Distinct Roles of Estrogen Receptors in the Regulation of Vitellogenin Expression in Orange-Spotted Grouper (Epinephelus coioides)
Abstract
:1. Introduction
2. Results
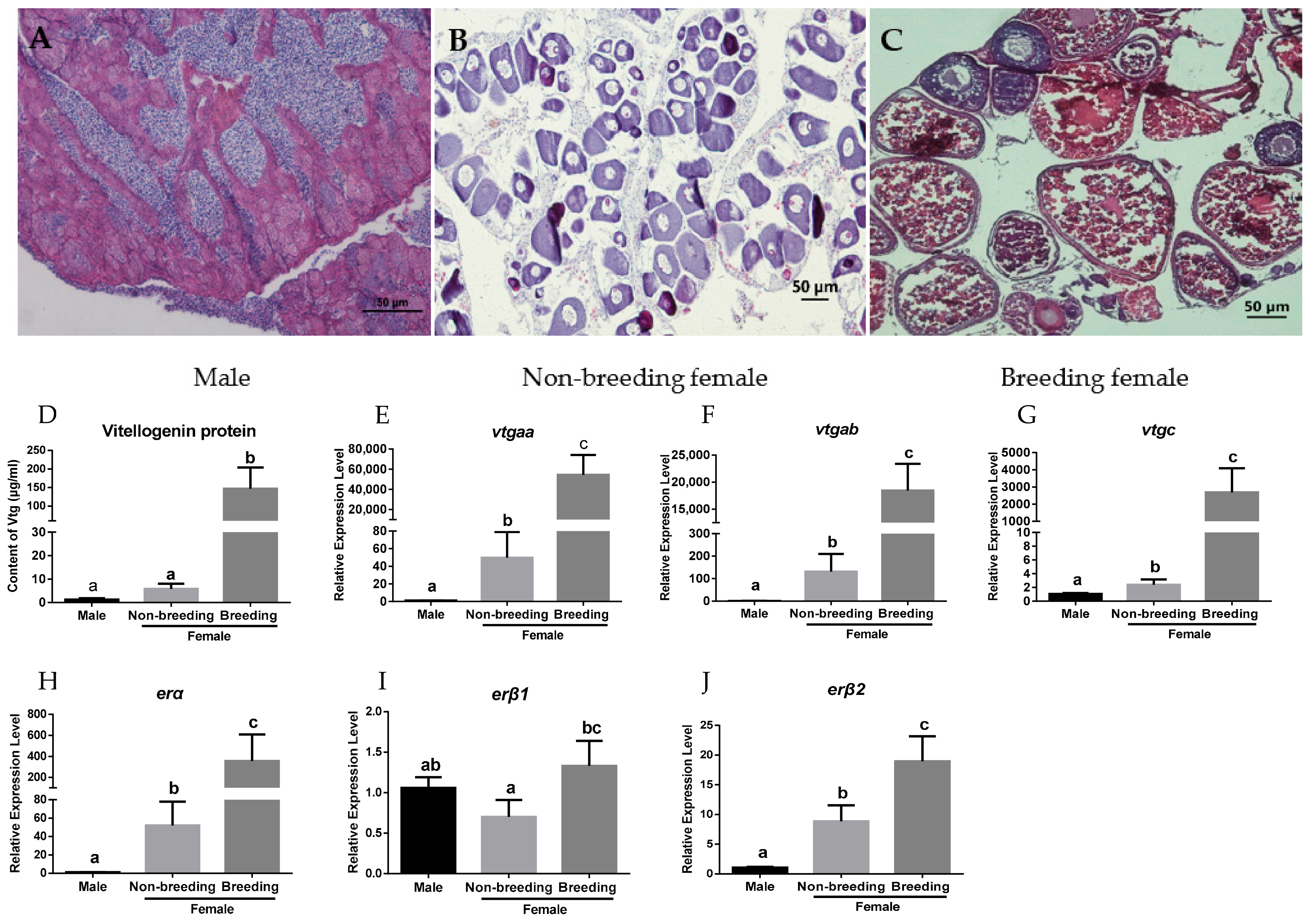
2.1. Expression Profiles of vtgs and ers in the Liver of Orange-Spotted Grouper
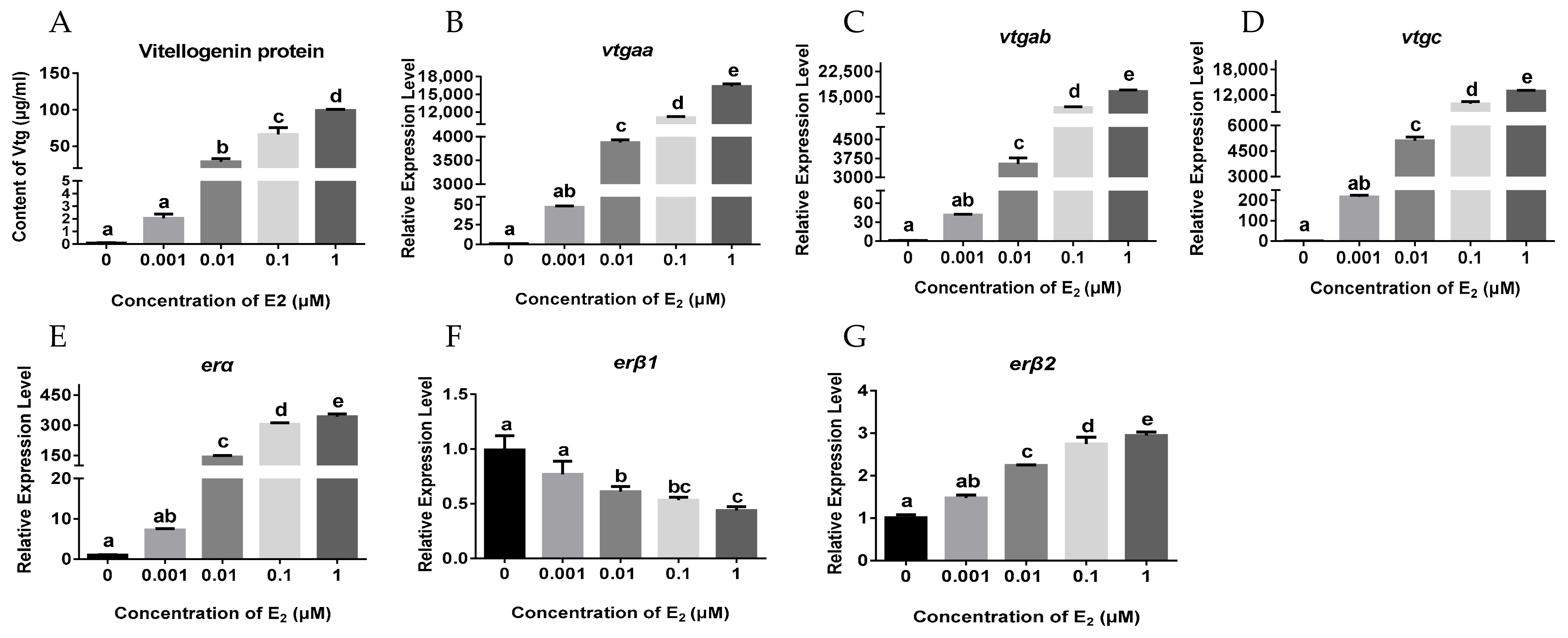
2.2. Effects of E2 on the Hepatic Expression of vtgs and ers in Juvenile Female Orange-Spotted Grouper
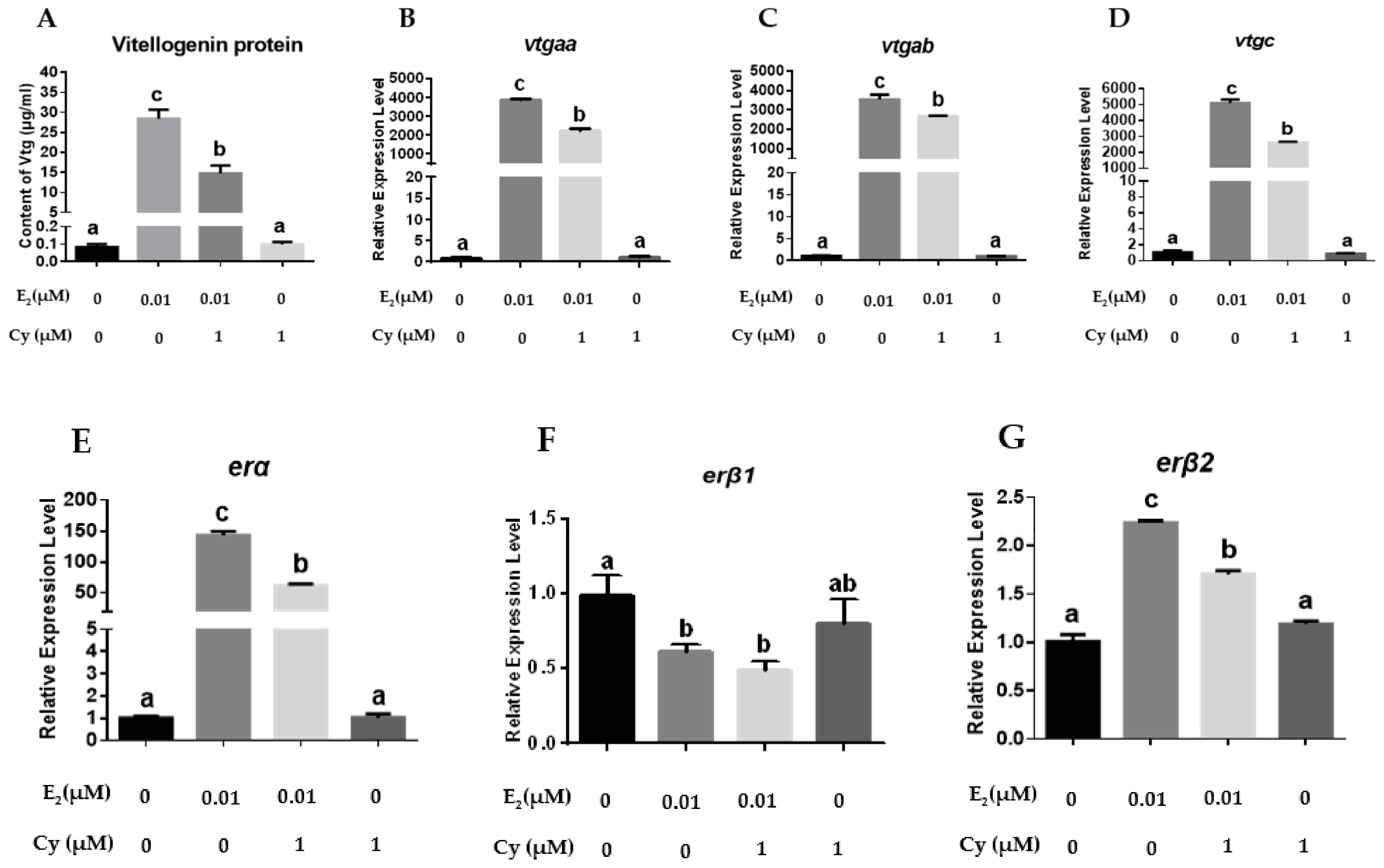
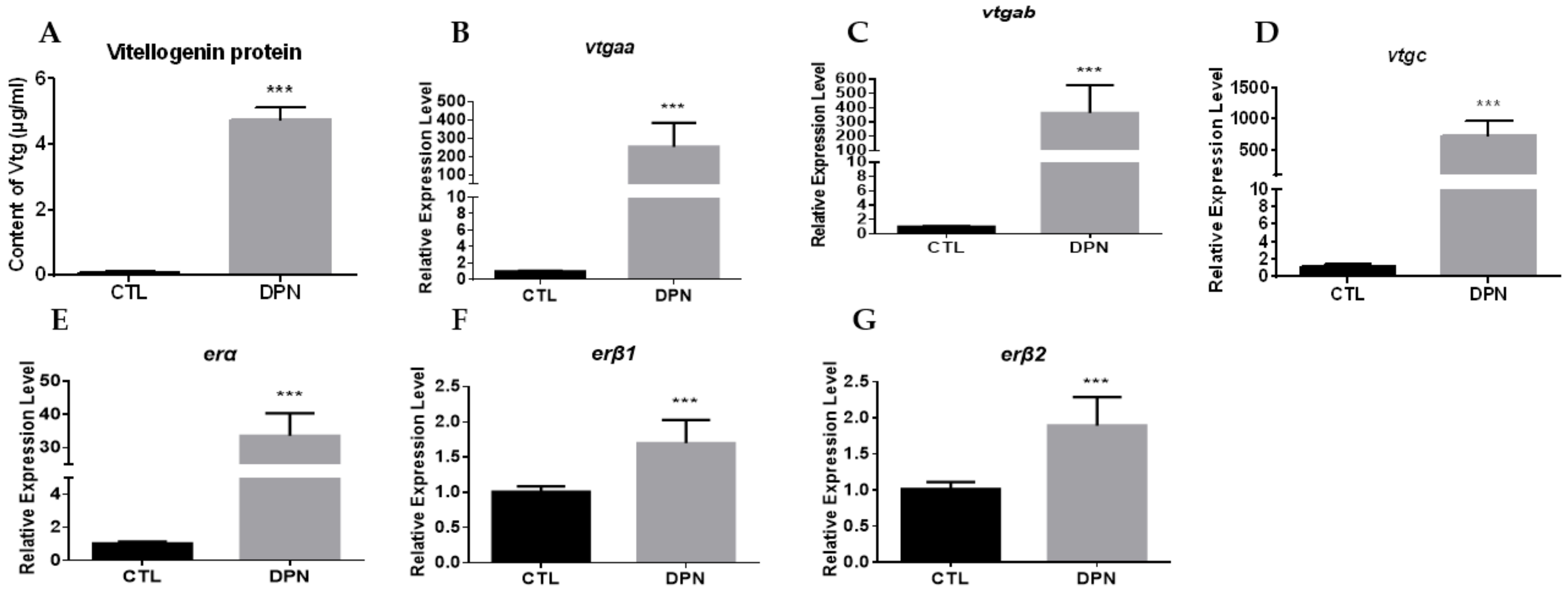
2.3. Effects of ER Antagonists or Agonist on the Expression of vtgs and ers in Primary Hepatocytes
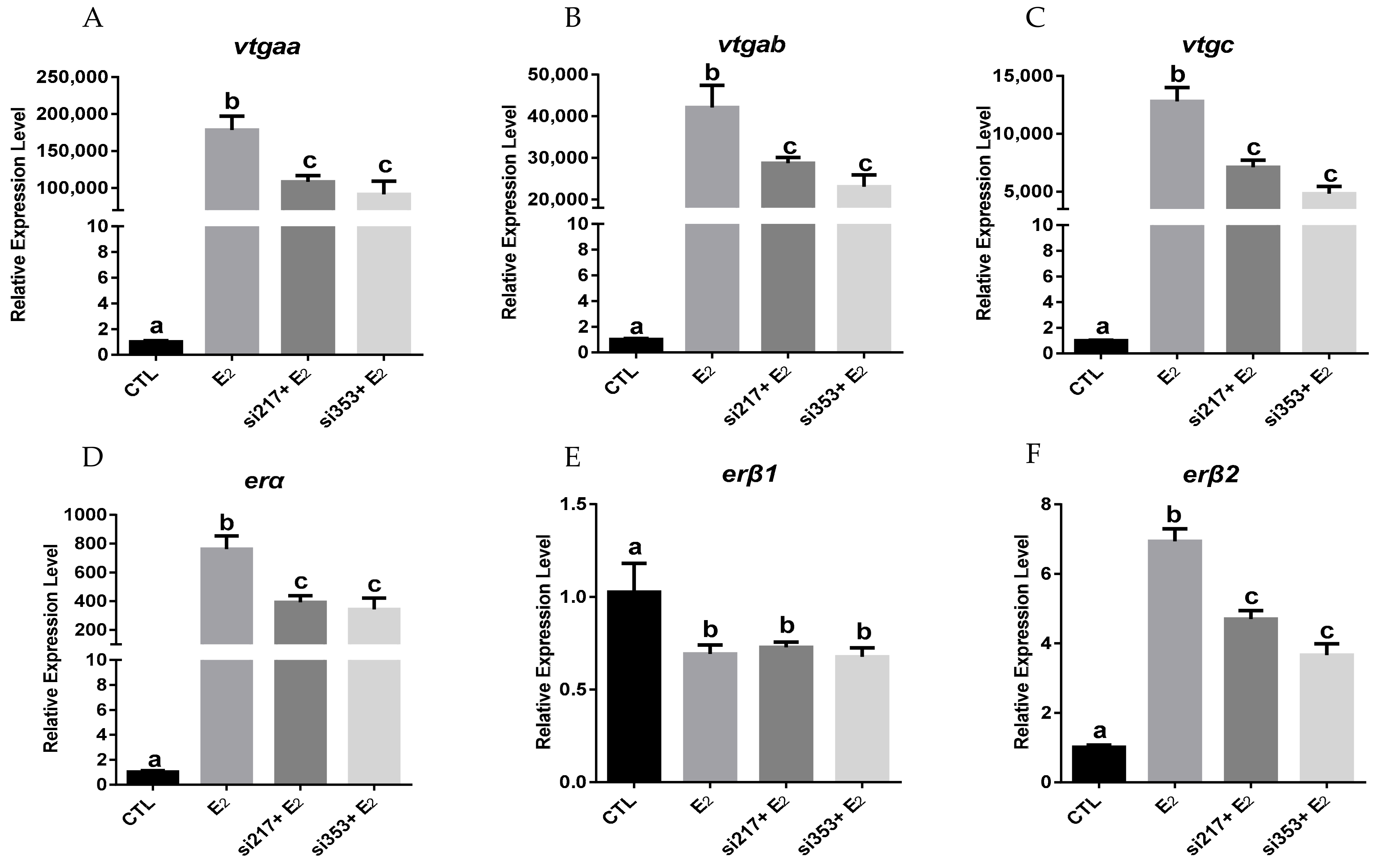
2.4. Effects of erβ2 Knockdown by siRNA on the Expression of vtgs and ers in Primary Hepatocytes
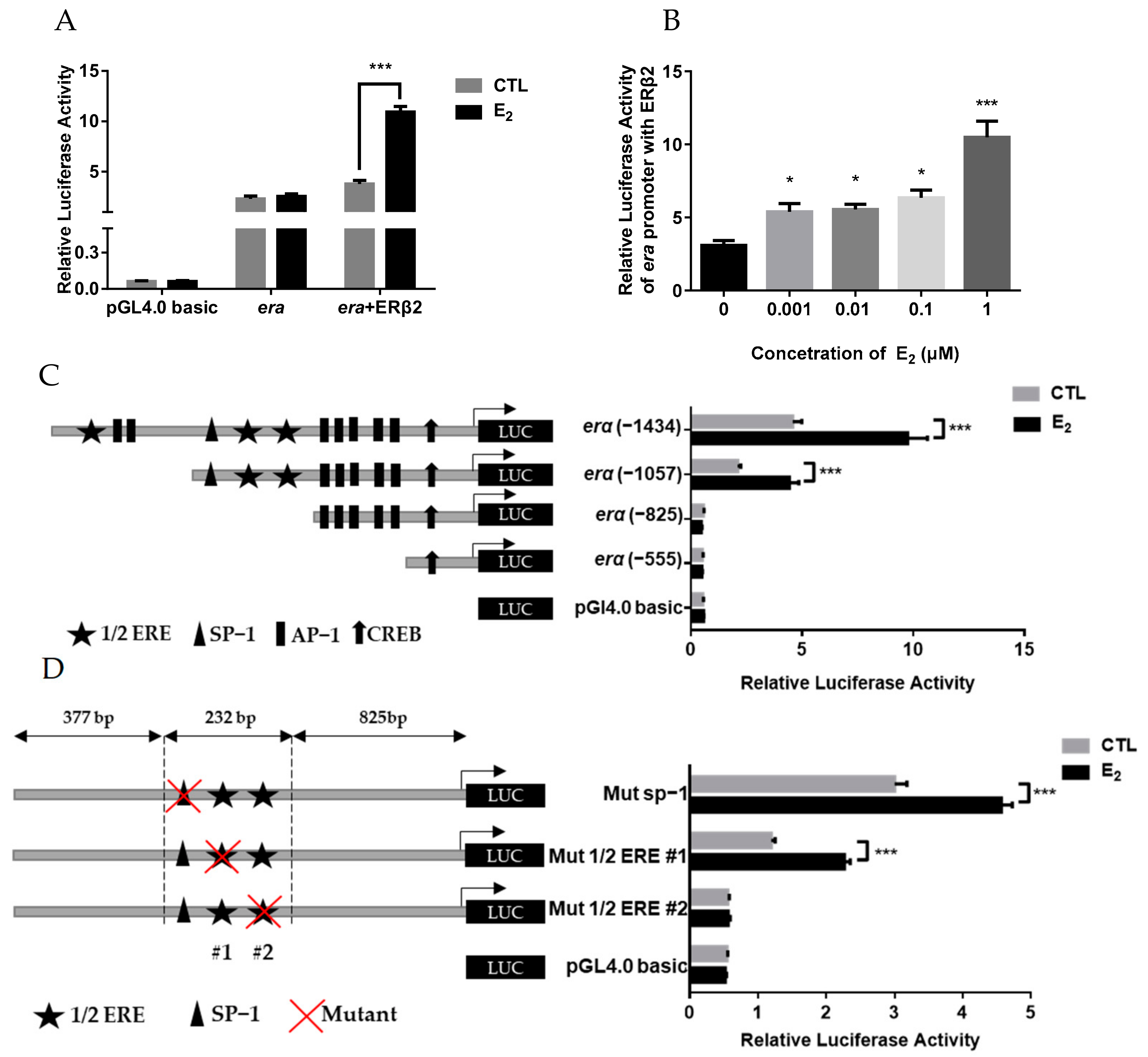
2.5. Estrogen Stimulation on erα Promoter Activity via ERβ2
3. Discussion
4. Materials and Methods
4.1. Animal and Sample Collection
4.2. Chemicals
4.3. Intraperitoneal Injection Experiment
4.4. Isolation, Primary Culture, and Static Incubation of Grouper Hepatocytes
4.5. Knockdown Experiment
4.6. Quantitative Real-Time PCR
4.7. Plasmid Construction and In Silico Analysis of Promoter
4.8. Dual-Luciferase Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goulas, A.; Triplett, E.L.; Taborsky, G. Isolation and characterization of a vitellogenin cDNA from rainbow trout (Oncorhynchus mykiss) and the complete sequence of a phosvitin coding segment. DNA Cell Biol. 1996, 15, 605–616. [Google Scholar] [CrossRef]
- Jalabert, B. Particularities of reproduction and oogenesis in teleost fish compared to mammals. Reprod. Nutr. Dev. 2005, 45, 261–279. [Google Scholar] [CrossRef]
- Panprommin, D.; Poompuang, S.; Srisapoome, P. Molecular characterization and seasonal expression of the vitellogenin gene from Günther’s walking catfish Clarias macrocephalus. Aquaculture 2008, 276, 60–68. [Google Scholar] [CrossRef]
- Yilmaz, O.; Patinote, A.; Com, E.; Pineau, C.; Bobe, J. Knock out of specific maternal vitellogenins in zebrafish (Danio rerio) evokes vital changes in egg proteomic profiles that resemble the phenotype of poor quality eggs. BMC Genom. 2021, 22, 308. [Google Scholar] [CrossRef]
- Finn, R.N.; Kristoffersen, B.A. Vertebrate vitellogenin gene duplication in relation to the “3R hypothesis”: Correlation to the pelagic egg and the oceanic radiation of teleosts. PLoS ONE 2007, 2, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakmak, G.; Togan, I.; Severcan, F. 17Beta-estradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FT-IR spectroscopy: A comparative study with nonylphenol. Aquat. Toxicol. 2006, 77, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Roman-Blas, J.A.; Castaneda, S.; Largo, R.; Herrero-Beaumont, G. Osteoarthritis associated with estrogen deficiency. Arthritis Res. Ther. 2009, 11, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, C.Y.; Habibi, H.R. Molecular cloning of estrogen receptor α and expression pattern of estrogen receptor subtypes in male and female goldfish. Mol. Cell. Endocrinol. 2003, 204, 169–177. [Google Scholar] [CrossRef]
- Menuet, A.; Page, Y.L.; Torres, O.; Kern, L.; Pakdel, F. Analysis of the estrogen regulation of the zebrafish estrogen receptor (ER) reveals distinct effects of ERalpha, ERbeta1 and ERbeta2. J. Mol. Endocrinol. 2004, 32, 975–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabo-Attwood, T.; Kroll, K.J.; Denslow, N.D. Differential expression of largemouth bass (Micropterus salmoides) estrogen receptor isotypes alpha, beta, and gamma by estradiol. Mol. Cell. Endocrinol. 2004, 218, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.K.; Katsu, Y.; Iguchi, T.; Lerner, D.T.; Hirano, T.; Grau, E.G. Transcriptional activity and biological effects of mammalian estrogen receptor ligands on three hepatic estrogen receptors in Mozambique tilapia. J. Steroid Biochem. Mol. Biol. 2010, 122, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.K.; Pierce, A.L.; Hiramatsu, N.; Sullivan, C.V.; Hirano, T.; Grau, E.G. Gender-specific expression of multiple estrogen receptors, growth hormone receptors, insulin-like growth factors and vitellogenins, and effects of 17 beta-estradiol in the male tilapia (Oreochromis mossambicus). Gen. Comp. Endocrinol. 2008, 156, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Filby, A.L.; Tyler, C.R. Molecular characterization of estrogen receptors 1, 2a, and 2b and their tissue and ontogenic expression profiles in fathead minnow (Pimephales promelas). Biol. Reprod. 2005, 73, 648–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce-Derricott, J.; Nagler, J.J.; Cloud, J.G. Regulation of hepatic estrogen receptor isoform mRNA expression in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2009, 161, 73–78. [Google Scholar] [CrossRef]
- Andreassen, T.K.; Korsgaard, B. Characterization of a cytosolic estrogen receptor and its up-regulation by 17β-estradiol and the xenoestrogen 4-tert-octylphenol in the liver of eelpout (Zoarces viviparus). Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2000, 125, 300–313. [Google Scholar] [CrossRef]
- Mosconi, G.; Carnevali, O.; Habibi, H.R.; Sanyal, R.; Polzonetti-Magni, A.M. Hormonal mechanisms regulating hepatic vitellogenin synthesis in the gilthead sea bream, Sparus aurata. Am. J. Physiol. 2002, 283, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, M.B.; Thornton, J.W.; Crews, D.; Skipper, J.K. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proc. Natl. Acad. Sci. USA 2000, 20, 10751–10756. [Google Scholar] [CrossRef] [Green Version]
- Hoegg, S.; Brinkmann, H.; Taylor, J.S.; Meyer, A. Phylogenetic Timing of the Fish-Specific Genome Duplication Correlates with the Diversification of Teleost Fish. J. Mol. Evol. 2004, 59, 190–203. [Google Scholar] [CrossRef] [Green Version]
- Nelson, E.R.; Wiehler, W.B.; Cole, W.C.; Habibi, H.R. Homologous regulation of estrogen receptor subtypes in goldfish (Carassius auratus). Mol. Reprod. Dev. 2007, 74, 1105–1112. [Google Scholar] [CrossRef]
- Leanos-Castaneda, O.; Van Der Kraak, G. Functional characterization of estrogen receptor subtypes, ERalpha and ERbeta, mediating vitellogenin production in the liver of rainbow trout. Toxicol. Appl. Pharmacol. 2007, 224, 116–125. [Google Scholar] [CrossRef]
- Nelson, E.R.; Habibi, H.R. Functional significance of nuclear estrogen receptor subtypes in the liver of goldfish. Endocrinology 2010, 151, 1668–1676. [Google Scholar] [CrossRef] [Green Version]
- Griffin, L.B.; January, K.E.; Ho, K.W. Morpholino-mediated knockdown of ERα, ERβa, and ERβb mRNAs in zebrafish (Danio rerio) embryos reveals differential regulation of estrogen-inducible genes. Endocrinology 2013, 154, 4158–4169. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.X.; Wang, Y.H.; Wang, J.; Zhuang, X.; You, Y.Z. Molecular phylogenetic relationships of 30 grouper species in China Seas based on 16S rDNA fragment sequences. Acta Zool. Sin. 2006, 52, 504–513. [Google Scholar]
- Ding, S.; Liu, Q.; Haohao, W.U.; Meng, Q.U. A review of research advances on the biology and artificial breeding of groupers. J. Fish. Sci. China 2018, 25, 737–752. [Google Scholar] [CrossRef]
- Zhao, H.H.; Liu, X.C.; Liufu, Y.Z.; Wang, Y.X.; Lin, H.R. Seasonal cycles of ovarian development and serum sex steroid levels of female grouper Epinephelus coioides. Acta Sci. Nat. Univ. Sunyatseni 2003, 42, 56–76. [Google Scholar]
- Nelson, E.R.; Habibi, H.R. Estrogen receptor function and regulation in fish and other vertebrates. Gen. Comp. Endocrinol 2013, 192, 15–24. [Google Scholar] [CrossRef]
- Cui, X.F.; Zhao, Y.; Chen, H.P.; Deng, S.P.; Jiang, D.N.; Wu, T.L.; Zhu, C.H.; Li, G.L. Cloning, expression and functional characterization on vitellogenesis of estrogen receptors in Scatophagus argus. Gen. Comp. Endocrinol. 2017, 246, 37–45. [Google Scholar] [CrossRef]
- Le Drean, Y.; Lazennec, G.; Kern, L.; Saligaut, D.; Pakdel, F.; Valotaire, Y. Characterization of an estrogen-responsive element implicated in regulation of the rainbow trout estrogen receptor gene. J. Mol. Endocrinol. 1995, 15, 37–47. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q.; Li, G.; He, M.; Tang, H.; Zhang, H.; Yang, X.; Liu, X.; Lin, H. Molecular mechanism of feedback regulation of 17beta-estradiol on two kiss genes in the protogynous orange-spotted grouper (Epinephelus coioides). Mol. Reprod. Dev. 2017, 84, 495–507. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, X.; Shi, H.; Chen, J.; Han, C.; Huang, H.; Lin, H.; Zhang, Y.; Li, S. Induction of oocyte maturation and changes in the biochemical composition, physiology and molecular biology of oocytes during maturation and hydration in the orange-spotted grouper (Epinephelus coioides). Aquaculture 2020, 522, 735–745. [Google Scholar] [CrossRef]


| Gene | Gene Accession | siRNA (Sense Strand) |
|---|---|---|
| erβ2 | GU721078.1 | GCCCAUCUGUGCUGAGCUATT |
| erβ2 | GU721078.1 | GCCUCUCGUCUACAAUGAATT |
| NC | UUGUCCGAACGUGUCACGUTT |
| Gene | Primer Name | 5′ to 3′ Sequence |
|---|---|---|
| vtgaa | vtgaa-F | GTATCCCAACAAGTTCCAGAGG |
| vtgaa-R | GGACGATGATGGCAAAGGTAG | |
| vtgab | vtgab-F | GCTGCCCGCCTGAAGATTAC |
| vtgab-R | CCTTTGCCAGGTTTATTTCG | |
| vtgc | vtgc-F | CTGCGAGCAATGCCTTAT |
| vtgc-R | GGAATGGCCTTGAGATGG | |
| erα | erα-F | GGACACCATCACAGATGCTCTC |
| erα-R | CTCTGTTTGGGCTCTGGTGGCTG | |
| erβ1 | erβ1-F | GACAAGAACCGCCGTAAGAGC |
| erβ1-R | GAGAAGATAAGTTTCCCTGGATG | |
| erβ2 | erβ2-F | TCACCAACCTGGCAGACAAGGAG |
| erβ2-R | GTACACAGATTGTAGTTAAGGAG | |
| beta-actin | actin-F | ACCATCGGCAATGAGAGGTT |
| actin-R | ACATCTGCTGGAAGGTGGAC |
| Gene | Primer Name | 5′ to 3′ Sequence |
|---|---|---|
| Primers used for rapid amplification of cDNA ends | ||
| erα | erα-race-R1 | AGTCATTGTGACCCTGAATGCTCC |
| erα-race-R2 | CATACTGTATGCCTCGTCACTG | |
| Primers used for Site-directed mutagenesis | ||
| erα | Mut-sp1-F | CCACCtcgataaagCATTTGGGATTGATTGTGTAATTATTG |
| Mut-sp1-R | ATGctttatcgaGGTGGCTATTTTAATCAGATGCTG | |
| erα | Mut-ERE1-F | GAGATTgacttcCCTGCTTTGTTTGCTGTGTTATG |
| Mut-ERE1-R | AGCAGGgaagtcAATCTCATTCCAACAGCCAATAATT | |
| erα | Mut-ERE2-F | TTATGGgacttcGGCAGTAAAACACACTGCTGTTTG |
| Mut-ERE2-R | ACTGCCgaagtcCCATAACACAGCAAACAAAGCAG | |
| Primers used for plasmid construction | ||
| erα | erα-1434-F | TGGCCTAACTGGCCGGTACCGAGCTCCTGTTGTAACTGGT |
| erα-1434-R | TCTTGATATCCTCGAGGTCACTGAAGGGGGCACGA | |
| erα-1057-F | TGGCCTAACTGGCCGGTACCCAATGAAATGTCATGAGG | |
| erα-825-F | TGGCCTAACTGGCCGGTACCGCATCTTTAATTTGTTTATC | |
| erα-555-F | TGGCCTAACTGGCCGGTACCTTACTGCAGAGTCTCAGG | |
| erβ2 | erβ2-ORF-F | CTAGCTAGCATGGCCTCGTCCCCTGAGCT |
| erβ2-ORF-R | CCGGAATTCCTACTGGTTCCACTGATGGA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Zhao, T.; Wei, Q.; Lin, H.; Zhang, Y.; Li, S. Distinct Roles of Estrogen Receptors in the Regulation of Vitellogenin Expression in Orange-Spotted Grouper (Epinephelus coioides). Int. J. Mol. Sci. 2022, 23, 8632. https://doi.org/10.3390/ijms23158632
Ye Z, Zhao T, Wei Q, Lin H, Zhang Y, Li S. Distinct Roles of Estrogen Receptors in the Regulation of Vitellogenin Expression in Orange-Spotted Grouper (Epinephelus coioides). International Journal of Molecular Sciences. 2022; 23(15):8632. https://doi.org/10.3390/ijms23158632
Chicago/Turabian StyleYe, Zhifeng, Tingting Zhao, Qianhao Wei, Haoran Lin, Yong Zhang, and Shuisheng Li. 2022. "Distinct Roles of Estrogen Receptors in the Regulation of Vitellogenin Expression in Orange-Spotted Grouper (Epinephelus coioides)" International Journal of Molecular Sciences 23, no. 15: 8632. https://doi.org/10.3390/ijms23158632
APA StyleYe, Z., Zhao, T., Wei, Q., Lin, H., Zhang, Y., & Li, S. (2022). Distinct Roles of Estrogen Receptors in the Regulation of Vitellogenin Expression in Orange-Spotted Grouper (Epinephelus coioides). International Journal of Molecular Sciences, 23(15), 8632. https://doi.org/10.3390/ijms23158632






