sandra Pérez-Torras. 2022. "Inborn Errors of Nucleoside Transporter (NT)-Encoding Genes (SLC28 and SLC29)" International Journal of Molecular Sciences 23, no. 15: 8770. https://doi.org/10.3390/ijms23158770
APA StylePastor-Anglada, M., Mata-Ventosa, A., & Pérez-Torras, S. (2022). Inborn Errors of Nucleoside Transporter (NT)-Encoding Genes (SLC28 and SLC29). International Journal of Molecular Sciences, 23(15), 8770. https://doi.org/10.3390/ijms23158770








