Post-Transplant Cyclophosphamide after Matched Sibling and Unrelated Donor Hematopoietic Stem Cell Transplantation in Pediatric Patients with Acute Myeloid Leukemia
Abstract
:1. Introduction
2. Results
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Discussion
4. Methods and Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doherty, E.E.; Redell, M.; Sasa, G.; Yassine, K.; John, T.D.; Craddock, J.; Wu, M.; Wang, T.; Martinez, C.A.; Krance, R.A.; et al. Outcomes after Allogeneic Hematopoietic Stem Cell Transplantation for Pediatric Acute Myeloid Leukemia in the Contemporary Era. Blood. 2019, 134 (Suppl. S1), 2056. [Google Scholar] [CrossRef]
- Gatza, E.; Reddy, P.; Choi, S.W. Prevention and Treatment of Acute Graft-versus-Host Disease in Children, Adolescents, and Young Adults. Biol. Blood Marrow Transpl. 2020, 26, e101–e112. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Mikulska, M.; Styczynski, J.; de la Camara, R. Coronavirus Disease COVID-19: Ebmt Recommendations Version 17 2022 May 20. 2022. Available online: https://www.ebmt.org/sites/default/files/2022-02/EBMT%20COVID-19%20guidelines%20v.17.2.pdf (accessed on 2 February 2022).
- Kanakry, C.G.; O’Donnell, P.V.; Furlong, T.; Lima, M.J.d.; Wei, W.; Medeot, M.; Mielcarek, M.; Champlin, R.E.; Jones, R.J.; Thall, P.F.; et al. Multi-Institutional Study of Post-Transplantation Cyclophosphamide As Single-Agent Graft-Versus-Host Disease Prophylaxis After Allogeneic Bone Marrow Transplantation Using Myeloablative Busulfan and Fludarabine Conditioning. J. Clin. Oncol. 2014, 32, 3497–3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raiola, A.M.; Dominietto, A.; Ghiso, A.; Di Grazia, C.; Lamparelli, T.; Gualandi, F.; Bregante, S.; Van Lint, M.T.; Geroldi, S.; Luchetti, S.; et al. Unmanipulated Haploidentical Bone Marrow Transplantation and Posttransplantation Cyclophosphamide for Hematologic Malignancies after Myeloablative Conditioning. Biol. Blood Marrow Transplant. 2013, 19, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Luznik, L.; Fuchs, E.J. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol. Res. 2010, 47, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.; Galimard, J.-E.; Labopin, M.; Afanasyev, B.; Angelucci, E.; Ciceri, F.; Blaise, D.; Cornelissen, J.J.; Meijer, E.; Diez-Martin, J.L.; et al. Post-transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: A comparative study of the ALWP EBMT. J. Hematol. Oncol. 2020, 13, 46. [Google Scholar] [CrossRef]
- Rimando, J.C.; McCurdy, S.R.; Luznik, L. How We Prevent GVHD in High Risk Patients: Post Transplant Cyclophosphamide and Beyond. Blood 2022, 35405017. [Google Scholar] [CrossRef]
- Bailen, R.; Pascual-Cascon, M.J.; Guerreiro, M.; Lopez-Corral, L.; Chinea, A.; Bermudez, A.; Sampol, A.; Heras, I.; García-Torres, E.; Torres, M.; et al. Post-Transplantation Cyclophosphamide After HLA Identical Compared to Haploidentical Donor Transplant in Acute Myeloid Leukemia: A Study on Behalf of GETH-TC. Transpl. Cell 2022, 28, 204.e1–204.e10. [Google Scholar] [CrossRef]
- Ruggeri, A.; Galimard, J.E.; Paina, O.; Fagioli, F.; Tbakhi, A.; Yesilipek, A.; Navarro, J.M.F.; Faraci, M.; Hamladji, R.-M.; Skorobogatova, E.; et al. Outcomes of Unmanipulated Haploidentical Transplantation Using Post-Transplant Cyclophosphamide (PT-Cy) in Pediatric Patients with Acute Lymphoblastic Leukemia. Transpl. Cell 2021, 27, 424.e1–424.e9. [Google Scholar] [CrossRef]
- Sharma, A.; Rastogi, N.; Chatterjee, G.; Kapoor, R.; Nivargi, S.; Yadav, S.P. Haploidentical Stem Cell Transplantation With Post-transplant Cyclophosphamide for Pediatric Acute Leukemia is Safe and Effective. J. Pediatr Hematol Oncol. 2021, 43, e1033–e1036. [Google Scholar]
- Popat, U.R.; Mehta, R.S.; Bassett, R.; Olson, A.L.; Alousi, A.M.; Anderlini, P.; Alatrash, G.; Bashir, Q.; Ciurea, S.O.; Hosing, C.; et al. Post-transplant cyclophosphamide in matched and haploidentical transplant recipients receiving myeloablative timed sequential busulfan conditioning regimen: Results of a phase II study. J. Clin. Oncol. 2019, 37 (Suppl. S15), 7007. [Google Scholar] [CrossRef]
- Pagliardini, T.; Castagna, L.; Harbi, S.; Della Porta, M.; Rey, J.; Fürst, S.; Bramanti, S.; Saillard, C.; Legrand, F.; Maisano, V.; et al. Thiotepa, Fludarabine, and Busulfan Conditioning Regimen before T Cell-Replete Haploidentical Transplantation with Post-Transplant Cyclophosphamide for Acute Myeloid Leukemia: A Bicentric Experience of 100 Patients. Biol. Blood Marrow Transplant. 2019, 25, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Duléry, R.; Bastos, J.; Paviglianiti, A.; Malard, F.; Brissot, E.; Battipaglia, G.; Médiavilla, C.; Giannotti, F.; Banet, A.; Van de Wyngaert, Z.; et al. Thiotepa, Busulfan, and Fludarabine Conditioning Regimen in T Cell-Replete HLA-Haploidentical Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1407–1415. [Google Scholar] [CrossRef]
- Luznik, L.; Bolaños-Meade, J.; Zahurak, M.; Chen, A.R.; Smith, B.D.; Brodsky, R.; Huff, C.A.; Borrello, I.; Matsui, W.; Powell, J.D.; et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 2010, 115, 3224–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mielcarek, M.; Furlong, T.; O’Donnell, P.V.; Storer, B.E.; McCune, J.S.; Storb, R.; Carpenter, P.A.; Flowers, M.E.; Appelbaum, F.R.; Martin, P.J. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood 2016, 127, 1502–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraiser, L.H.; Kanekal, S.; Kehrer, J.P. Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs 1991, 42, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Jacobsohn, D.A. Acute graft-versus-host disease in children. Bone Marrow Transplant. 2008, 41, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Cieri, N.; Peccatori, J.; Oliveira, G.; Greco, R.; Marktel, S.; Lunghi, F.; Ciceri, F.; Bonini, C. Tracking T Cell Dynamics in the First Month After Haplo-HSCT with Post-Transplant Cyclophosphamide Reveals a Predominant Contribution of Memory Stem T Cells to the Early Phase of Immune Reconstitution. Blood 2013, 122, 4615. [Google Scholar] [CrossRef]
- Williams, L.; Cirrone, F.; Cole, K.; Abdul-Hay, M.; Luznik, L.; Al-Homsi, A.S. Post-transplantation Cyclophosphamide: From HLA-Haploidentical to Matched-Related and Matched-Unrelated Donor Blood and Marrow Transplantation. Front. Immunol. 2020, 11, 636. [Google Scholar] [CrossRef]
- Motallebnezhad, M.; Jadidi-Niaragh, F.; Qamsari, E.S.; Bagheri, S.; Gharibi, T.; Yousefi, M. The immunobiology of myeloid-derived suppressor cells in cancer. Tumor Biol. 2016, 37, 1387–1406. [Google Scholar] [CrossRef]
- Oshrine, B.; Innamarato, P.; Branthoover, H.; Nagle, L.; Verdugo, P.; Pilon-Thomas, S.; Beatty, M. Early Recovery of Myeloid-Derived Suppressor Cells After Allogeneic Hematopoietic Transplant: Comparison of Post-Transplantation Cyclophosphamide to Standard Graft-Versus-Host Disease Prophylaxis. Transpl. Cell 2022, 28, 203.e1–203.e7. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Ross, D.B.; Panoskaltsis-Mortari, A.; Kanakry, C.G.; Blazar, B.R.; Levy, R.B.; Luznik, L. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood 2014, 124, 2131–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Dandoy, C.E.; Chen, M.; Kim, S.; Mulroney, C.M.; Kharfan-Dabaja, M.A.; Ganguly, S.; Maziarz, R.T.; Kanakry, C.G.; Kanakry, J.A.; et al. Post-Transplantation Cyclophosphamide Is Associated with an Increase in Non-Cytomegalovirus Herpesvirus Infections in Patients with Acute Leukemia and Myelodysplastic Syndrome. Transplant. Cell. Ther. 2022, 28, 48.e1–48.e10. [Google Scholar] [CrossRef] [PubMed]
- Mohty, R.; Brissot, E.; Battipaglia, G.; Ruggeri, A.; Dulery, R.; Bonnin, A.; Médiavilla, C.; Sestili, S.; Belhocine, R.; Vekhoff, A.; et al. Infectious complications after post-transplantation cyclophosphamide and anti-thymocyte globulin-based haploidentical stem cell transplantation. Br. J. Haematol. 2019, 187, e64–e68. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.S.; Saliba, R.M.; Ghanem, S.; Alousi, A.M.; Rondon, G.; Anderlini, P.; Al-Atrash, G.; Bashir, Q.; Hosing, C.M.; Im, J.S.; et al. Haploidentical vs. Matched Unrelated vs. Matched Sibling Donor HCT with Post-Transplantation Cyclophosphamide. Transpl. Cell 2022, 28, 395.e1–395.e11. [Google Scholar]
- Retière, C.; Willem, C.; Guillaume, T.; Vié, H.; Gautreau-Rolland, L.; Scotet, E.; Saulquin, X.; Gagne, K.; Béné, M.C.; Imbert, B.-M.; et al. Impact on early outcomes and immune reconstitution of high-dose post-transplant cyclophosphamide vs. anti-thymocyte globulin after reduced intensity conditioning peripheral blood stem cell allogeneic transplantation. Oncotarget 2018, 9, 11451–11464. [Google Scholar] [CrossRef] [Green Version]
- Yong, M.K.; Cameron, P.U.; Slavin, M.A.; Cheng, A.C.; Morrissey, C.O.; Bergin, K.; Spencer, A.; Ritchie, D.; Lewin, S.R. Low T-Cell Responses to Mitogen Stimulation Predicts Poor Survival in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2017, 8, 1506. [Google Scholar] [CrossRef] [Green Version]
- Norian, R.; Delirezh, N.; Azadmehr, A. Evaluation of proliferation and cytokines production by mitogen-stimulated bovine peripheral blood mononuclear cells. Vet Res Forum. 2015, 6, 265–271. [Google Scholar]
- Chan, A.; Hong, D.-L.; Atzberger, A.; Kollnberger, S.; Filer, A.D.; Buckley, C.D.; McMichael, A.; Enver, T.; Bowness, P. CD56bright Human NK Cells Differentiate into CD56dim Cells: Role of Contact with Peripheral Fibroblasts. J. Immunol. 2007, 179, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Okada, R.; Kondo, T.; Matsuki, F.; Takata, H.; Takiguchi, M. Phenotypic classification of human CD4+ T cell subsets and their differentiation. Int. Immunol. 2008, 20, 1189–1199. [Google Scholar] [CrossRef]
- Dawes, R.; Petrova, S.; Liu, Z.; Wraith, D.; Beverley, P.C.L.; Tchilian, E.Z. Combinations of CD45 isoforms are crucial for immune function and disease. J. Immunol. 2006, 176, 3417–3425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patient | Age (Years)/Sex | Diagnosis | Remission Status Pre HSCT | Pre-Transplant Complications | Stem Cell Source | HLA Match | Cell Dose | Sex Match R/D | ABO (R/D) | CMV R/D | GVHD Prophylaxis | Neutrophil/ Platelets (>20,000/µ) Engraftment (Days) | Follow-Up (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17/M | Primary refractory high risk acute myeloid leukemia with 11q23 rearrangement and FLT3-ITD (allelic ratio 0.02) | CR1 | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection treated with remdesivir | PBSC/MUD | 10/10 | TNC: 6.1 × 108/kg CD34+: 8.1 × 106/kg | M/F | A+/B+ | +/+ | IV PTCy 50 mg/kg on days +3 and +4 followed by MMF and tacrolimus starting day +5 | +16/+25 | +182 |
| 2 | 11/M | Relapsed refractory acute myeloid leukemia with reciprocal translocation 11q23 and 19p13.1, FLT3-ITD with allelic ratio of 0.91, NPM1 negative, FLT3-TKD negative | CR2 | Pulmonary toxoplasmosis | BM/MSD | 14/14 | TNC: 1.99 × 108/kg CD34+: 2.3 × 106/kg | M/F | A+/A+ | +/+ | IV PTCy 50 mg/kg on days +3 and +4 followed by MMF and tacrolimus starting day +5 | +17/+31 | +194 |
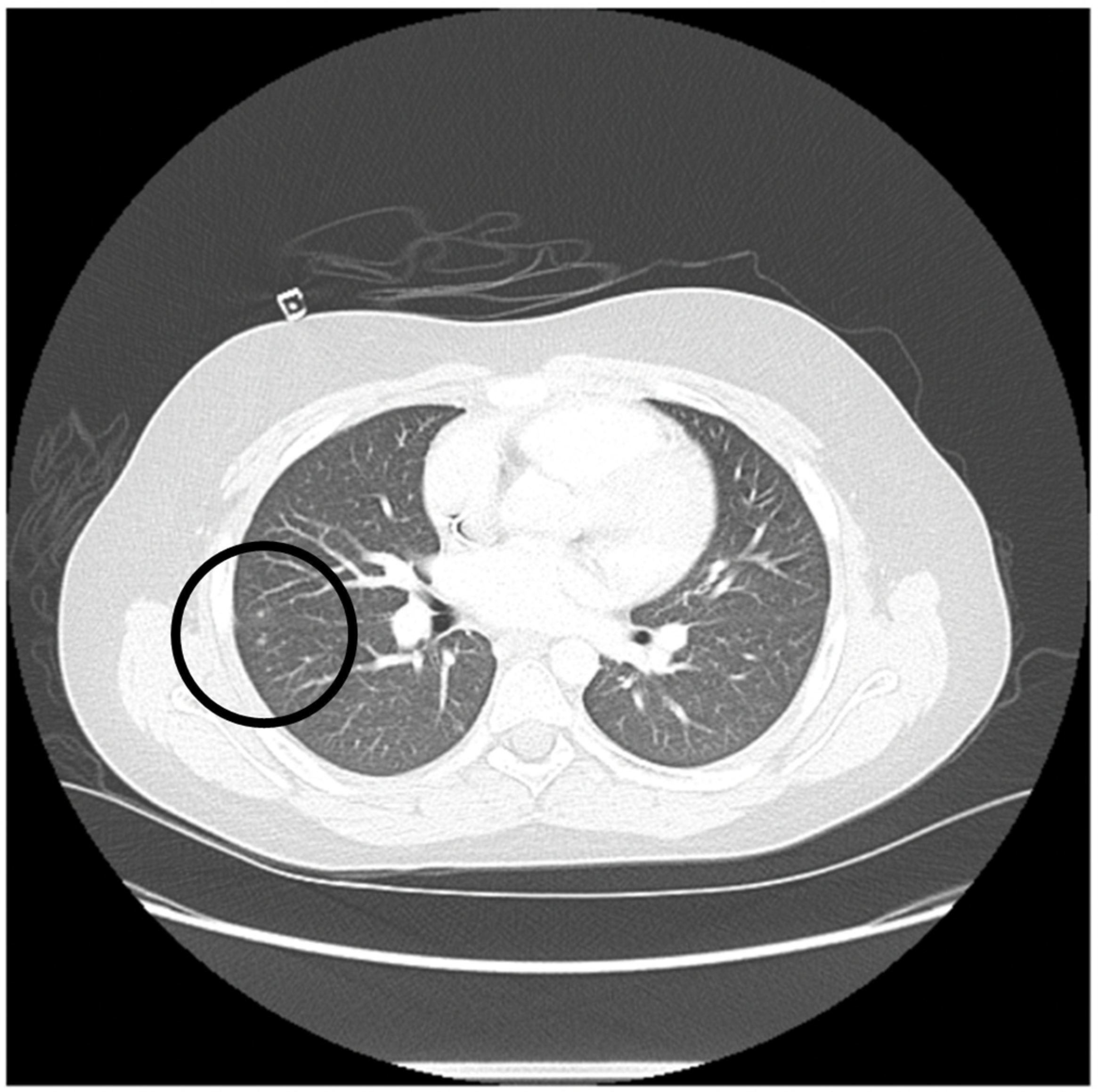
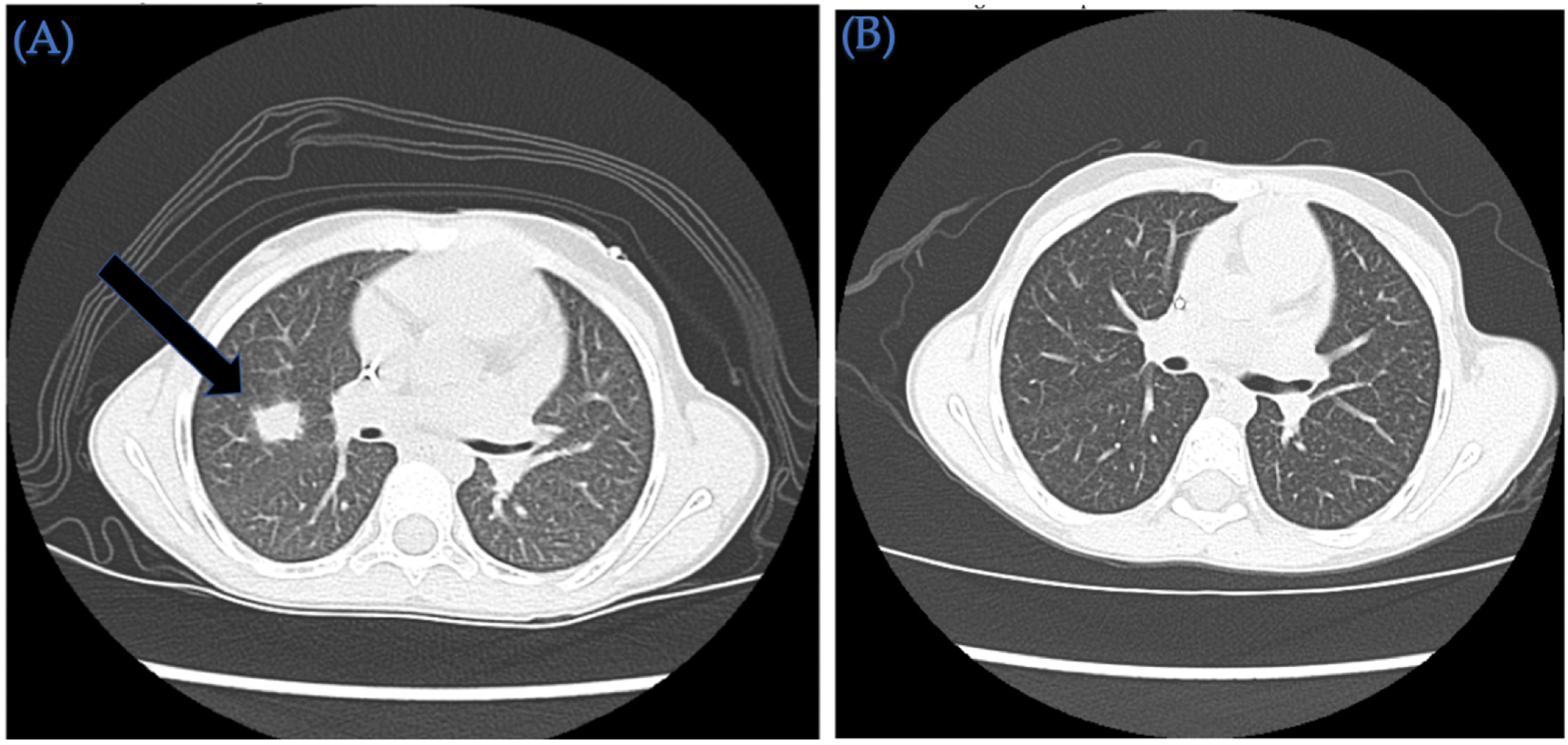
| 3 | 6/F | Very high risk acute myeloid leukemia with t(6;9) (p23;q34) and DEK/NUP214 fusion and FLT3-ITD positive (allelic ratio 0.75) | CR1 | Invasive pulmonary aspergillus infection requiring triple anti-fungal therapy (Voriconazole, liposomal amphotericin and caspofungin) SARS-CoV-2 infection treated with remdesivir | PBSC/MUD | 10/10 | TNC: 14.6 × 108/kg CD34+: 7.6 × 106/kg | F/M | O+/O+ | +/+ | IV PTCy 50 mg/kg on day +3 and +4 followed by MMF and tacrolimus starting day +5 | +16/+31 | +183 |
| Patient | Post-HSCT Complications | Treatment Administered | Result |
|---|---|---|---|
| 1 | Transient adenoviremia on day +40 (465 copies/mL) | Supportive care, No specific anti-viral therapy | Complete resolution |
| 2 | Asymptomatic intermittent low-level CMV viremia (<34.5 IU/mL) and mildly symptomatic BK viruria (transient microscopic hematuria and dysuria). | Supportive care, No specific anti-viral therapy | Complete resolution |
| 3 | Staphylococcus epidermidis bacteremia on day +8 | 2 weeks of IV vancomycin therapy | Complete resolution |
| Lymphocyte or Natural Killer Cell Subtype (Cells/Microliter) | Patient Number | 1 | 2 | 3 |
|---|---|---|---|---|
| Day Following Stem Cell Transplantation | +79 | +93 | +78 | |
| CD4/CD8 ratio (Normal: >1.0) | 3.83 | 0.68 | 2.36 | |
| CD3+ T cells (Normal range: 1000–2200) | 328 | 310 | 365 | |
| CD4+ T-helper cells (Normal range: 530–1300) | 253 | 117 | 238 | |
| CD4 + Subsets | CD4 Naïve (CD3+CD4+CD45RO−CD62L+) | 10 | 7 | 46 |
| CD4 Central memory (CD3+CD4+CD45RO+CD62L+) | 208 | 53 | 163 | |
| CD4 Effector memory (CD3+CD4+CD45RO+CD62L-) | 33 | 52 | 26 | |
| CD4 Terminally differentiated effector (CD3+CD4+CD45RO−CD62L-) | 1 | 0 | 1 | |
| CD8+ Cytotoxic T cells (Normal range: 330–920) | 66 | 172 | 101 | |
| CD8+ Subsets | CD8 Naïve (CD3+CD8+CD45RO−CD62L+) | 12 | 14 | 17 |
| CD8 Central Memory (CD3+CD8+CD45RO+CD62L+) | 28 | 38 | 63 | |
| CD8 Effector Memory (CD3+CD8+CD45RO+CD62L-) | 14 | 87 | 11 | |
| CD8 Terminally differentiated effector (CD3+CD8+CD45RO−CD62L-) | 8 | 29 | 1 | |
| CD19+ T-cells (Normal range: 110–570) | 153 | 111 | 191 | |
| CD19+ Subsets | Naïve B cells (CD19+CD27-) | 143 | 100 | 179 |
| Class-switched memory B cells (CD19+CD27+IgM-) | 1 | 5 | 1 | |
| IgM memory B cells (CD19+CD27+IgM+) | 2 | 3 | 4 | |
| CD56+ Subsets (Normal range: >60) | CD56+CD3- | 77 | 72 | 82 |
| CD56bright+CD3- | 59 | 40 | 41 | |
| CD56dim+CD3- | 18 | 32 | 41 | |
| Regulatory T cells (Tregs) | CD3+CD4+CD25+CD127- | 14 | 12 | 18 |
| Naïve Tregs | CD3+CD4+CD25+CD127-CD45RO-CD62L+ | 1 | 2 | 1 |
| Central memory Tregs | CD3+CD4+CD25+CD127-CD45RO+CD62L+ | 12 | 8 | 16 |
| Mitogen Proliferation Assay | (%) | (%) | (%) | |
|---|---|---|---|---|
| PHACD45 (Normal Range: ≥49.94%) | 56.4 | 37.7 | 73.8 | |
| PWCD19 (Normal Range: ≥3.9%) | 16.4 | 10.2 | 25.0 | |
| PWCD3 (Normal Range: ≥3.5%) | 22.3 | 17.8 | 23.8 | |
| PWCD45 (Normal Range: ≥4.5%) | 15.2 | 12.3 | 19.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikh, I.N.; Alqahtani, S.; Ragoonanan, D.; Tewari, P.; Petropoulos, D.; Mahadeo, K.M.; Popat, U.; Shpall, E.J.; Khazal, S. Post-Transplant Cyclophosphamide after Matched Sibling and Unrelated Donor Hematopoietic Stem Cell Transplantation in Pediatric Patients with Acute Myeloid Leukemia. Int. J. Mol. Sci. 2022, 23, 8748. https://doi.org/10.3390/ijms23158748
Sheikh IN, Alqahtani S, Ragoonanan D, Tewari P, Petropoulos D, Mahadeo KM, Popat U, Shpall EJ, Khazal S. Post-Transplant Cyclophosphamide after Matched Sibling and Unrelated Donor Hematopoietic Stem Cell Transplantation in Pediatric Patients with Acute Myeloid Leukemia. International Journal of Molecular Sciences. 2022; 23(15):8748. https://doi.org/10.3390/ijms23158748
Chicago/Turabian StyleSheikh, Irtiza N., Shaikha Alqahtani, Dristhi Ragoonanan, Priti Tewari, Demetrios Petropoulos, Kris M. Mahadeo, Uday Popat, Elizabeth J. Shpall, and Sajad Khazal. 2022. "Post-Transplant Cyclophosphamide after Matched Sibling and Unrelated Donor Hematopoietic Stem Cell Transplantation in Pediatric Patients with Acute Myeloid Leukemia" International Journal of Molecular Sciences 23, no. 15: 8748. https://doi.org/10.3390/ijms23158748
APA StyleSheikh, I. N., Alqahtani, S., Ragoonanan, D., Tewari, P., Petropoulos, D., Mahadeo, K. M., Popat, U., Shpall, E. J., & Khazal, S. (2022). Post-Transplant Cyclophosphamide after Matched Sibling and Unrelated Donor Hematopoietic Stem Cell Transplantation in Pediatric Patients with Acute Myeloid Leukemia. International Journal of Molecular Sciences, 23(15), 8748. https://doi.org/10.3390/ijms23158748






