Kaposi’s Sarcoma-Associated Herpesvirus ORF50 Protein Represses Cellular MDM2 Expression via Suppressing the Sp1- and p53-Mediated Transactivation
Abstract
1. Introduction
2. Results
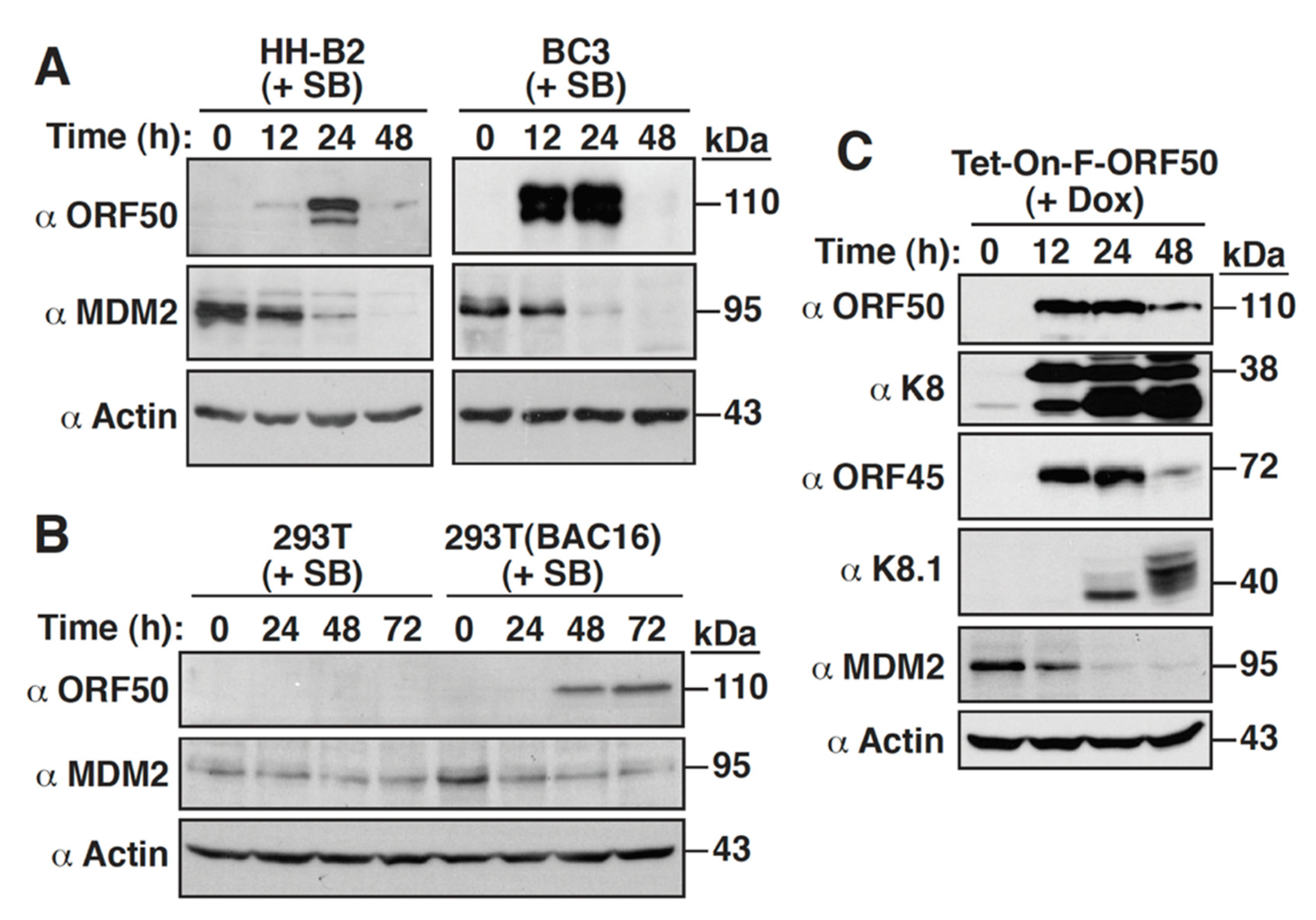
2.1. MDM2 Is Downregulated during the KSHV Lytic Cycle
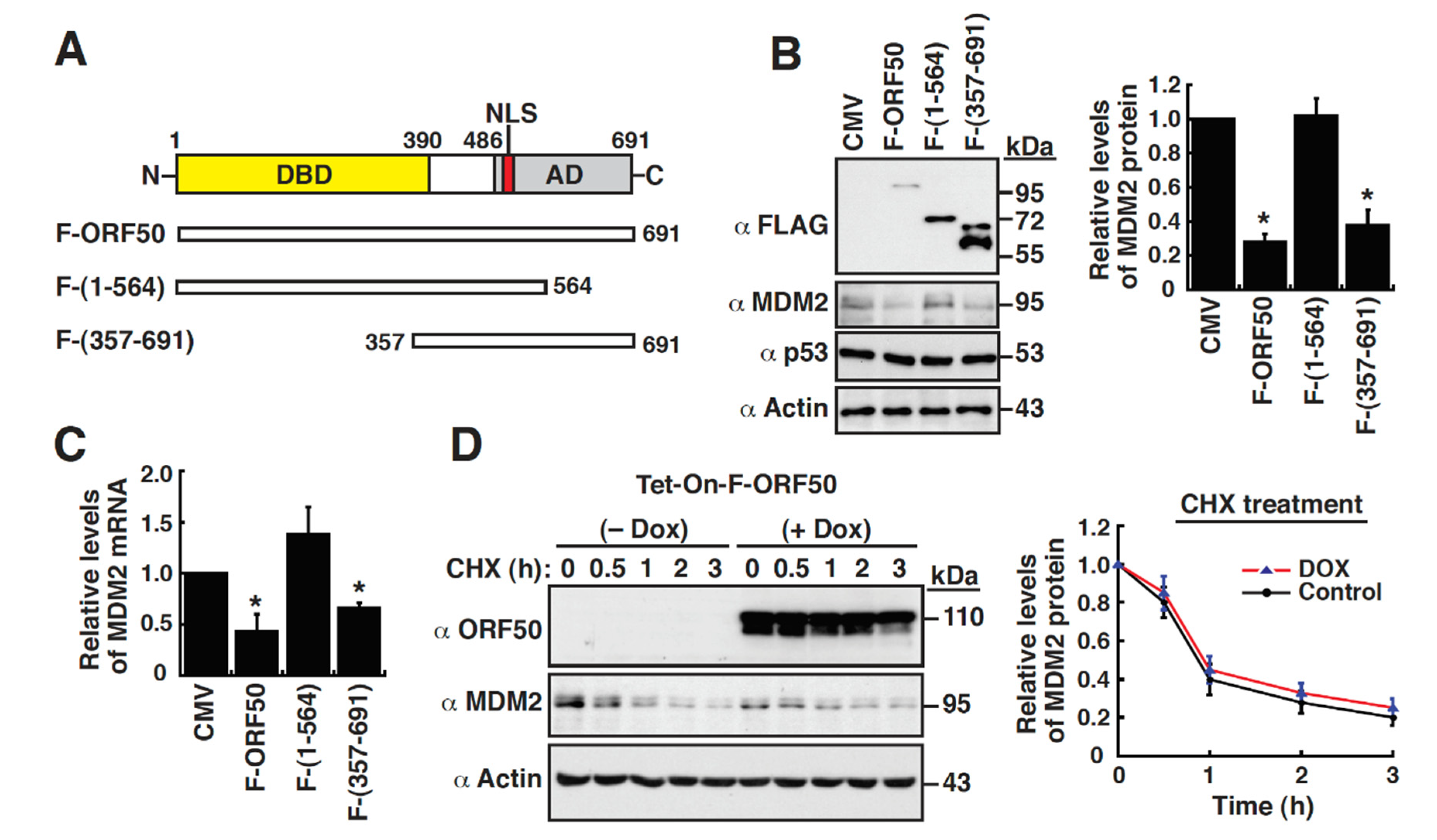
2.2. ORF50 Protein Downregulates MDM2 Expression Independently of Other Viral Factors
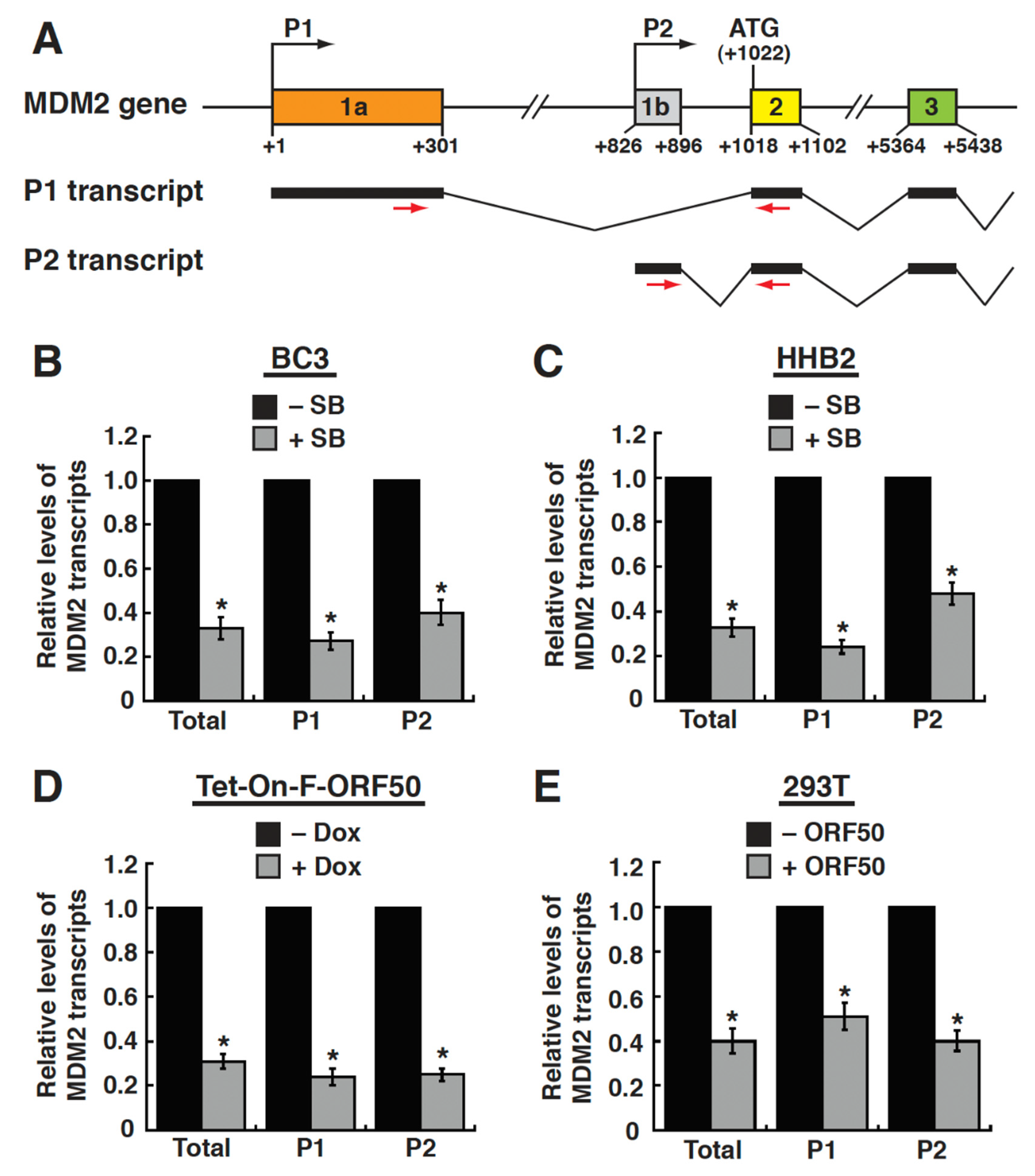
2.3. Both the Upstream Promoter (P1) and the Internal Promoter (P2) of the MDM2 Gene Are Repressed by ORF50
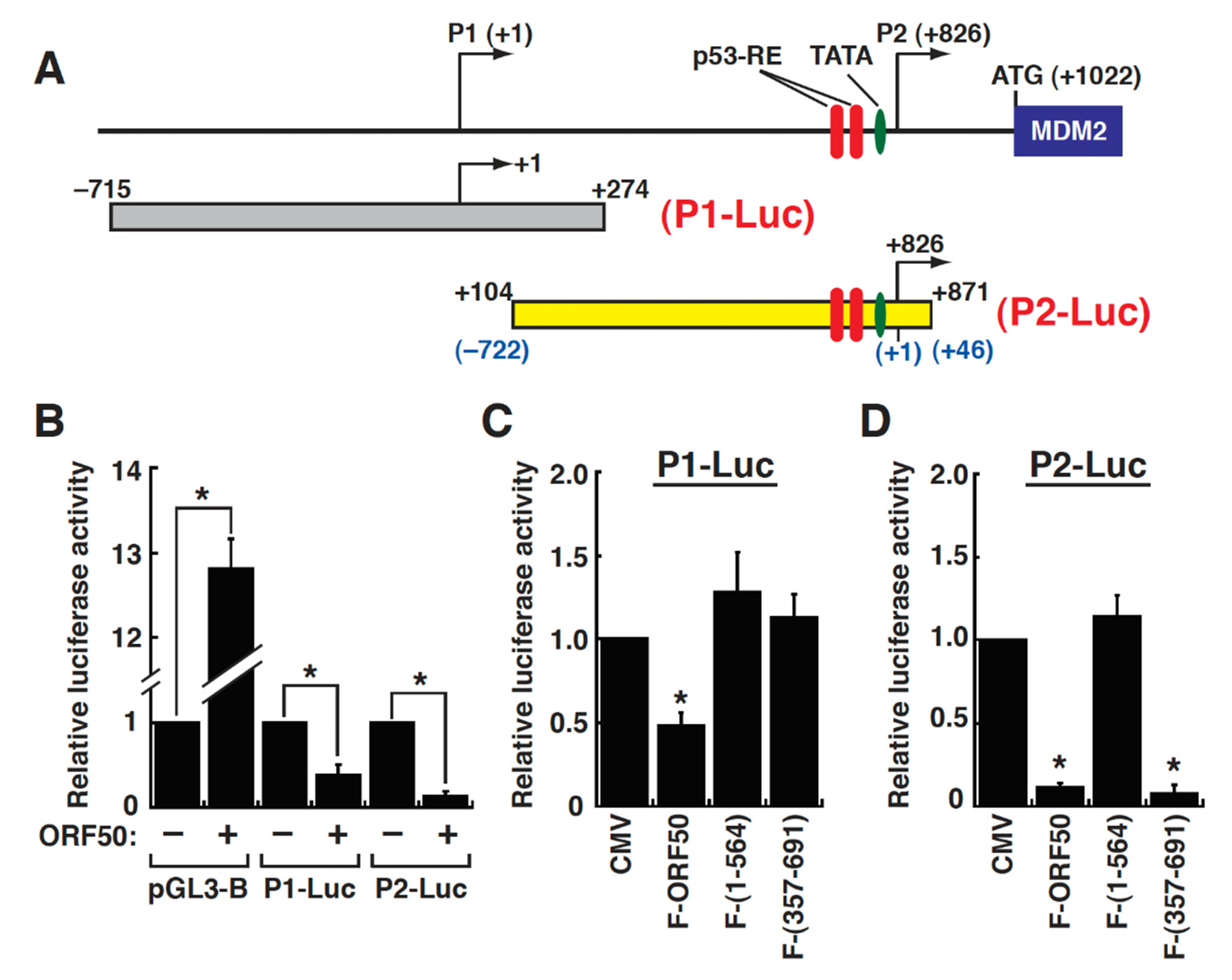
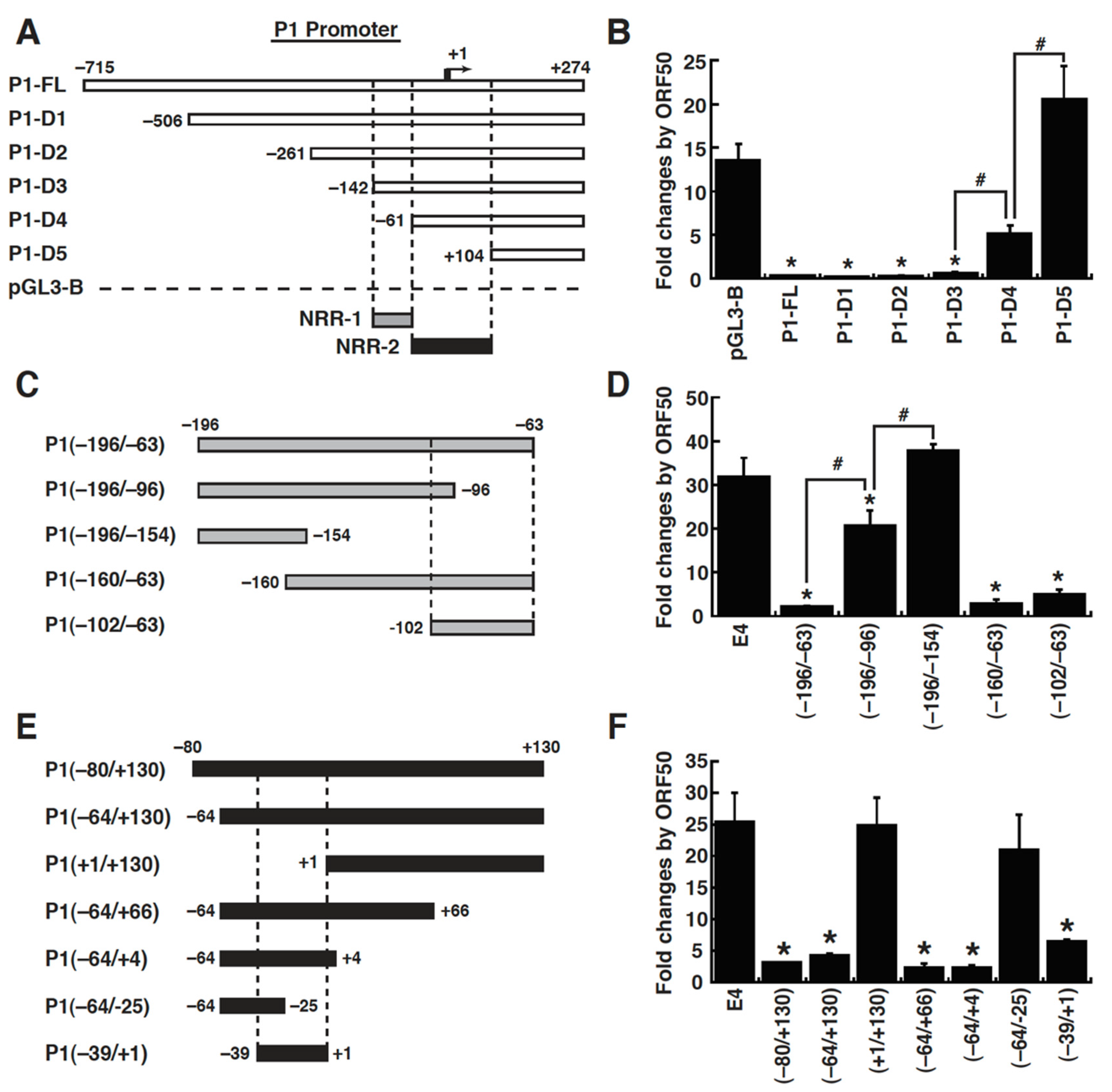
2.4. Mapping of the ORF50-Dependent Negative Response Elements in the P1 Promoter of the MDM2 Gene
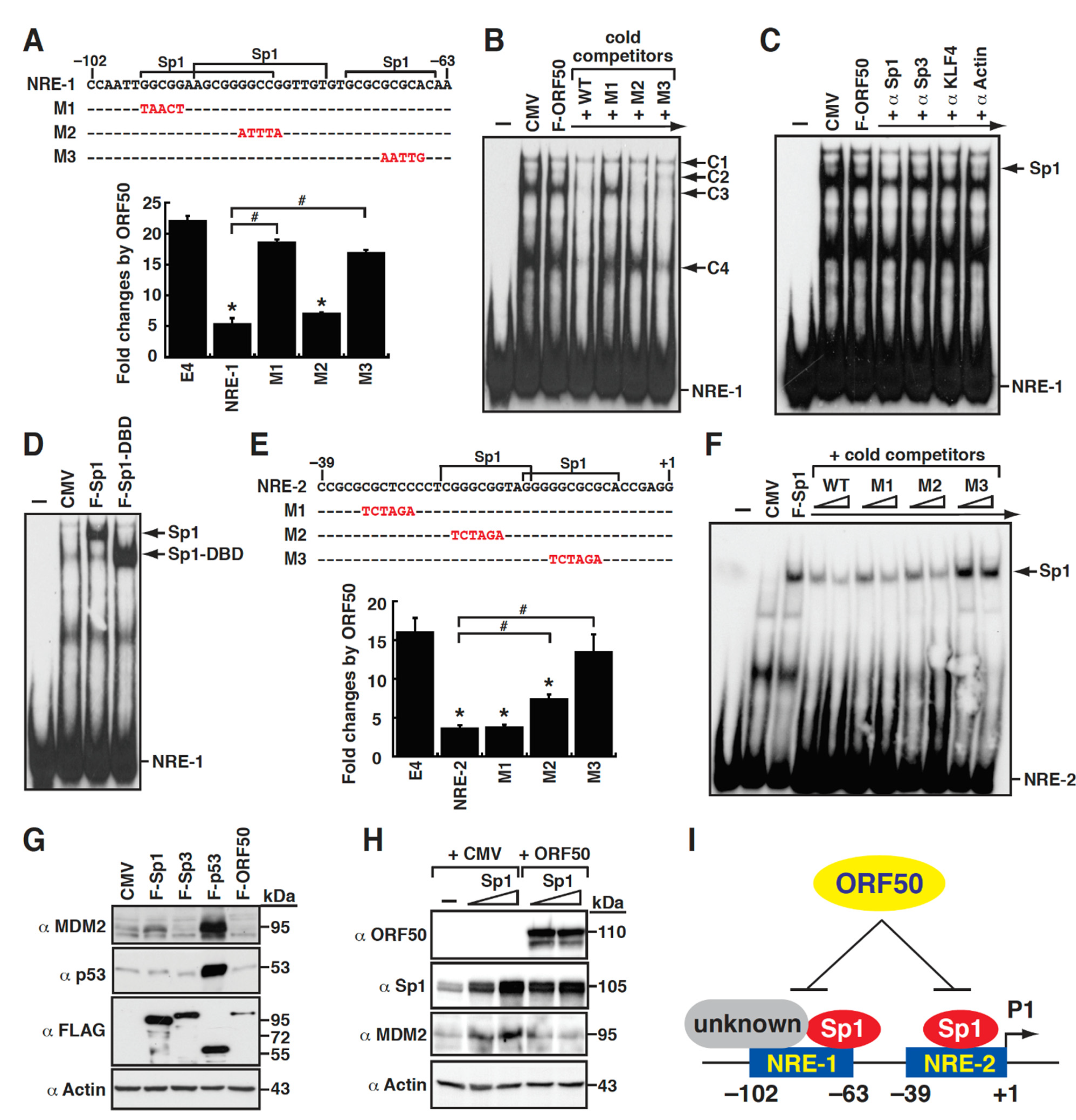
2.5. Repression of the MDM2 P1 Promoter by ORF50 Is Mediated through Inhibition of Sp1-Mediated Transactivation
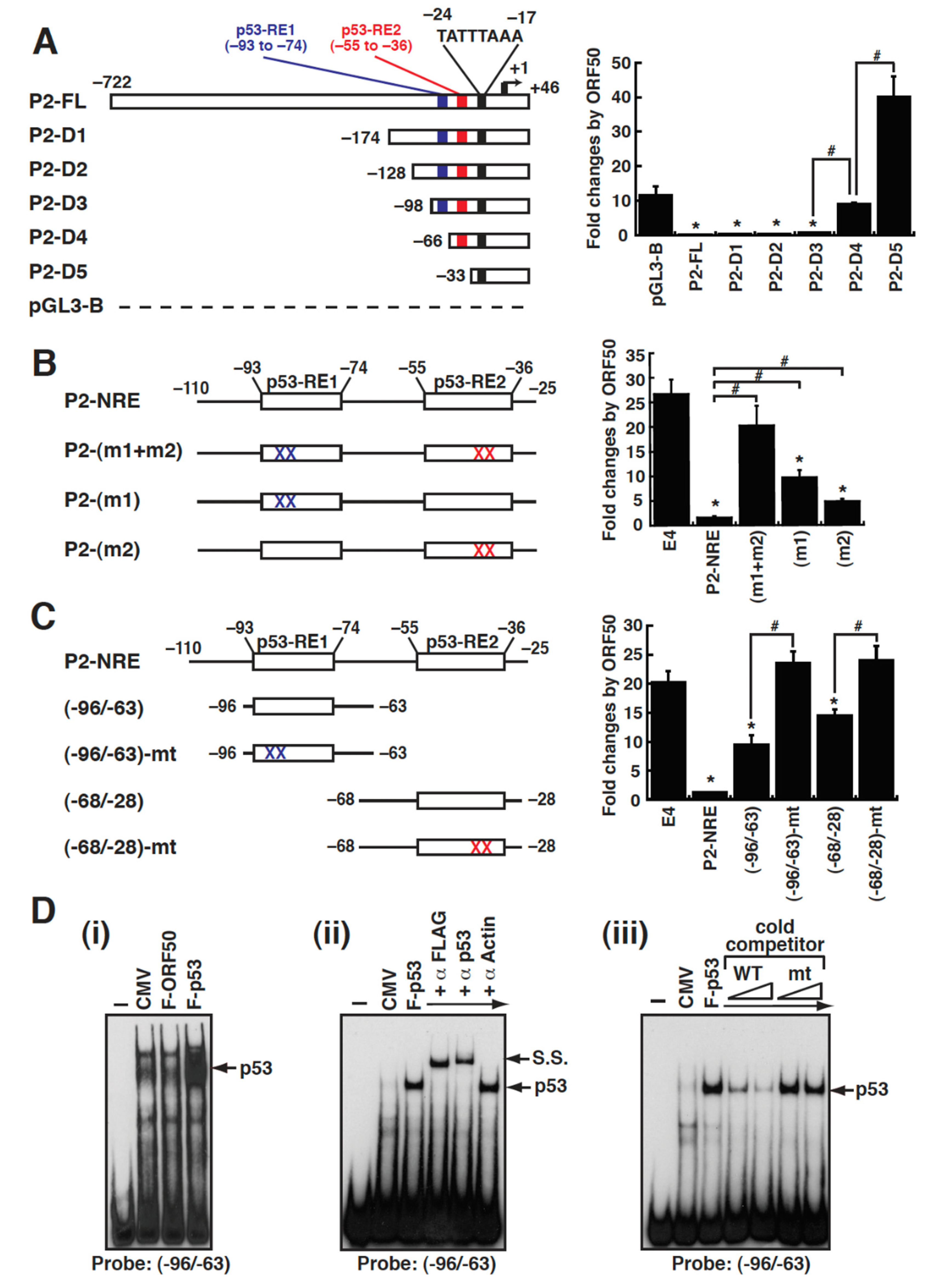
2.6. Defining the ORF50-Dependent Negative Response Element in the MDM2 P2 Promoter
2.7. Repression of the MDM2 P2 Promoter by ORF50 Is Mediated through Inhibiting p53 Transactivation
3. Discussion
3.1. Negative Reciprocal Regulation between ORF50 and MDM2
3.2. ORF50 Protein Represses the P1 and P2 Promoters of the MDM2 Gene
4. Materials and Methods
4.1. Cell Cultures, Reagents, and Transfections
4.2. Plasmid Construction
4.3. Western Blot Analysis
4.4. Quantitative Reverse Transcription PCR (RT-qPCR)
4.5. Cycloheximide Chase Assay
4.6. Luciferase Reporter Assays
4.7. Electrophoretic Mobility Shift Assay (EMSA)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| HHV8 | human herpesvirus 8 |
| ORF50 | open reading frame 50 |
| MDM2 | murine double-minute 2 homolog |
| KS | Kaposi’s sarcoma |
| PEL | primary effusion lymphoma |
| RTA | replication and transcription activator |
| siRNA | small interfering RNA |
| SB | sodium butyrate |
| RT-qPCR | quantitative reverse transcription polymerase chain reaction |
| NRR | negative response region |
| NRE | negative response element |
| EMSA | electrophoretic mobility shift assay |
| DBD | DNA-binding domain |
| NLS | nuclear localization signal |
References
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Chang, Y.; Moore, P.S.; Said, J.W.; Knowles, D.M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 1995, 332, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Soulier, J.; Grollet, L.; Oksenhendler, E.; Cacoub, P.; Cazals-Hatem, D.; Babinet, P.; d’Agay, M.F.; Clauvel, J.P.; Raphael, M.; Degos, L.; et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995, 86, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Dourmishev, L.A.; Dourmishev, A.L.; Palmeri, D.; Schwartz, R.A.; Lukac, D.M. Molecular genetics of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 175–212. [Google Scholar] [CrossRef]
- Broussard, G.; Damania, B. Regulation of KSHV Latency and Lytic Reactivation. Viruses 2020, 12, 1034. [Google Scholar] [CrossRef]
- Sun, R.; Lin, S.-F.; Gradoville, L.; Yuan, Y.; Zhu, F.; Miller, G. A viral gene that activates lytic cycle expression of Kaposi’s sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 1998, 95, 10866–10871. [Google Scholar] [CrossRef]
- Lukac, D.M.; Renne, R.; Kirshner, J.R.; Ganem, D. Reactivation of Kaposi’s sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 1998, 252, 304–312. [Google Scholar] [CrossRef]
- Chang, P.J.; Miller, G. Autoregulation of DNA binding and protein stability of Kaposi’s sarcoma-associated herpesvirus ORF50 protein. J. Virol. 2004, 78, 10657–10673. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Wu, M.; Geng, Y.; Wood, C. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8 ORF50 gene product contains a potent C-terminal activation domain which activates gene expression via a specific target sequence. Arch. Virol. 2001, 146, 1415–1426. [Google Scholar] [CrossRef]
- Chang, P.J.; Shedd, D.; Gradoville, L.; Cho, M.S.; Chen, L.W.; Chang, J.; Miller, G. Open reading frame 50 protein of Kaposi’s sarcoma-associated herpesvirus directly activates the viral PAN and K12 genes by binding to related response elements. J. Virol. 2002, 76, 3168–3178. [Google Scholar] [CrossRef]
- Chang, P.J.; Shedd, D.; Miller, G. Two subclasses of Kaposi’s sarcoma-associated herpesvirus lytic cycle promoters distinguished by open reading frame 50 mutant proteins that are deficient in binding to DNA. J. Virol. 2005, 79, 8750–8763. [Google Scholar] [CrossRef]
- Liang, Y.; Chang, J.; Lynch, S.J.; Lukac, D.M.; Ganem, D. The lytic switch protein of KSHV activates gene expression via functional interaction with RBP-Jkappa (CSL), the target of the Notch signaling pathway. Genes Dev. 2002, 16, 1977–1989. [Google Scholar] [CrossRef]
- Guito, J.; Lukac, D.M. KSHV Rta Promoter Specification and Viral Reactivation. Front. Microbiol. 2012, 3, 30. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Chen, L.-W.; Peng, K.-T.; Hung, C.-H.; Chang, P.-J.; Wang, S.-S. Sp3 Transcription Factor Cooperates with the Kaposi’s Sarcoma-Associated Herpesvirus ORF50 Protein to Synergistically Activate Specific Viral and Cellular Gene Promoters. J. Virol. 2020, 94, e01143-20. [Google Scholar] [CrossRef]
- Purushothaman, P.; Uppal, T.; Verma, S.C. Molecular Biology of KSHV Lytic Reactivation. Viruses 2015, 7, 116–153. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, S.E.; Hayward, G.S. The KSHV Immediate-Early Transcription Factor RTA Encodes Ubiquitin E3 Ligase Activity that Targets IRF7 for Proteosome-Mediated Degradation. Immunity 2005, 22, 59–70. [Google Scholar] [CrossRef]
- Izumiya, Y.; Kobayashi, K.; Kim, K.Y.; Pochampalli, M.; Izumiya, C.; Shevchenko, B.; Wang, D.H.; Huerta, S.B.; Martinez, A.; Campbell, M.; et al. Kaposi’s sarcoma-associated herpesvirus K-Rta exhibits SUMO-targeting ubiquitin ligase (STUbL) like activity and is essential for viral reactivation. PLoS Pathog. 2013, 9, e1003506. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, H.; Hu, W. The regulation of MDM2 oncogene and its impact on human cancers. Acta Biochim. Biophys. Sin. 2014, 46, 180–189. [Google Scholar] [CrossRef]
- Oliner, J.D.; Saiki, A.; Caenepeel, S. The Role of MDM2 Amplification and Overexpression in Tumorigenesis. Cold Spring Harb. Perspect. Med. 2016, 6, a026336. [Google Scholar] [CrossRef]
- Manfredi, J.J. The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 2010, 24, 1580–1589. [Google Scholar] [CrossRef]
- Moll, U.M.; Petrenko, O. The MDM2-p53 interaction. Mol. Cancer Res. 2003, 1, 1001–1008. [Google Scholar] [PubMed]
- Zauberman, A.; Flusberg, D.; Haupt, Y.; Barak, Y.; Oren, M. A functional p53-responsive intronic promoter is contained within the human mdm2 gene. Nucleic. Acids. Res. 1995, 23, 2584–2592. [Google Scholar] [CrossRef]
- Chang, C.-J.; Freeman, D.J.; Wu, H. PTEN Regulates Mdm2 Expression through the P1 Promoter. J. Biol. Chem. 2004, 279, 29841–29848. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, G.; Viiliäinen, J.; Turunen, M.; Diaz, R.; Lyly, L.; Pekkonen, P.; Rantala, J.; Ojala, K.; Sarek, G.; Teesalu, M.; et al. Oncogenic Herpesvirus Utilizes Stress-Induced Cell Cycle Checkpoints for Efficient Lytic Replication. PLOS Pathog. 2016, 12, e1005424. [Google Scholar] [CrossRef]
- Chang, T.-H.; Wang, S.-S.; Chen, L.-W.; Shih, Y.-J.; Chang, L.-K.; Liu, S.-T.; Chang, P.-J. Regulation of the Abundance of Kaposi’s Sarcoma-Associated Herpesvirus ORF50 Protein by Oncoprotein MDM. PLOS Pathog. 2016, 12, e1005918. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, Z.; Zhao, L.; Li, Q.; Liao, D. Downregulation of Mdm2 and Mdm4 enhances viral gene expression during adenovirus infection. Cell Cycle 2012, 11, 582–593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Izumi, T.; Takaori-Kondo, A.; Shirakawa, K.; Higashitsuji, H.; Itoh, K.; Io, K.; Matsui, M.; Iwai, K.; Kondoh, H.; Sato, T.; et al. MDM2 is a novel E3 ligase for HIV-1 Vif. Retrovirology 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Ronsard, L.; Lata, S.; Trivedi, S.; Banerjea, A.C. HIV-1 Tat potently stabilises Mdm2 and enhances viral replication. Biochem. J. 2017, 474, 2449–2464. [Google Scholar] [CrossRef]
- Pizzorno, M.A.; Dubois, J.; Machado, D.; Cartet, G.; Traversier, A.; Julien, T.; Lina, B.; Bourdon, J.-C.; Rosa-Calatrava, M.; Terrier, O. Influenza A viruses alter the stability and antiviral contribution of host E3-ubiquitin ligase Mdm2 during the time-course of infection. Sci. Rep. 2018, 8, 3746. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, S.; Guo, X.; Liu, S.; Jin, Y.; He, T.; Bi, D.; Zhang, P.; Lin, B.; An, X.; et al. An Integrative Analysis Reveals a Central Role of P53 Activation via MDM2 in Zika Virus Infection Induced Cell Death. Front. Cell. Infect. Microbiol. 2017, 7, 327. [Google Scholar] [CrossRef]
- Ye, F.; Lattif, A.A.; Xie, J.; Weinberg, A.; Gao, S. Nutlin-3 induces apoptosis, disrupts viral latency and inhibits expression of angiopoietin-2 in Kaposi sarcoma tumor cells. Cell Cycle 2012, 11, 1393–1399. [Google Scholar] [CrossRef]
- Bodzak, E.; Blough, M.D.; Lee, P.W.; Hill, R. p53 binding to the p21 promoter is dependent on the nature of DNA damage. Cell Cycle 2008, 7, 2535–2543. [Google Scholar] [CrossRef]
- Aneja, K.K.; Yuan, Y. Reactivation and Lytic Replication of Kaposi’s Sarcoma-Associated Herpesvirus: An Update. Front. Microbiol. 2017, 8, 613. [Google Scholar] [CrossRef]
- Gradoville, L.; Gerlach, J.; Grogan, E.; Shedd, D.; Nikiforow, S.; Metroka, C.; Miller, G. Kaposi’s sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 2000, 74, 6207–6212. [Google Scholar] [CrossRef]
- Arvanitakis, L.; Mesri, E.A.; Nador, R.G.; Said, J.W.; Asch, A.S.; Knowles, D.M.; Cesarman, E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 1996, 88, 2648–2654. [Google Scholar] [CrossRef]
- Graham, F.L.; Smiley, J.; Russell, W.C.; Nairn, R. Characteristics of a Human Cell Line Transformed by DNA from Human Adenovirus Type 5. J. Gen. Virol. 1977, 36, 59–74. [Google Scholar] [CrossRef]
- Brulois, K.F.; Chang, H.; Lee, A.S.; Ensser, A.; Wong, L.Y.; Toth, Z.; Lee, S.H.; Lee, H.R.; Myoung, J.; Ganem, D.; et al. Construction and manipulation of a new Kaposi’s sarcoma-associated herpesvirus bacterial artificial chromosome clone. J. Virol. 2012, 86, 9708–9720. [Google Scholar] [CrossRef]
- Chang, P.J.; Chen, L.W.; Shih, Y.C.; Tsai, P.H.; Liu, A.C.; Hung, C.H.; Liou, J.Y.; Wang, S.S. Role of the cellular transcription factor YY1 in the latent-lytic switch of Kaposi’s sarcoma-associated herpesvirus. Virology 2011, 413, 194–204. [Google Scholar] [CrossRef][Green Version]
- Chang, P.J.; Shedd, D.; Miller, G. A mobile functional region of Kaposi’s sarcoma-associated herpesvirus ORF50 protein independently regulates DNA binding and protein abundance. J. Virol. 2008, 82, 9700–9716. [Google Scholar] [CrossRef]
- Wang, S.S.; Chen, L.W.; Chen, L.Y.; Tsai, H.H.; Shih, Y.C.; Yang, C.T.; Chang, P.J. Transcriptional regulation of the ORF61 and ORF60 genes of Kaposi’s sarcoma-associated herpesvirus. Virology 2010, 397, 311–321. [Google Scholar] [CrossRef][Green Version]
- Chen, L.-Y.; Chen, L.-W.; Hung, C.-H.; Lin, C.-L.; Wang, S.-S.; Chang, P.-J. Caspase-Mediated Cleavage of the Transcription Factor Sp3: Possible Relevance to Cancer and the Lytic Cycle of Kaposi’s Sarcoma-Associated Herpesvirus. Microbiol. Spectr. 2022, 10, e01464-21. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.J.; Boonsiri, J.; Wang, S.S.; Chen, L.Y.; Miller, G. Binding of RBP-Jkappa (CSL) protein to the promoter of the Kaposi’s sarcoma-associated herpesvirus ORF47 (gL) gene is a critical but not sufficient determinant of transactivation by ORF50 protein. Virology 2010, 398, 38–48. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-I.; Wang, S.-S.; Hung, C.-H.; Chang, P.-J.; Chen, L.-W. Kaposi’s Sarcoma-Associated Herpesvirus ORF50 Protein Represses Cellular MDM2 Expression via Suppressing the Sp1- and p53-Mediated Transactivation. Int. J. Mol. Sci. 2022, 23, 8673. https://doi.org/10.3390/ijms23158673
Lin C-I, Wang S-S, Hung C-H, Chang P-J, Chen L-W. Kaposi’s Sarcoma-Associated Herpesvirus ORF50 Protein Represses Cellular MDM2 Expression via Suppressing the Sp1- and p53-Mediated Transactivation. International Journal of Molecular Sciences. 2022; 23(15):8673. https://doi.org/10.3390/ijms23158673
Chicago/Turabian StyleLin, Chia-I, Shie-Shan Wang, Chien-Hui Hung, Pey-Jium Chang, and Lee-Wen Chen. 2022. "Kaposi’s Sarcoma-Associated Herpesvirus ORF50 Protein Represses Cellular MDM2 Expression via Suppressing the Sp1- and p53-Mediated Transactivation" International Journal of Molecular Sciences 23, no. 15: 8673. https://doi.org/10.3390/ijms23158673
APA StyleLin, C.-I., Wang, S.-S., Hung, C.-H., Chang, P.-J., & Chen, L.-W. (2022). Kaposi’s Sarcoma-Associated Herpesvirus ORF50 Protein Represses Cellular MDM2 Expression via Suppressing the Sp1- and p53-Mediated Transactivation. International Journal of Molecular Sciences, 23(15), 8673. https://doi.org/10.3390/ijms23158673





