Selective Recovery of Cadmium, Cobalt, and Nickel from Spent Ni–Cd Batteries Using Adogen® 464 and Mesoporous Silica Derivatives
Abstract
:1. Introduction
2. Results and Discussion
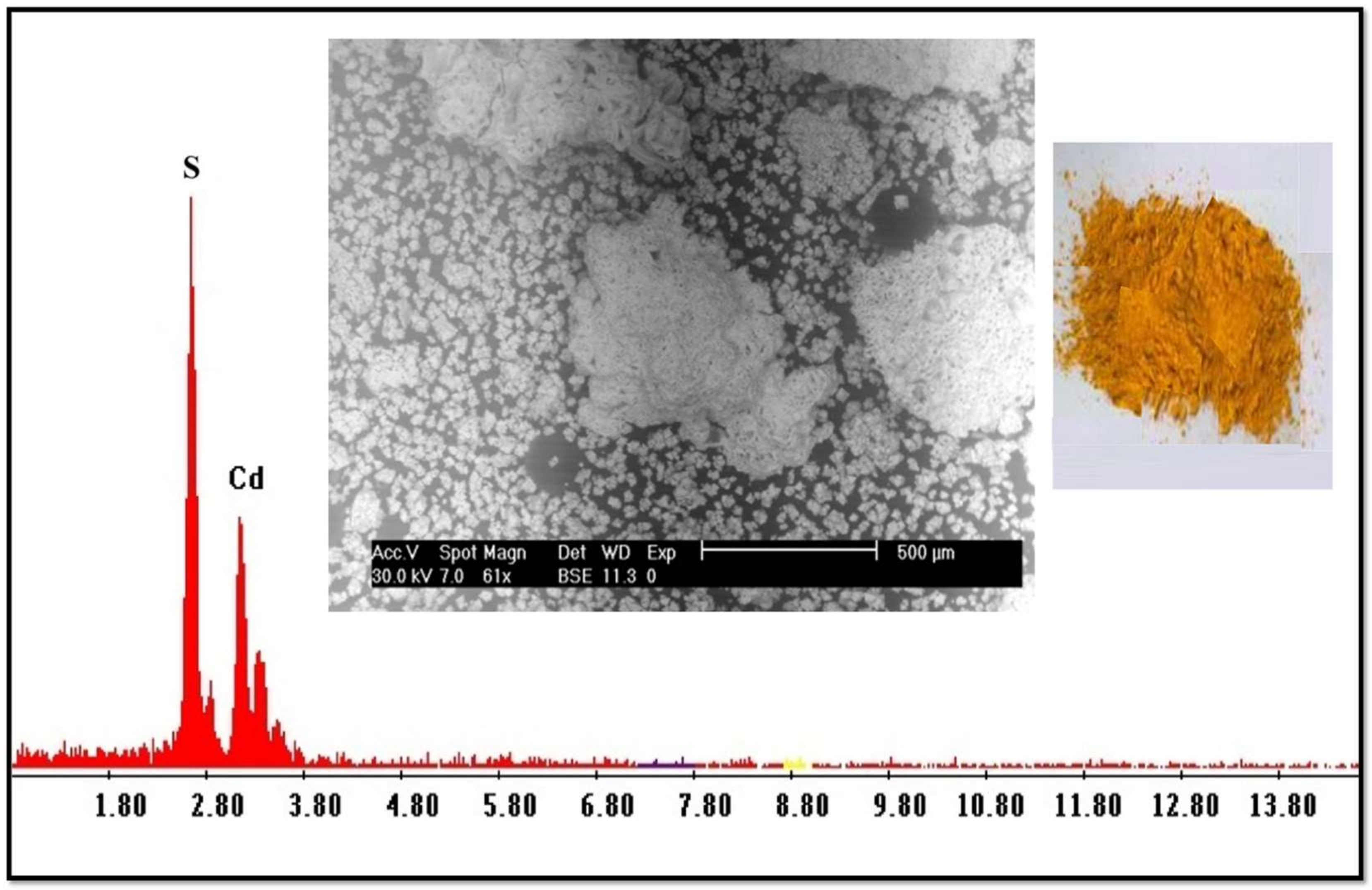
2.1. Precipitation of Iron
2.2. Factors Controlling Adsorption of Cadmium
2.2.1. Effect of pH
2.2.2. Effect of Time
2.2.3. Effect of Adogen 464 Concentration
2.2.4. Effect of Sulfate Concentration
2.2.5. Effect of Organic to Aqueous Ratio
2.2.6. Effect of Temperature
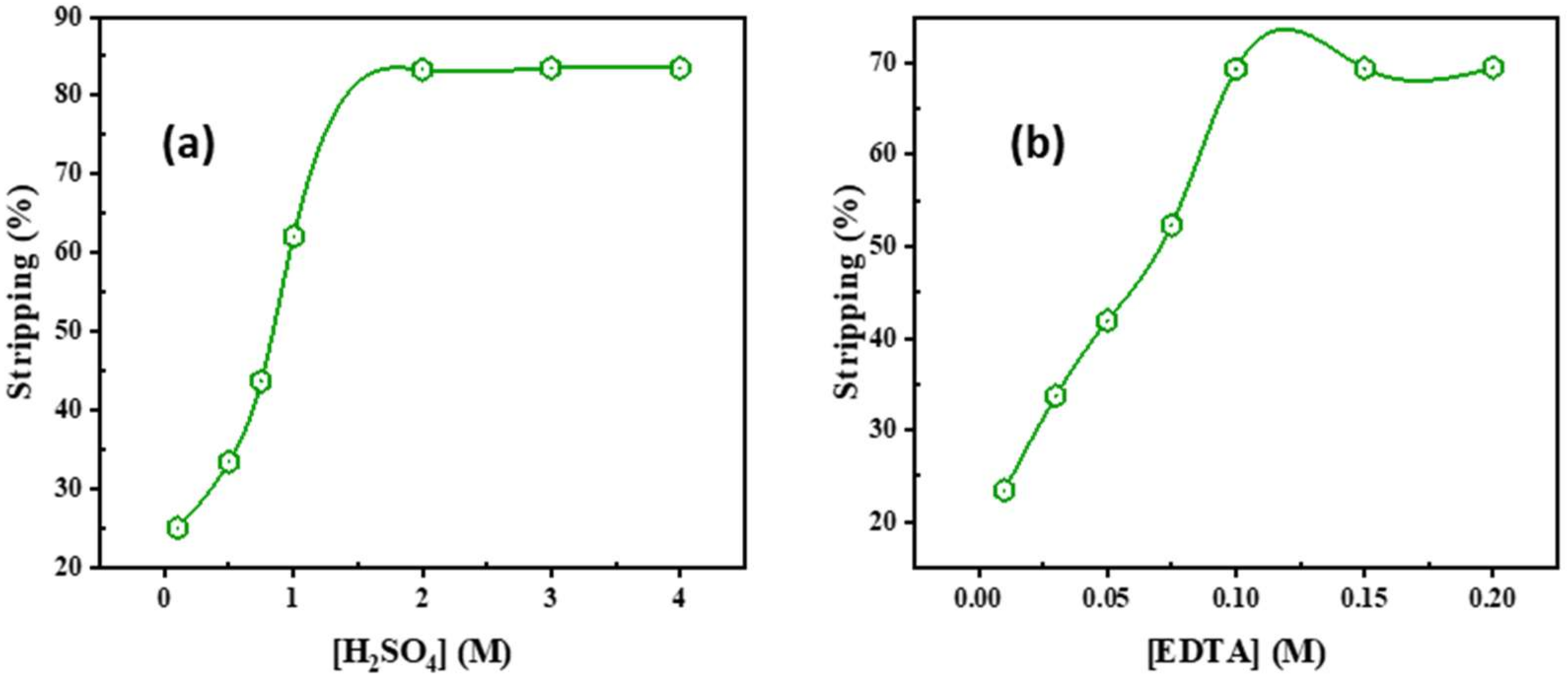
2.3. Cadmium Stripping from Cadmium/Adogen 464 and Preciptation
2.4. Adsorption of Cobalt Using Prepared Silica Adsorbant
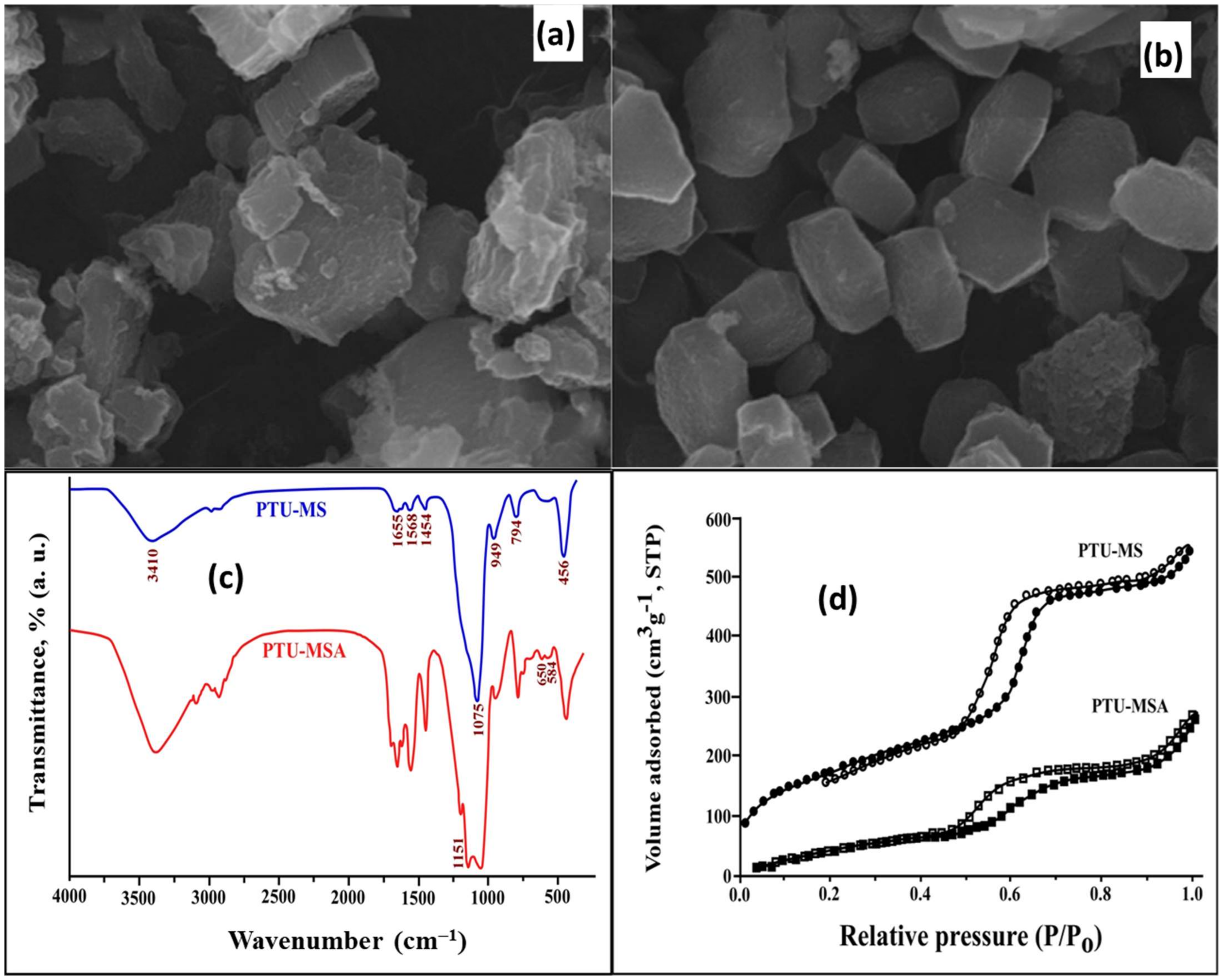
2.4.1. Characterization of PTU-MSA
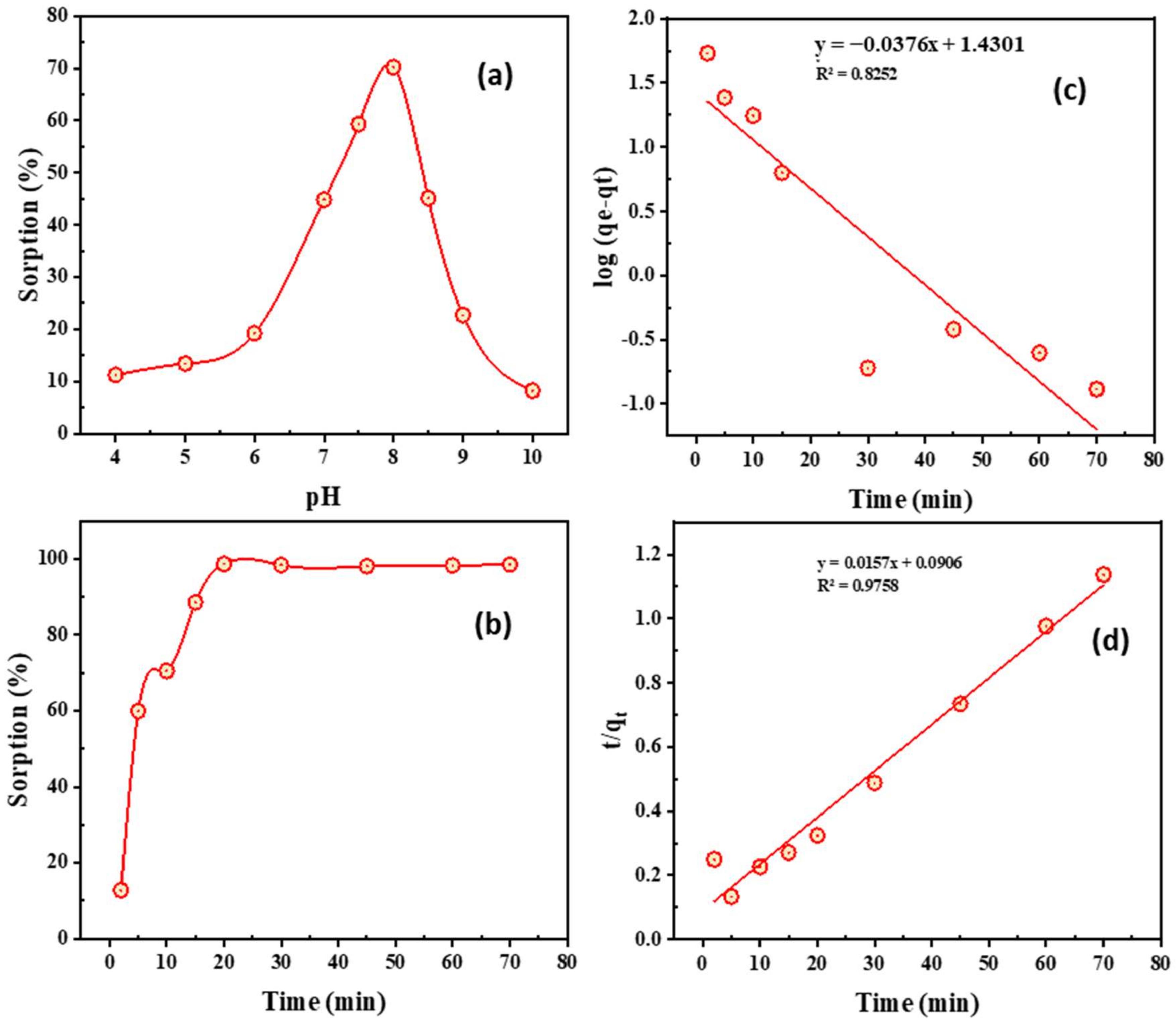
2.4.2. Factors Controlling the Adsorption Process of Cobalt
- 1.
- Impact of pH
- 2.
- Impact of time and kinetics
- 3.
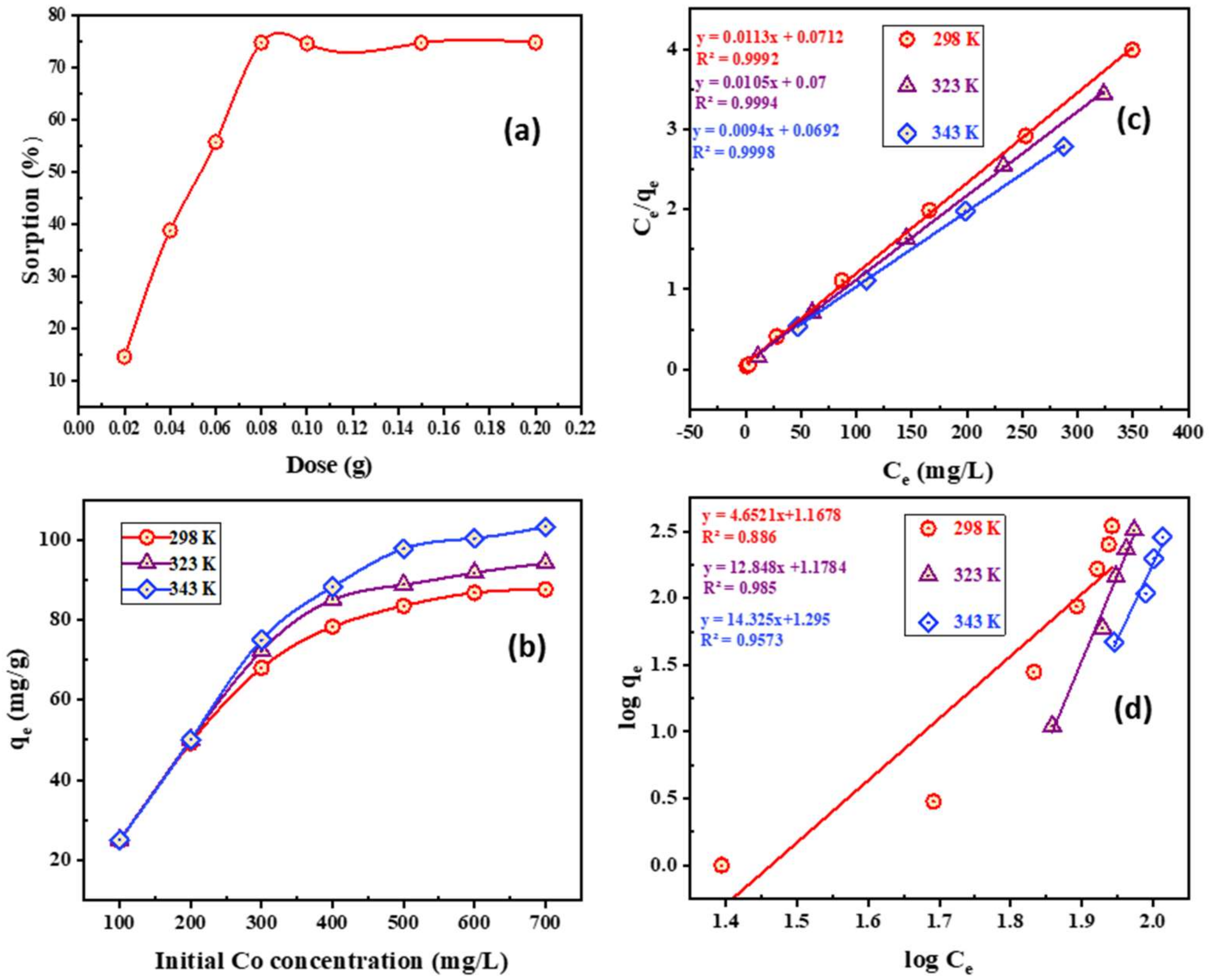
- Impact of PTU-MSA dose
- 4.
- Impact of initial Co2+ concentration and adsorption isotherms features
- 5.
- Thermodynamic parameters for systems of cobalt adsorption
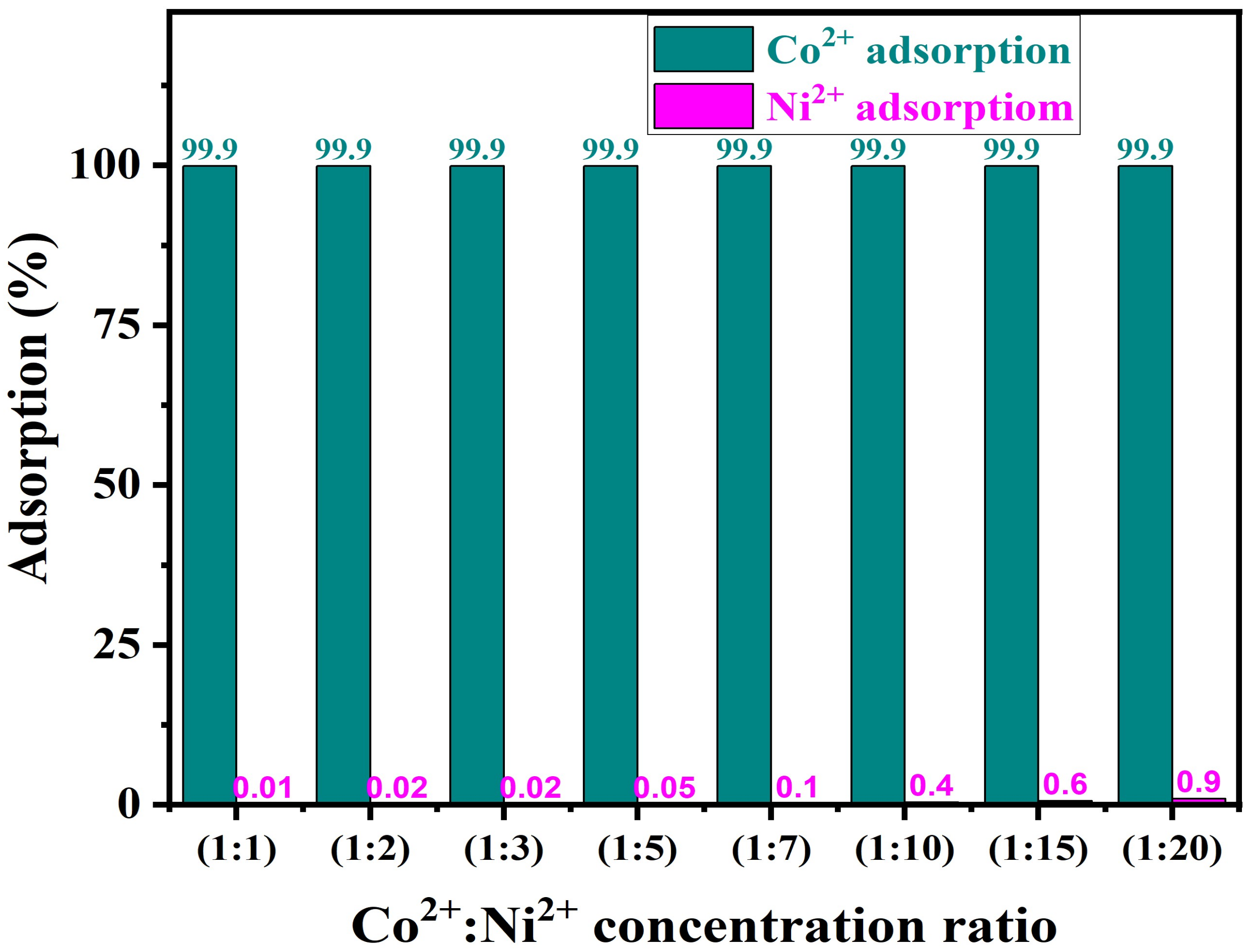
- 6.
- Impact of Ni2+ ion concentration on PTU-MSA selectivity for Co2+ adsorption
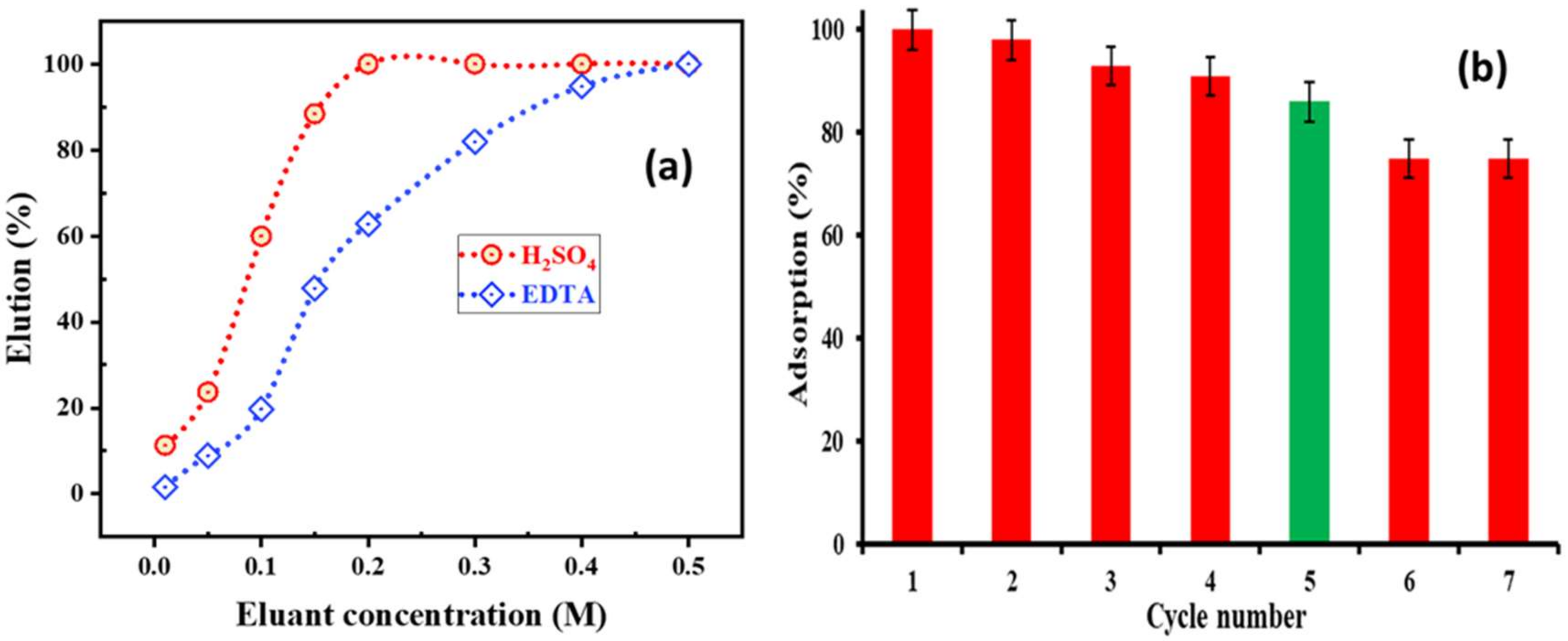
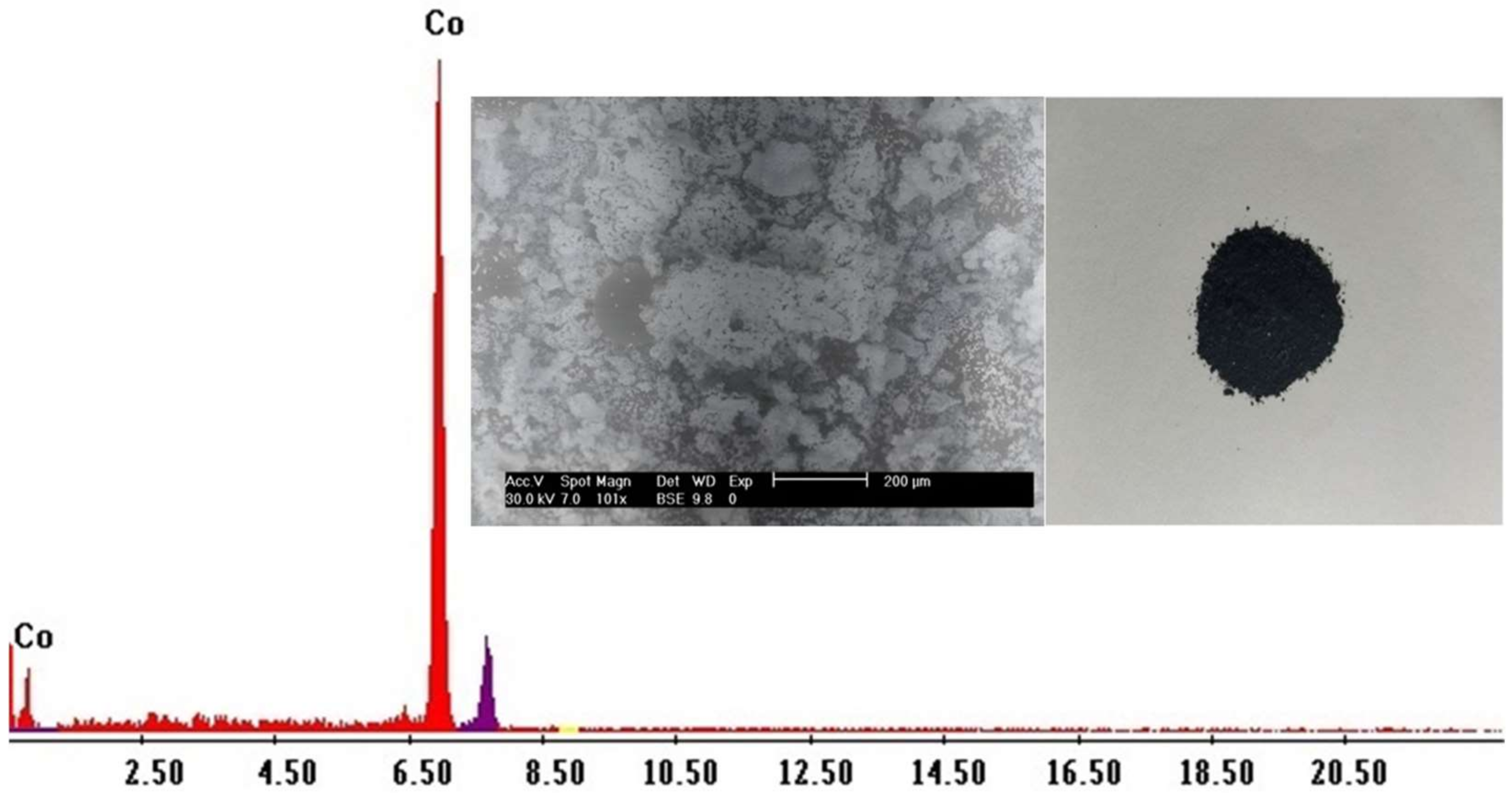
2.5. Desorption and Precipitation of Cobalt
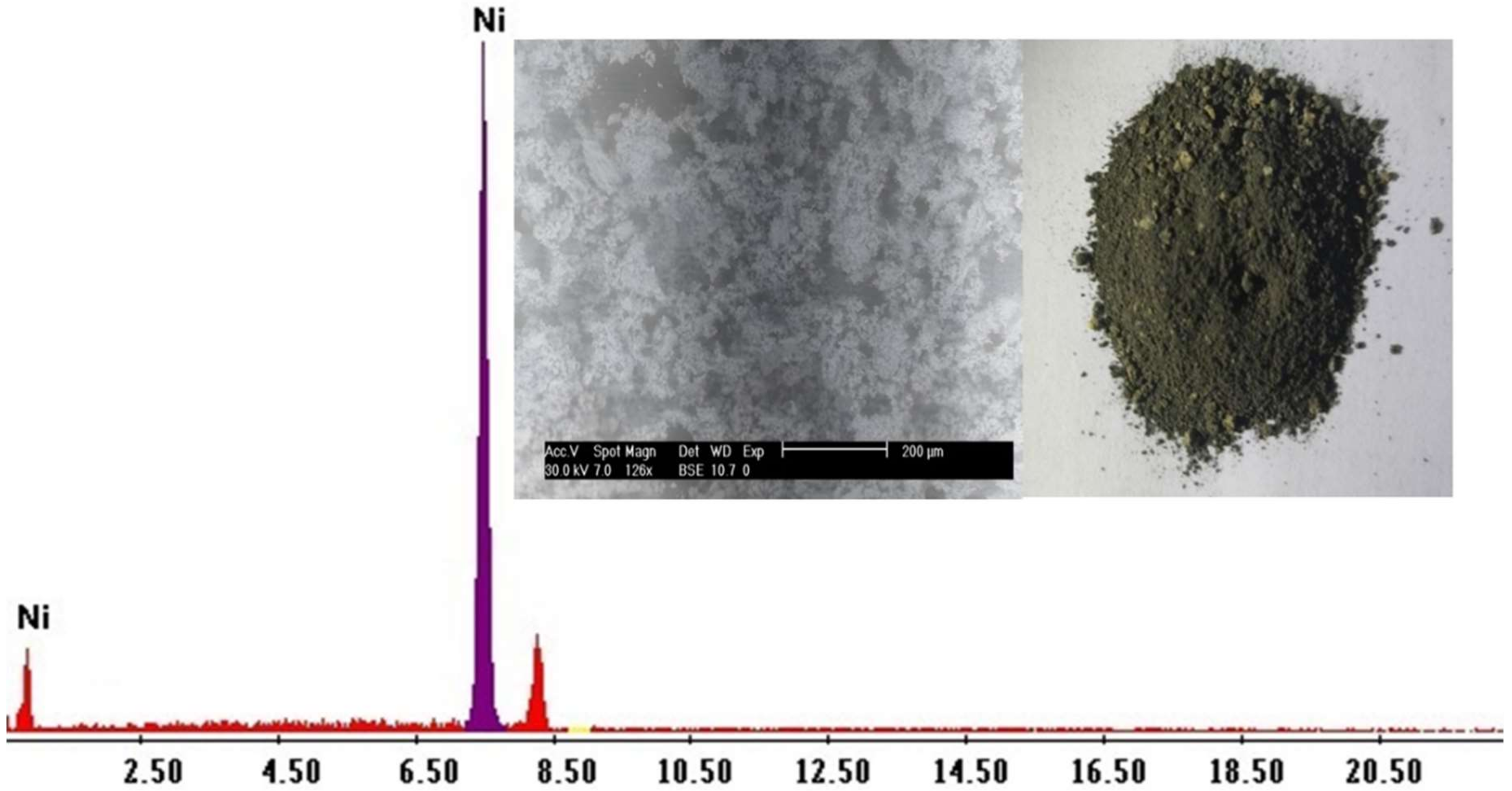
2.6. Nickel Recovery
3. Materials and Methods
3.1. Chemicals and Instruments
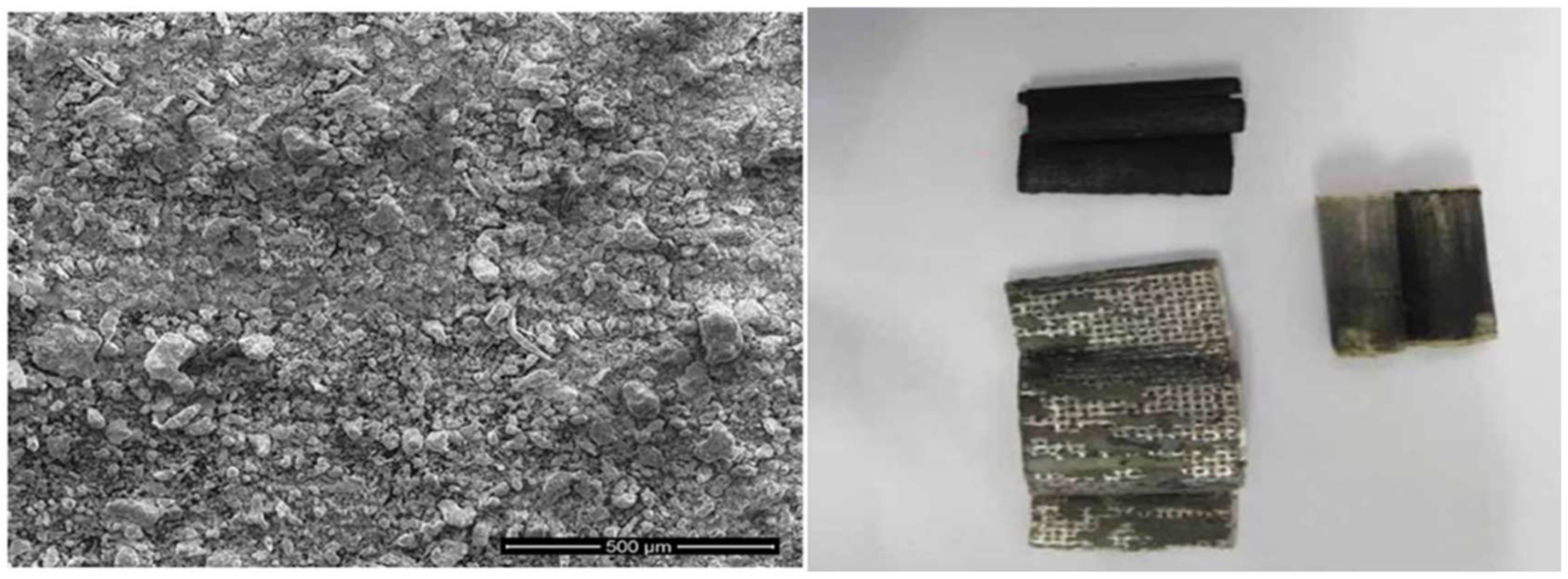
3.2. Preparation of Cd–Ni Batteries Leachate
3.3. Preparation of Adogen® 464 for Extraction Process
3.4. Extraction Processes and Measurements
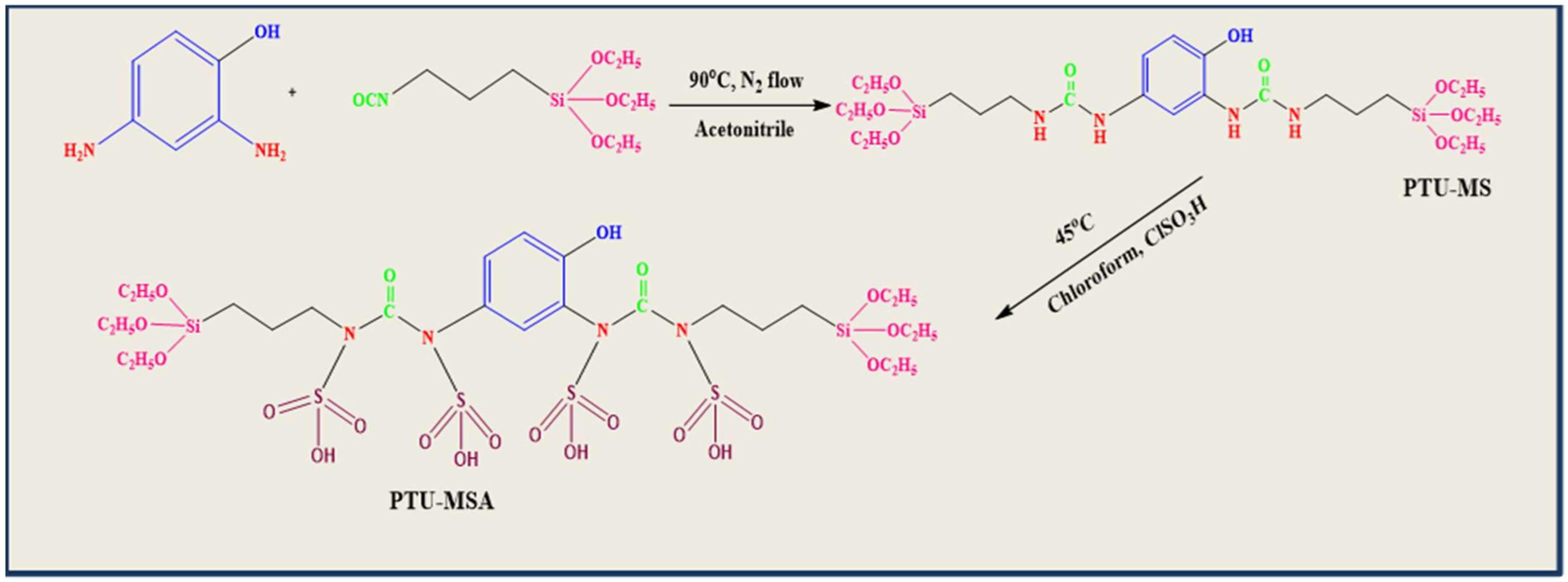
3.5. Preparation of Precursor, 1,1′-(4-hydroxy-1,3-phenylene)bis(3-(3-(triethoxysilyl)propyl)urea (PTU)
3.6. Synthesis of PTU Bridged Mesoporous Organosilica (PTU-MS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plachy, J. Cadmium Recycling in the United States in 2000 [Electronic Resource] by Jozef Plachy; U.S. Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 2003. [Google Scholar]
- Dehghani-Sanij, A.R.; Tharumalingam, E.; Dusseault, M.B.; Fraser, R. Study of energy storage systems and environmental challenges of batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Weshahy, A.R.; Gouda, A.A.; Atia, B.M.; Sakr, A.K.; Al-Otaibi, J.S.; Almuqrin, A.; Hanfi, M.Y.; Sayyed, M.I.; El Sheikh, R.; Radwan, H.A.; et al. Efficient Recovery of Rare Earth Elements and Zinc from Spent Ni–Metal Hydride Batteries: Statistical Studies. Nanomaterials 2022, 12, 2305. [Google Scholar] [CrossRef] [PubMed]
- Mason-Jones, K.; von Blottnitz, H. Flows and fates of nickel–cadmium batteries in the City of Cape Town. Miner. Eng. 2010, 23, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, C.A.; Margarido, F. Leaching behaviour of electrode materials of spent nickel–cadmium batteries in sulphuric acid media. Hydrometallurgy 2004, 72, 111–118. [Google Scholar] [CrossRef]
- Hazotte, C.; Leclerc, N.; Diliberto, S.; Meux, E.; Lapicque, F. End-of-life nickel–cadmium accumulators: Characterization of electrode materials and industrial Black Mass. Environ. Technol. 2015, 36, 796–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrow, H.; Keating, J. Overview paper on effective recycling of Ni–Cd batteries. In Proceedings of the OECD Workshop on the Effective Collection and Recycling of Nickel–Cadmium Batteries, Lyon, France, 23–25 September 1997; pp. 23–25. [Google Scholar]
- Bernardes, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Tenório, J.A.S. Recycling of nickel–cadmium batteries using coal as reducing agent. J. Power Sources 2006, 157, 600–604. [Google Scholar] [CrossRef]
- Sullivan, J.L.; Gaines, L. Status of life cycle inventories for batteries. Energy Convers. Manag. 2012, 58, 134–148. [Google Scholar] [CrossRef]
- Sohn, J.-S.; Shin, S.; Kang, K.-S.; Choi, M.-J. Trend on the Recycling Technologies for the used Lithium Battery by the Patent Analysis. J. Korean Inst. Resour. Recycl. 2007, 16, 50–60. [Google Scholar]
- Espinosa, D.C.R.; Tenório, J.A.S. Fundamental aspects of recycling of nickel–cadmium batteries through vacuum distillation. J. Power Sources 2004, 135, 320–326. [Google Scholar] [CrossRef]
- Huang, K.; Li, J.; Xu, Z. A Novel Process for Recovering Valuable Metals from Waste Nickel−Cadmium Batteries. Environ. Sci. Technol. 2009, 43, 8974–8978. [Google Scholar] [CrossRef]
- Rudnik, E.; Nikiel, M. Hydrometallurgical recovery of cadmium and nickel from spent Ni–Cd batteries. Hydrometallurgy 2007, 89, 61–71. [Google Scholar] [CrossRef]
- Reddy, B.R.; Priya, D.N. Chloride leaching and solvent extraction of cadmium, cobalt and nickel from spent nickel–cadmium, batteries using Cyanex 923 and 272. J. Power Sources 2006, 161, 1428–1434. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Bourdot Dutra, A.J. Hydrometallurgical route to recover nickel, cobalt and cadmium from spent Ni–Cd batteries. J. Power Sources 2012, 220, 286–291. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.H.; Thi, L.D.; Qureshi, T.I. Recycling of NiCd batteries by hydrometallurgical process on small scale. J. Chem. Soc. Pak. 2011, 33, 853–857. [Google Scholar]
- Jha, M.K.; Kumar, V.; Jeong, J.; Lee, J.-C. Review on solvent extraction of cadmium from various solutions. Hydrometallurgy 2012, 111, 1–9. [Google Scholar] [CrossRef]
- Abdel Geleel, M.; Atwa, S.T.; Sakr, A.K. Removal of Cr (III) from aqueous waste using Spent Activated Clay. J. Am. Sci. 2013, 9, 256–262. [Google Scholar] [CrossRef]
- Gotfryd, L.; Cox, M. The selective recovery of cadmium(II) from sulfate solutions by a counter-current extraction–stripping process using a mixture of diisopropylsalicylic acid and Cyanex® 471X. Hydrometallurgy 2006, 81, 226–233. [Google Scholar] [CrossRef]
- Tanong, K.; Tran, L.-H.; Mercier, G.; Blais, J.-F. Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the unsorted spent batteries using solvent extraction, electrodeposition and precipitation methods. J. Clean. Prod. 2017, 148, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Vander Hoogerstraete, T.; Onghena, B.; Binnemans, K. Homogeneous Liquid–Liquid Extraction of Rare Earths with the Betaine—Betainium Bis(trifluoromethylsulfonyl)imide Ionic Liquid System. Int. J. Mol. Sci. 2013, 14, 21353–21377. [Google Scholar] [CrossRef] [Green Version]
- Mahandra, H.; Singh, R.; Gupta, B. Liquid-liquid extraction studies on Zn(II) and Cd(II) using phosphonium ionic liquid (Cyphos IL 104) and recovery of zinc from zinc plating mud. Sep. Purif. Technol. 2017, 177, 281–292. [Google Scholar] [CrossRef]
- Lerum, H.V.; Sand, S.; Eriksen, D.Ø.; Wibetoe, G.; Omtvedt, J.P. Comparison of single-phase and two-phase measurements in extraction, separation and back-extraction of Cd, Zn and Co from a multi-element matrix using Aliquat 336. J. Radioanal. Nucl. Chem. 2020, 324, 1203–1214. [Google Scholar] [CrossRef]
- Zhang, L.; Hessel, V.; Peng, J. Liquid-liquid extraction for the separation of Co(II) from Ni(II) with Cyanex 272 using a pilot scale Re-entrance flow microreactor. Chem. Eng. J. 2018, 332, 131–139. [Google Scholar] [CrossRef]
- Flieger, J.; Feder-Kubis, J.; Tatarczak-Michalewska, M. Chiral Ionic Liquids: Structural Diversity, Properties and Applications in Selected Separation Techniques. Int. J. Mol. Sci. 2020, 21, 4253. [Google Scholar] [CrossRef]
- Park, J.; Jung, Y.; Kusumah, P.; Lee, J.; Kwon, K.; Lee, C.K. Application of Ionic Liquids in Hydrometallurgy. Int. J. Mol. Sci. 2014, 15, 15320–15343. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Shimomura, T.; Murakami, S.; Maeda, T.; Nakamura, T. Liquid-liquid extraction of divalent manganese, cobalt, copper, zinc and cadmium from aqueous chloride solutions by tricaprylmethylammonium chloride. Hydrometallurgy 1984, 12, 245–254. [Google Scholar] [CrossRef]
- Daud, H.; Cattrall, R.W. The extraction of Hg(II) from potassium iodide solutions and the extraction of Cu(II), Zn(II) and Cd(II) from hydrochloric acid solutions by aliquat 336 dissolved in chloroform. J. Inorg. Nucl. Chem. 1981, 43, 779–785. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Dou, X.; Yuan, H.; Yang, M. Granular ferric hydroxide adsorbent for phosphate removal: Demonstration preparation and field study. Water Sci. Technol. 2015, 72, 2179–2186. [Google Scholar] [CrossRef]
- Ritcey, G.M.; Ashbrook, A.W. Solvent Extraction: Principles and Applications to Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Atia, B.M.; Khawassek, Y.M.; Hussein, G.M.; Gado, M.A.; El-Sheify, M.A.; Cheira, M.F. One-pot synthesis of pyridine dicarboxamide derivative and its application for uranium separation from acidic medium. J. Environ. Chem. Eng. 2021, 9, 105726. [Google Scholar] [CrossRef]
- Atia, B.M.; Gado, M.A.; Cheira, M.F.; El-Gendy, H.S.; Yousef, M.A.; Hashem, M.D. Direct synthesis of a chelating carboxamide derivative and its application for thorium extraction from Abu Rusheid ore sample, South Eastern Desert, Egypt. Int. J. Environ. Anal. Chem. 2021, 1–24. [Google Scholar] [CrossRef]
- Ibrahium, H.A.; Atia, B.M.; Awwad, N.S.; Nayl, A.A.; Radwan, H.A.; Gado, M.A. Efficient preparation of phosphazene chitosan derivatives and its applications for the adsorption of molybdenum from spent hydrodesulfurization catalyst. J. Dispers. Sci. Technol. 2022, 1–16. [Google Scholar] [CrossRef]
- Ibrahium, H.A.; Awwad, N.S.; Gado, M.A.; Hassanin, M.A.; Nayl, A.A.; Atia, B.M. Physico-Chemical Aspects on Uranium and Molybdenum Extraction from Aqueous Solution by Synthesized Phosphinimine Derivative Chelating Agent. J. Inorg. Organomet. Polym. Mater. 2022, 1–18. [Google Scholar] [CrossRef]
- Alharbi, A.; Gouda, A.A.; Atia, B.M.; Gado, M.A.; Alluhaybi, A.A.; Alkabli, J. The Role of Modified Chelating Graphene Oxide for Vanadium Separation from Its Bearing Samples. Russ. J. Inorg. Chem. 2022, 67, 560–575. [Google Scholar] [CrossRef]
- Ibrahium, H.A.; Gado, M.A.; Elhosiny Ali, H.; Fathy, W.M.; Atia, B.M.; Awwad, N.S. Synthesis of chelating N-hydroxyl amine derivative and its application for vanadium separation from Abu Zeneima ferruginous siltstone ore, Southwestern Sinai, Egypt. Int. J. Environ. Anal. Chem. 2021, 1–23. [Google Scholar] [CrossRef]
- Ibrahium, H.A.; Gado, M.A.; Awwad, N.S.; Fathy, W.M. Selective separation of Yttrium and Uranium from Xenotime Concentrate. Z. Anorg. Allg. Chem. 2021, 647, 1568–1577. [Google Scholar] [CrossRef]
- Nayl, A.A. Extraction and separation of Co(II) and Ni(II) from acidic sulfate solutions using Aliquat 336. J. Hazard. Mater. 2010, 173, 223–230. [Google Scholar] [CrossRef]
- Sakr, A.K.; Snelling, H.V.; Young, N.A. Experimental evidence for the molecular molybdenum fluorides MoF to MoF6: A matrix isolation and DFT investigation. New J. Chem. 2022, 46, 9666–9684. [Google Scholar] [CrossRef]
- Chang, S.H.; Teng, T.T.; Ismail, N.; Alkarkhi, A.F.M. Selection of design parameters and optimization of operating parameters of soybean oil-based bulk liquid membrane for Cu(II) removal and recovery from aqueous solutions. J. Hazard. Mater. 2011, 190, 197–204. [Google Scholar] [CrossRef]
- Sayed, A.S.; Abdelmottaleb, M.; Cheira, M.F.; Abdel-Aziz, G.; Gomaa, H.; Hassanein, T.F. Date seed as an efficient, eco-friendly, and cost-effective bio-adsorbent for removal of thorium ions from acidic solutions. Aswan Univ. J. Environ. Stud. 2020, 1, 106–124. [Google Scholar] [CrossRef]
- Gado, M.A.; Atia, B.M.; Cheira, M.F.; Abdou, A.A. Thorium ions adsorption from aqueous solution by amino naphthol sulphonate coupled chitosan. Int. J. Environ. Anal. Chem. 2021, 101, 1419–1436. [Google Scholar] [CrossRef]
- Gado, M.; Rashad, M.; Kassab, W.; Badran, M. Highly Developed Surface Area Thiosemicarbazide Biochar Derived from Aloe Vera for Efficient Adsorption of Uranium. Radiochemistry 2021, 63, 353–363. [Google Scholar] [CrossRef]
- Atia, B.M.; Gado, M.A.; Abd El-Magied, M.O.; Elshehy, E.A. Highly efficient extraction of uranyl ions from aqueous solutions using multi-chelators functionalized graphene oxide. Sep. Sci. Technol. 2020, 55, 2746–2757. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, I.; Rodríguez-Pérez, A.; Pacheco-Castillo, N.C.; Enríquez-Domínguez, E.; Cárdenas-González, J.F.; Martínez-Juárez, V.-M. Removal of Cobalt (II) from Waters Contaminated by the Biomass of Eichhornia crassipes. Water 2021, 13, 1725. [Google Scholar] [CrossRef]
- Cheira, M.F.; Atia, B.M.; Kouraim, M.N. Uranium(VI) recovery from acidic leach liquor by Ambersep 920U SO4 resin: Kinetic, equilibrium and thermodynamic studies. J. Radiat. Res. Appl. Sci. 2017, 10, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Mercier, L.; Pinnavaia, T.J. Direct Synthesis of Hybrid Organic−Inorganic Nanoporous Silica by a Neutral Amine Assembly Route: Structure−Function Control by Stoichiometric Incorporation of Organosiloxane Molecules. Chem. Mater. 2000, 12, 188–196. [Google Scholar] [CrossRef]
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 1999, 402, 867–871. [Google Scholar] [CrossRef]
- Lim, M.H.; Blanford, C.F.; Stein, A. Synthesis and Characterization of a Reactive Vinyl-Functionalized MCM-41: Probing the Internal Pore Structure by a Bromination Reaction. J. Am. Chem. Soc. 1997, 119, 4090–4091. [Google Scholar] [CrossRef]
- Lim, M.H.; Blanford, C.F.; Stein, A. Synthesis of Ordered Microporous Silicates with Organosulfur Surface Groups and Their Applications as Solid Acid Catalysts. Chem. Mater. 1998, 10, 467–470. [Google Scholar] [CrossRef]
- Asefa, T.; Kruk, M.; MacLachlan, M.; Coombs, N.; Grondey, H.; Jaroniec, M.; Ozin, G. Sequential Hydroboration–Alcoholysis and Epoxidation–Ring Opening Reactions of Vinyl Groups in Mesoporous Vinylsilica. Adv. Funct. Mater. 2001, 11, 447–456. [Google Scholar] [CrossRef]
- Wahab, M.A.; Imae, I.; Kawakami, Y.; Kim, I.; Ha, C.-S. Functionalized periodic mesoporous organosilica fibers with longitudinal pore architectures under basic conditions. Microporous Mesoporous Mater. 2006, 92, 201–211. [Google Scholar] [CrossRef]
- Moreau, J.J.E.; Vellutini, L.; Wong Chi Man, M.; Bied, C. Shape-Controlled Bridged Silsesquioxanes: Hollow Tubes and Spheres. Chem. A Eur. J. 2003, 9, 1594–1599. [Google Scholar] [CrossRef]
- Wahab, M.A.; Guo, W.; Cho, W.-J.; Ha, C.-S. Synthesis and Characterization of Novel Amorphous Hybrid Silica Materials. J. Sol-Gel Sci. Technol. 2003, 27, 333–341. [Google Scholar] [CrossRef]
- Park, S.S.; Ha, C.-S. Organic–inorganic hybrid mesoporous silicas: Functionalization, pore size, and morphology control. Chem. Rec. 2006, 6, 32–42. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Awual, M.R.; Suzuki, S.; Taguchi, T.; Shiwaku, H.; Okamoto, Y.; Yaita, T. Radioactive cesium removal from nuclear wastewater by novel inorganic and conjugate adsorbents. Chem. Eng. J. 2014, 242, 127–135. [Google Scholar] [CrossRef]
- Awual, M.R.; Yaita, T.; Shiwaku, H. Design a novel optical adsorbent for simultaneous ultra-trace cerium(III) detection, sorption and recovery. Chem. Eng. J. 2013, 228, 327–335. [Google Scholar] [CrossRef]
- Yuan, G.; Tu, H.; Li, M.; Liu, J.; Zhao, C.; Liao, J.; Yang, Y.; Yang, J.; Liu, N. Glycine derivative-functionalized metal-organic framework (MOF) materials for Co(II) removal from aqueous solution. Appl. Surf. Sci. 2019, 466, 903–910. [Google Scholar] [CrossRef]
- Fan, G.; Lin, R.; Su, Z.; Lin, X.; Xu, R.; Chen, W. Removal of Cr (VI) from aqueous solutions by titanate nanomaterials synthesized via hydrothermal method. Can. J. Chem. Eng. 2017, 95, 717–723. [Google Scholar] [CrossRef]
- Yuan, G.; Tu, H.; Liu, J.; Zhao, C.; Liao, J.; Yang, Y.; Yang, J.; Liu, N. A novel ion-imprinted polymer induced by the glycylglycine modified metal-organic framework for the selective removal of Co(II) from aqueous solutions. Chem. Eng. J. 2018, 333, 280–288. [Google Scholar] [CrossRef]
- Negm, S.H.; Abd El-Hamid, A.A.M.; Gado, M.A.; El-Gendy, H.S. Selective uranium adsorption using modified acrylamide resins. J. Radioanal. Nucl. Chem. 2019, 319, 327–337. [Google Scholar] [CrossRef]
- Gado, M.A. Sorption of thorium using magnetic graphene oxide polypyrrole composite synthesized from natural source. Sep. Sci. Technol. 2018, 53, 2016–2033. [Google Scholar] [CrossRef]
- Cho, G.; Fung, B.M.; Glatzhofer, D.T.; Lee, J.-S.; Shul, Y.-G. Preparation and Characterization of Polypyrrole-Coated Nanosized Novel Ceramics. Langmuir 2001, 17, 456–461. [Google Scholar] [CrossRef]
- Tian, B.; Zerbi, G. Lattice dynamics and vibrational spectra of polypyrrole. J. Chem. Phys. 1990, 92, 3886–3891. [Google Scholar] [CrossRef]
- Sakr, A.K.; Al-Hamarneh, I.F.; Gomaa, H.; Abdel Aal, M.M.; Hanfi, M.Y.; Sayyed, M.I.; Khandaler, M.U.; Cheira, M.F. Removal of uranium from nuclear effluent using regenerated bleaching earth steeped in β-naphthol. Radiat. Phys. Chem. 2022, 110204. [Google Scholar] [CrossRef]
- Mahmud, H.N.M.E.; Kassim, A.; Zainal, Z.; Yunus, W.M.M. Fourier transform infrared study of polypyrrole–poly(vinyl alcohol) conducting polymer composite films: Evidence of film formation and characterization. J. Appl. Polym. Sci. 2006, 100, 4107–4113. [Google Scholar] [CrossRef]
- Gado, M.; Atia, B.; Morcy, A. The role of graphene oxide anchored 1-amino-2-naphthol-4-sulphonic acid on the adsorption of uranyl ions from aqueous solution: Kinetic and thermodynamic features. Int. J. Environ. Anal. Chem. 2019, 99, 996–1015. [Google Scholar] [CrossRef]
- Hassanin, M.A.; Negm, S.H.; Youssef, M.A.; Sakr, A.K.; Mira, H.I.; Mohammaden, T.F.; Al-Otaibi, J.S.; Hanfi, M.Y.; Sayyed, M.I.; Cheira, M.F. Sustainable Remedy Waste to Generate SiO2 Functionalized on Graphene Oxide for Removal of U(VI) Ions. Sustainability 2022, 14, 2699. [Google Scholar] [CrossRef]
- Gado, M.A.; Atia, B.M.; Cheira, M.F.; Elawady, M.E.; Demerdash, M. Highly efficient adsorption of uranyl ions using hydroxamic acid-functionalized graphene oxide. Radiochim. Acta 2021, 109, 743–757. [Google Scholar] [CrossRef]
- Cheira, M.F.; Mira, H.I.; Sakr, A.K.; Mohamed, S.A. Adsorption of U(VI) from acid solution on a low-cost sorbent: Equilibrium, kinetic, and thermodynamic assessments. Nucl. Sci. Tech. 2019, 30, 156. [Google Scholar] [CrossRef]
- Sakr, A.K.; Cheira, M.F.; Hassanin, M.A.; Mira, H.I.; Mohamed, S.A.; Khandaker, M.U.; Osman, H.; Eed, E.M.; Sayyed, M.I.; Hanfi, M.Y. Adsorption of Yttrium Ions on 3-Amino-5-Hydroxypyrazole Impregnated Bleaching Clay, a Novel Sorbent Material. Appl. Sci. 2021, 11, 10320. [Google Scholar] [CrossRef]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Cheira, M.F.; Almuqrin, A.; Hanfi, M.Y.; Sayyed, M.I. Rare Earth Group Separation after Extraction Using Sodium Diethyldithiocarbamate/Polyvinyl Chloride from Lamprophyre Dykes Leachate. Materials 2022, 15, 1211. [Google Scholar] [CrossRef]
- Radwan, H.A.; Gado, M.A.; El-Wahab, Z.H.A.; El-Sheikh, E.M.; Faheim, A.A.; Taha, R.H. Recovery of uranium from ferruginous Shale mineralization from Um Bogma formation, Egypt, via Duolite ES-467 chelating resin. Z. Anorg. Allg. Chem. 2021, 647, 396–412. [Google Scholar] [CrossRef]
- Garoub, M.; Gado, M. Separation of Cadmium Using a new Adsorbent of Modified Chitosan with Pyridine Dicarboxyamide derivative and application in different samples. Z. Anorg. Allg. Chem. 2022, 648, e202100222. [Google Scholar] [CrossRef]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Hanfi, M.Y.; Sayyed, M.I.; Al-Otaibi, J.S.; Cheira, M.F. Cetylpyridinium Bromide/Polyvinyl Chloride for Substantially Efficient Capture of Rare Earth Elements from Chloride Solution. Polymers 2022, 14, 954. [Google Scholar] [CrossRef]
- Radwan, H.A.; Faheim, A.A.; El-Sheikh, E.M.; Abd El-Wahab, Z.H.; Gado, M.A. Optimization of the leaching process for uranium recovery and some associated valuable elements from low-grade uranium ore. Int. J. Environ. Anal. Chem. 2021, 1–23. [Google Scholar] [CrossRef]
- Mahmoud, N.S.; Atwa, S.T.; Sakr, A.K.; Abdel Geleel, M. Kinetic and thermodynamic study of the adsorption of Ni (II) using Spent Activated clay Mineral. N. Y. Sci. J. 2012, 5, 62–68. [Google Scholar] [CrossRef]
- Korkmaz, K.; Alemrajabi, M.; Rasmuson, Å.; Forsberg, K. Recoveries of Valuable Metals from Spent Nickel Metal Hydride Vehicle Batteries via Sulfation, Selective Roasting, and Water Leaching. J. Sustain. Metall. 2018, 4, 313–325. [Google Scholar] [CrossRef] [Green Version]
- Kumbasar, R.A. Selective extraction and concentration of cobalt from acidic leach solution containing cobalt and nickel through emulsion liquid membrane using PC-88A as extractant. Sep. Purif. Technol. 2009, 64, 273–279. [Google Scholar] [CrossRef]
- Pietrelli, L.; Bellomo, B.; Fontana, D.; Montereali, M. Characterization and leaching of NiCd and NiMH spent batteries for the recovery of metals. Waste Manag. 2005, 25, 221–226. [Google Scholar] [CrossRef]
- Vassura, I.; Morselli, L.; Bernardi, E.; Passarini, F. Chemical characterisation of spent rechargeable batteries. Waste Manag. 2009, 29, 2332–2335. [Google Scholar] [CrossRef]
- Atia, B.M.; Sakr, A.K.; Gado, M.A.; El-Gendy, H.S.; Abdelazeem, N.M.; El-Sheikh, E.M.; Hanfi, M.Y.; Sayyed, M.I.; Al-Otaibi, J.S.; Cheira, M.F. Synthesis of a New Chelating Iminophosphorane Derivative (Phosphazene) for U(VI) Recovery. Polymers 2022, 14, 1687. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Shi, X.; Yang, J.; Yang, Q. Periodic Mesoporous Organosilicas with 1,4-Diethylenebenzene in the Mesoporous Wall: Synthesis, Characterization, and Bioadsorption Properties. J. Phys. Chem. C 2007, 111, 10948–10954. [Google Scholar] [CrossRef]



| Pseudo-1st Order | Exp. Capacity qe, mg/g | Pseudo-2nd Order | ||||
|---|---|---|---|---|---|---|
| qe | K1 | R2 | 49.712 | qe | K2 | R2 |
| 18.737 | 0.1029 | 0.9599 | 52.632 | 0.0153 | 0.9959 | |
| ΔH° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | ΔG° (kJ·mol−1) | |||||
|---|---|---|---|---|---|---|---|
| 9.981 | 0.0505 | 303 K | 313 K | 323 K | 333 K | 343 K | 353 K |
| −15.302 | −15.808 | −16.313 | −16.818 | −17.323 | −17.829 | ||
| Materials | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Diameter (Å) |
|---|---|---|---|
| PTU-MS | 634 | 0.71 | 68 |
| PTU-MSA | 357 | 0.42 | 55 |
| Pseudo-1st Order | Exp. Capacity qe (mg·g−1) | Pseudo-2nd Order | ||||
|---|---|---|---|---|---|---|
| qe | K1 | R2 | 61.69 | qe | K2 | R2 |
| 26.922 | 0.08659 | 0.8252 | 63.692 | 0.00272 | 0.9758 | |
| Temp °C | Langmuir Isotherm | qe (mg·g−1) | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|---|
| b | qmax | R2 | kf | 1/n | R2 | ||
| 25 | 14.045 | 88.496 | 0.9992 | 87.625 | 14.716 | 4.6521 | 0.886 |
| 50 | 14.288 | 95.24 | 0.9994 | 94.125 | 15.079 | 12.848 | 0.985 |
| 70 | 14.451 | 106.38 | 0.9998 | 103.2 | 19.724 | 14.325 | 0.9573 |
| ΔH° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | ΔG° (kJ·mol−1) | ||
|---|---|---|---|---|
| 15.921 | 7.018 | 298 K | 323 K | 343 K |
| −2.075 | −2.251 | −2.391 | ||
| Elements | Chemical Configuration of Powder Ni–Cd Batteries (g/kg) | Elements Concentration in Ni–Cd Batteries Leachate (g/L) | Leaching Efficiency, % |
|---|---|---|---|
| Ni | 21.89 | 20.80 | 94.90 |
| Cd | 13.31 | 13.31 | 99.90 |
| Co | 0.85 | 0.85 | 99.90 |
| Fe | 2.71 | 2.71 | 99.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weshahy, A.R.; Sakr, A.K.; Gouda, A.A.; Atia, B.M.; Somaily, H.H.; Hanfi, M.Y.; Sayyed, M.I.; El Sheikh, R.; El-Sheikh, E.M.; Radwan, H.A.; et al. Selective Recovery of Cadmium, Cobalt, and Nickel from Spent Ni–Cd Batteries Using Adogen® 464 and Mesoporous Silica Derivatives. Int. J. Mol. Sci. 2022, 23, 8677. https://doi.org/10.3390/ijms23158677
Weshahy AR, Sakr AK, Gouda AA, Atia BM, Somaily HH, Hanfi MY, Sayyed MI, El Sheikh R, El-Sheikh EM, Radwan HA, et al. Selective Recovery of Cadmium, Cobalt, and Nickel from Spent Ni–Cd Batteries Using Adogen® 464 and Mesoporous Silica Derivatives. International Journal of Molecular Sciences. 2022; 23(15):8677. https://doi.org/10.3390/ijms23158677
Chicago/Turabian StyleWeshahy, Ahmed R., Ahmed K. Sakr, Ayman A. Gouda, Bahig M. Atia, H. H. Somaily, Mohamed Y. Hanfi, M. I. Sayyed, Ragaa El Sheikh, Enass M. El-Sheikh, Hend A. Radwan, and et al. 2022. "Selective Recovery of Cadmium, Cobalt, and Nickel from Spent Ni–Cd Batteries Using Adogen® 464 and Mesoporous Silica Derivatives" International Journal of Molecular Sciences 23, no. 15: 8677. https://doi.org/10.3390/ijms23158677
APA StyleWeshahy, A. R., Sakr, A. K., Gouda, A. A., Atia, B. M., Somaily, H. H., Hanfi, M. Y., Sayyed, M. I., El Sheikh, R., El-Sheikh, E. M., Radwan, H. A., Cheira, M. F., & Gado, M. A. (2022). Selective Recovery of Cadmium, Cobalt, and Nickel from Spent Ni–Cd Batteries Using Adogen® 464 and Mesoporous Silica Derivatives. International Journal of Molecular Sciences, 23(15), 8677. https://doi.org/10.3390/ijms23158677








