Synthesis and Immunological Evaluation of Mannosylated Desmuramyl Dipeptides Modified by Lipophilic Triazole Substituents
Abstract
:1. Introduction
2. Results
2.1. Chemistry
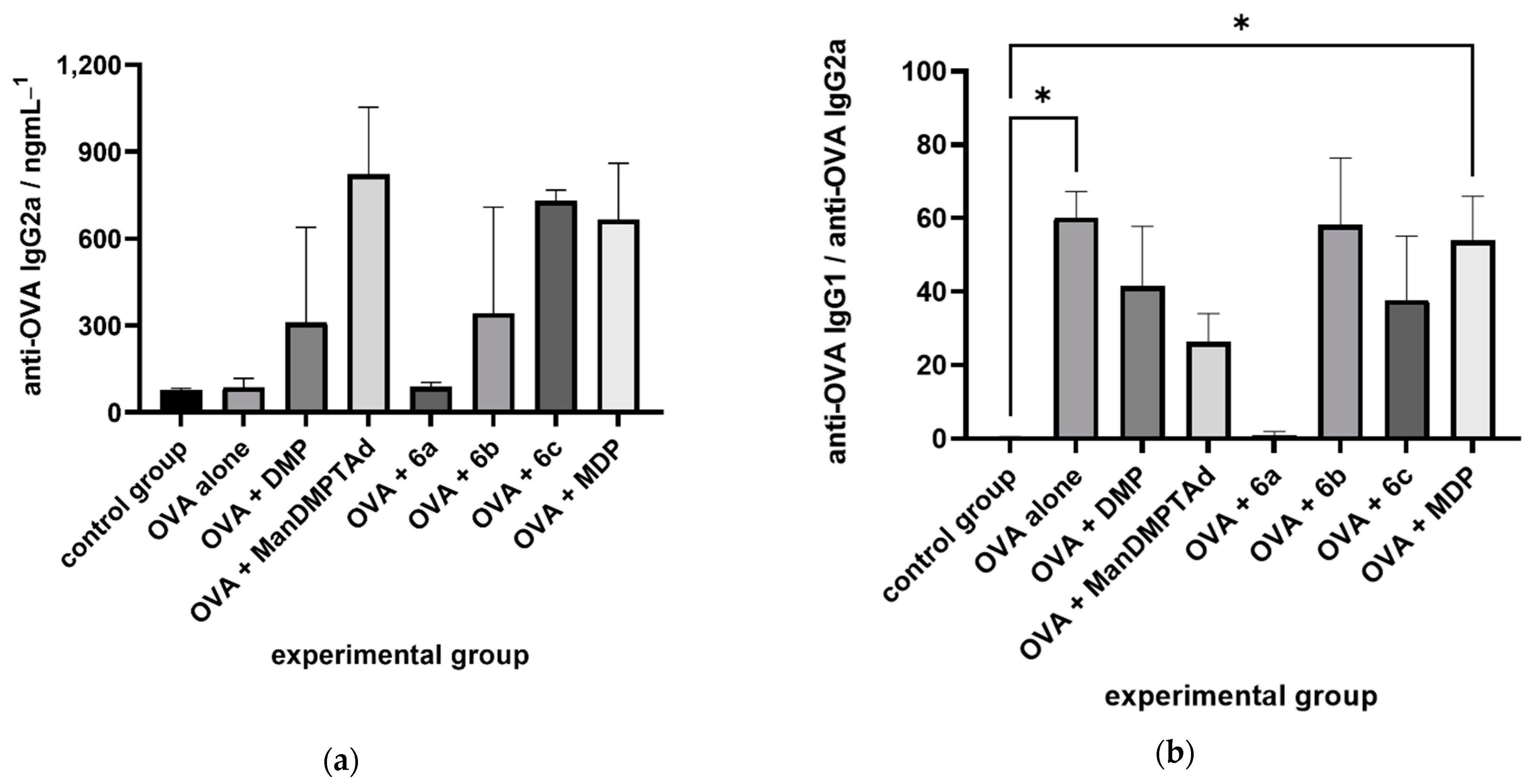
2.2. Immunological Evaluation
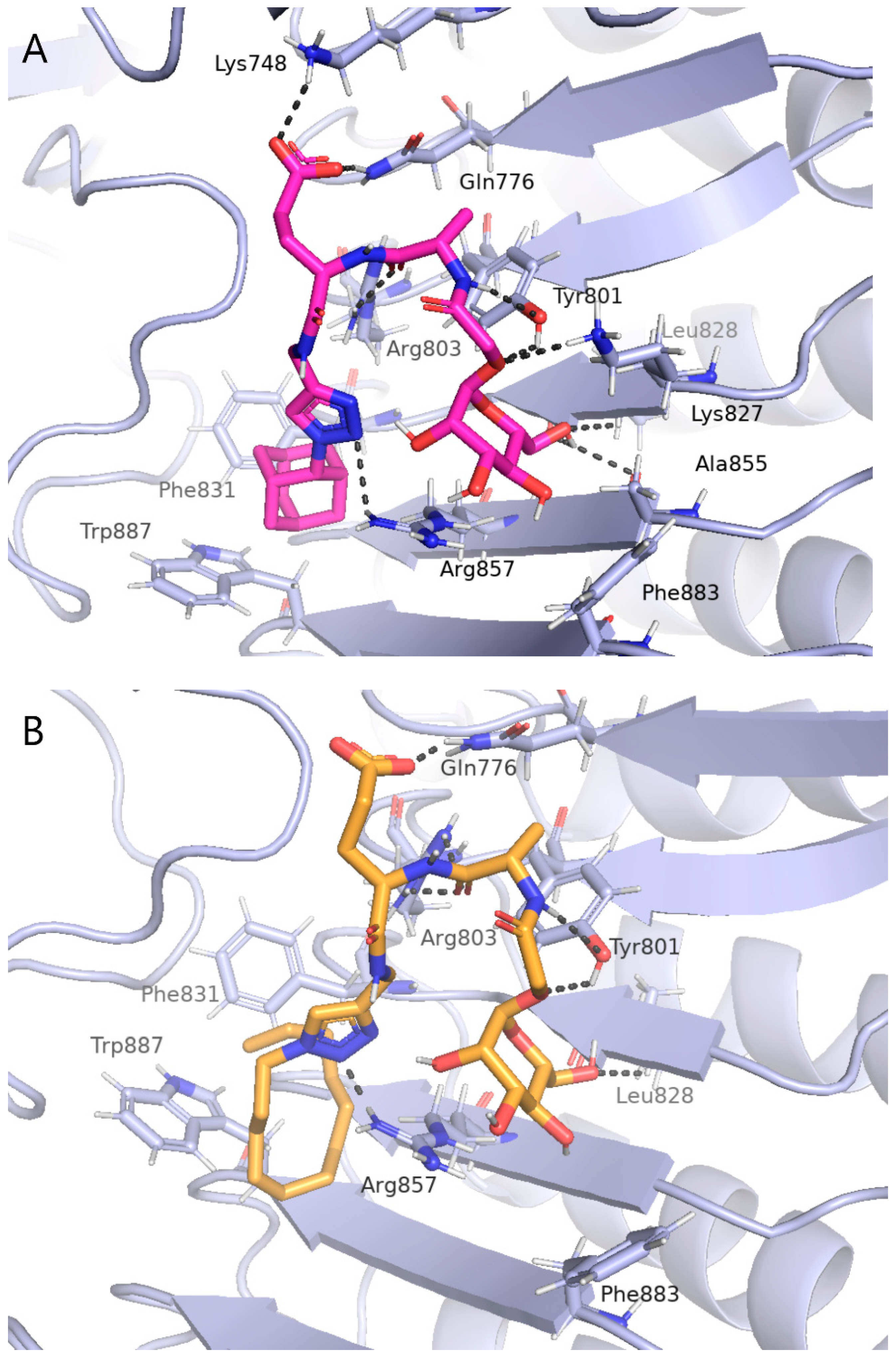
2.3. Computational Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis
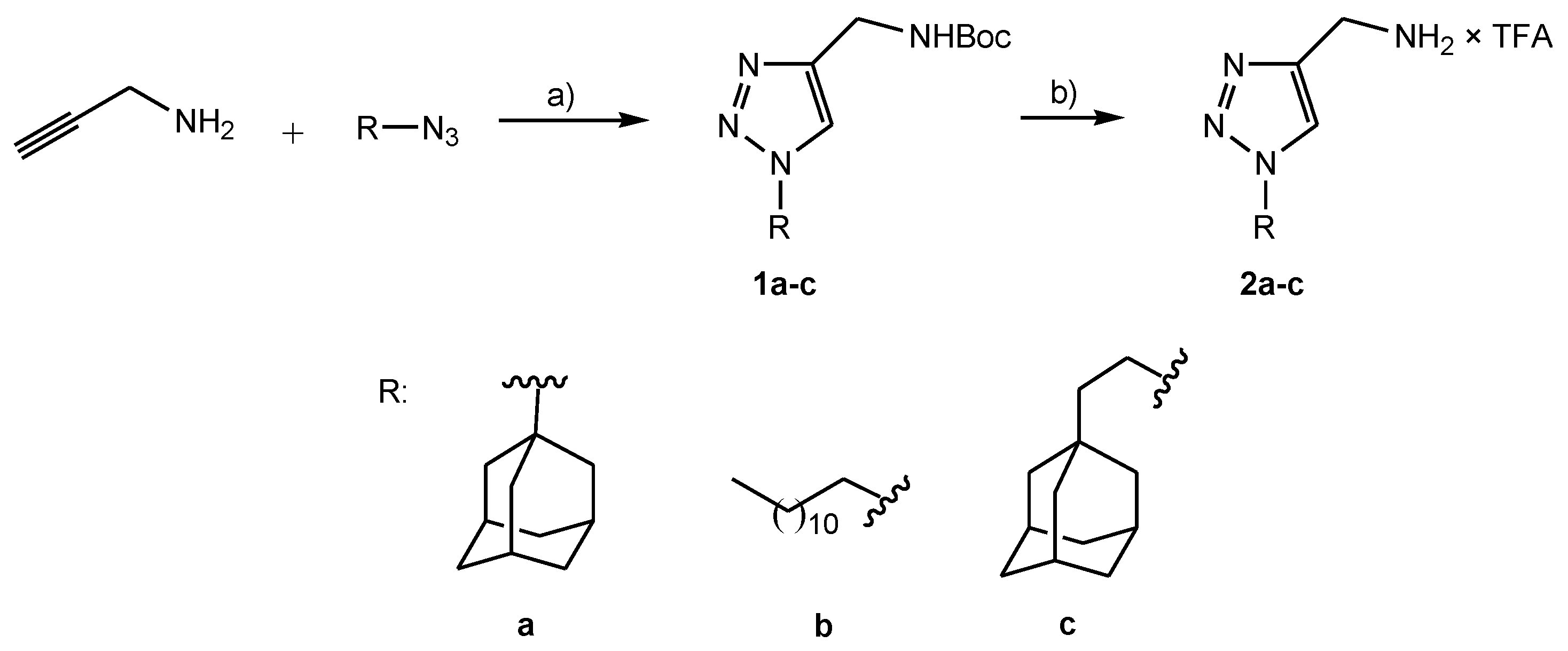
4.2.1. Preparation of Boc Protected Lipophilic Triazole Derivatives 1a–c
tert-Butyl-[1-(adamantan-1-yl)-1H-1,2,3-triazol-4-yl]methyl carbamate (1a)
tert-Butyl-[1-(dodecan-1-yl)-1H-1,2,3-triazol-4-yl]methyl carbamate (1b)
tert-Butyl-{1-[(2-(adamantan-1-yl)ethan-1-yl]-1H-1,2,3-triazol-4-yl}methyl carbamate (1c)
4.2.2. Preparation of Deprotected Lipophilic Triazole Derivatives 2a–c
[1-(adamantan-1-yl)-1H-1,2,3-triazol-4-yl]methylammonium trifluoroacetate (2a)
[1-(dodecan-1-yl)-1H-1,2,3-triazol-4-yl] methylammonium trifluoroacetate (2b)
{1-[2-(adamantan-1-yl)ethan-1-yl]-1H-1,2,3-triazol-4-yl}methylammonium trifluoroacetate (2c)
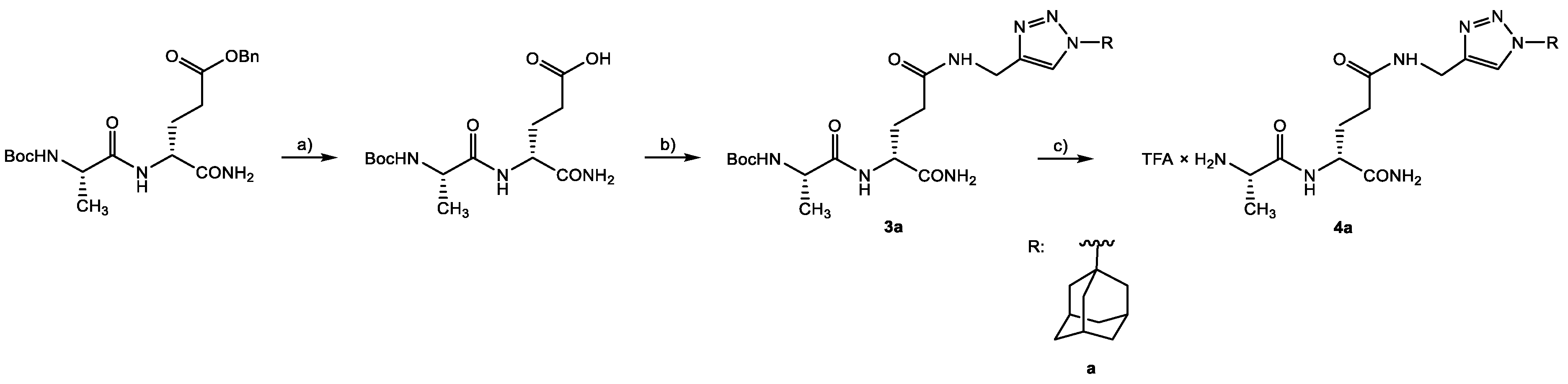
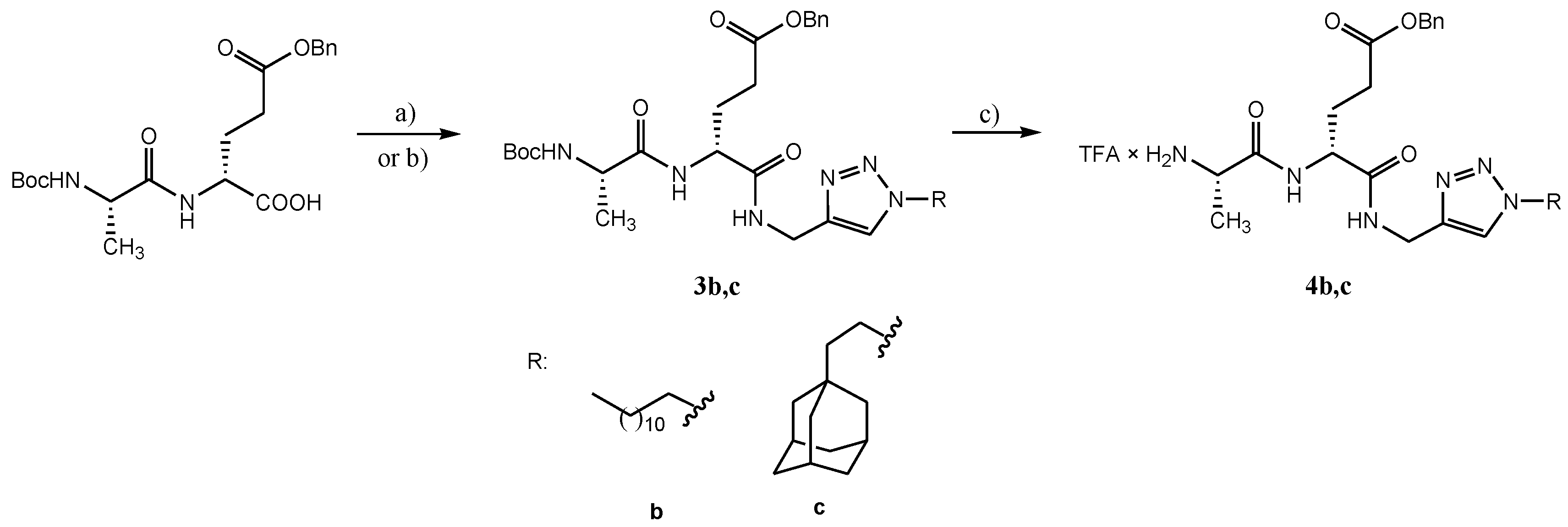
4.2.3. Preparation of Lipophilic Triazole Derivatives of Protected Desmuramyl Dipeptides 3a–c
N-[(adamantan-1-yl)-1H-1,2,3-triazole-4-yl]methyl-(4R)-4-[(2S)-2-(tert-butyloxycarbonylamino)]propanamido}-4-carbamoylbutanoate (3a)
Benzyl-(4R)-4-{1-[(dodecan-1-yl)-1H-1,2,3-triazol-4-yl]methylaminocarbonyl}-4-[(2S)-2-(tert-butyloxycarbonylamino)propanamido]butanoate (3b)
Benzyl-(4R)-4-{1-[(2-(adamantan-1-yl)ethan-1-yl)-1H-1,2,3-triazol-4-yl]methyl-aminocabonyl}-4-[(2S)-2-(tert-butyloxycarbonylamino)propanamido]butanoate (3c)
4.2.4. Preparation of Deprotected Lipophilic Triazole Derivatives Od Desmuramyl dipeptide 4a–c
(1S)-1-{[N-(1R)-{3-(adamantan-1-yl)-1H-1,2,3-triazol-4-yl]methylaminocarbonyl}-1-carbamoylpropan-1-yl]aminocarbonyl}ethylammonium trifluoracetate (4a)
(1S)-1-{[N-(1R)-{3-benzyloxycarbonyl-1-{[1-(dodecan-1-yl)-1H-1,2,3-triazol-4-yl]methylaminocarbonyl}propan-1-yl]aminocarbonyl}ethylammonium trifluoracetate (4b)
(1S)-1-{[N-(1R)-{3-benzyloxycarbonyl-1-{[1-(2-(adamantan-1-yl)ethan-1-yl]-1H-1,2,3-triazol-4-yl]methylaminocarbonyl}propan-1yl]aminocarbonyl}ethylammonium trifluoracetate (4c)
4.2.5. Preparation of Protected Mannose Glycoconjugates of Lipophilic Triazole Derivatives of Desmuramyl Dipeptides 5a–c
N-[(adamantan-1-yl)-1H-1,2,3-triazole-4-yl]methyl-(4R)-4-{(2S)-2-[(2,3,4,6-tetra-O-benzyl-α-D-mannopyranosyloxy)ethanamido]propanamido}-4-carbamoylbutanoate (5a)
Benzyl-(4R)-4-{[1-(dodecane-1-yl)-1H-1,2,3-triazole-4-yl]methylaminocarbonyl}-4-(2S)-2-[2,3,4,6-tetra-O-benzyl-α-D-mannopyranosyloxy)ethanamido]propanamido}butanoate (5b)
Benzyl-(4R)-4-{N-{1-[(2-(adamantan-1-yl)ethan-1-yl]-1H-1,2,3-triazole-4-il}methylcarbamoyl}-4-{(2S)-2-[(2,3,4,6-tetra-O-benzyl-α-D-mannopyranosyloxy)ethanamido]propanamido}butanoate (5c)
4.2.6. Preparation of Deprotected Mannose Glycoconjugates of Lipophilic Triazole Derivatives of Desmuramyl Dipeptides 6a–c
N-[(adamantan-1-yl)-1H-1,2,3-triazole-4-yl]methyl-(4R)-4-{(2S)-2-[(α-D-mannopyranosyloxy)ethanamido]propanamido}-4-carbamoylbutanoate (6a)
(4R)-4-{N-[1-(dodecan-1-yl)-1H-1,2,3-triazol-4-yl]methylcarbamoyl}-4-{(2S)-2-[(α-D-mannopyranosyloxy)ethanamido]propanamido}butanoic acid (6b)
(4R)-4-{N-{1-[(2-(adamantan-1-yl)ethan-1-yl]-1H-1,2,3-triazol-4-yl}methylcarbamoyl}-4-{(2S)-2-[(α-D-mannopyranosyloxy)ethanamido]propanamido}butanoic acid (6c)
4.3. Computational Studies
4.4. Experiments In Vivo
4.5. Enzyme Immunoassays
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awate, S.; Babiuk, L.A.B.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, A.E.; Hantho, J.D.; Mancini, R.J. Synthetic Agonists of NOD-like, RIG-I-like, and C-Type Lectin Receptors for Probing the Inflammatory Immune Response. Future Med. Chem. 2017, 9, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Traub, S.; von Aulock, S.; Hartung, T.; Hermann, C. Invited Review: MDP and Other Muropeptides—Direct and Synergistic Effects on the Immune System. J. Endotoxin Res. 2006, 12, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Kingeter, L.M.; Lin, X. C-Type Lectin Receptor-Induced NF-ΚB Activation in Innate Immune and Inflammatory Responses. Cell. Mol. Immunol. 2012, 9, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, S.; Ohto, U.; Shibata, T.; Miyake, K.; Shimizu, T. Crystal Structure of NOD2 and Its Implications in Human Disease. Nat. Commun. 2016, 7, 11813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.-A.; Khanam, R.; Wasim Qasim, M.; Wang, Y.; Jiang, Z.-H. Improved Synthesis of D-Isoglutamine: Rapid Access to Desmuramyl Analogues of Muramyl Dipeptide for the Activation of Intracellular NOD2 Receptor and Vaccine Adjuvant Applications. Eur. J. Org. Chem. 2021, 2021, 6688–6699. [Google Scholar] [CrossRef]
- Gobec, M.; Tomašič, T.; Štimac, A.; Frkanec, R.; Trontelj, J.; Anderluh, M.; Mlinarič-Raščan, I.; Jakopin, Ž. Discovery of Nanomolar Desmuramylpeptide Agonists of the Innate Immune Receptor Nucleotide-Binding Oligomerization Domain-Containing Protein 2 (NOD2) Possessing Immunostimulatory Properties. J. Med. Chem. 2018, 61, 2707–2724. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.-A.; Ulanova, M.; Bai, B.; Yalamati, D.; Jiang, Z.-H. Design, Synthesis and Immunological Evaluation of Novel Amphiphilic Desmuramyl Peptides. Eur. J. Med. Chem. 2017, 141, 26–36. [Google Scholar] [CrossRef]
- Willems, M.M.J.H.P.; Zom, G.G.; Meeuwenoord, N.; Khan, S.; Ossendorp, F.; Overkleeft, H.S.; Marel, G.A.; Filippov, D.V.; Codée, J.D.C. Lipophilic Muramyl Dipeptide–Antigen Conjugates as Immunostimulating Agents. ChemMedChem 2016, 11, 190–198. [Google Scholar] [CrossRef]
- Ribić, R.; Habjanec, L.; Frkanec, R.; Vranešić, B.; Tomić, S. Influence of Mannosylation on Immunostimulating Activity of Adamant-1-Yl Tripeptide. Chem. Biodivers. 2012, 9, 1373–1381. [Google Scholar] [CrossRef]
- Filipic, M.; Kuhar, P.; Zegura, B.; Urleb, U.; Gobec, S. Determination of Cytotoxic Activity of Adamantyl-Desmuramyl Dipeptides. Pharmazie 2003, 58, 442–443. [Google Scholar] [PubMed]
- Ribić, R.; Stojković, R.; Milković, L.; Antica, M.; Cigler, M.; Tomić, S. Design, Synthesis and Biological Evaluation of Immunostimulating Mannosylated Desmuramyl Peptides. Beilstein J. Org. Chem. 2019, 15, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.H.; Gringhuis, S.I. Signalling through C-Type Lectin Receptors: Shaping Immune Responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Gazi, U.; Martinez-Pomares, L. Influence of the Mannose Receptor in Host Immune Responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Peroković, V.P.; Car, Ž.; Draženović, J.; Stojković, R.; Milković, L.; Antica, M.; Škalamera, Đ.; Tomić, S.; Ribić, R. Design, Synthesis, and Biological Evaluation of Desmuramyl Dipeptides Modified by Adamantyl-1,2,3-Triazole. Molecules 2021, 26, 6352. [Google Scholar] [CrossRef]
- Habjanec, L.; Halassy, B.; Tomašić, J. Comparative Study of Structurally Related Peptidoglycan Monomer and Muramyl Dipeptide on Humoral IgG Immune Response to Ovalbumin in Mouse. Int. Immunopharmacol. 2010, 10, 751–759. [Google Scholar] [CrossRef]
- Maršavelski, A.; Paurević, M.; Ribić, R. Mannosylated Adamantane-Containing Desmuramyl Peptide Recognition by the NOD2 Receptor: A Molecular Dynamics Study. Org. Biomol. Chem. 2021, 19, 7001–7012. [Google Scholar] [CrossRef]
- Cheng, W.-C.; You, T.-Y.; Teo, Z.-Z.; Sayyad, A.A.; Maharana, J.; Guo, C.-W.; Liang, P.-H.; Lin, C.-S.; Meng, F.-C. Further Insights on Structural Modifications of Muramyl Dipeptides to Study the Human NOD2 Stimulating Activity. Chem.–Asian J. 2020, 15, 3836–3844. [Google Scholar] [CrossRef]
- Gobec, M.; Mlinarič-Raščan, I.; Dolenc, M.S.; Jakopin, Ž. Structural Requirements of Acylated Gly-l-Ala-d-Glu Analogs for Activation of the Innate Immune Receptor NOD2. Eur. J. Med. Chem. 2016, 116, 1–12. [Google Scholar] [CrossRef]
- Guzelj, S.; Nabergoj, S.; Gobec, M.; Pajk, S.; Klančič, V.; Slütter, B.; Frkanec, R.; Štimac, A.; Šket, P.; Plavec, J.; et al. Structural Fine-Tuning of Desmuramylpeptide NOD2 Agonists Defines Their In Vivo Adjuvant Activity. J. Med. Chem. 2021, 64, 7809–7838. [Google Scholar] [CrossRef] [PubMed]
- Ribić, R.; Habjanec, L.; Vranešić, B.; Frkanec, R.; Tomić, S. Synthesis and Immunostimulating Properties of Novel Adamant-1-Yl Tripeptides. Chem. Biodivers. 2012, 9, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Mifamurtide: CGP 19835, CGP 19835A, L-MTP-PE, Liposomal MTP-PE, MLV 19835A, MTP-PE, Muramyltripeptide Phosphatidylethanolamine. Drugs RD 2008, 9, 131–135. [CrossRef] [PubMed]
- Štimac, A.; Šegota, S.; Dutour Sikirić, M.; Ribić, R.; Frkanec, L.; Svetličić, V.; Tomić, S.; Vranešić, B.; Frkanec, R. Surface Modified Liposomes by Mannosylated Conjugates Anchored via the Adamantyl Moiety in the Lipid Bilayer. Biochim. Biophys. Acta BBA-Biomembr. 2012, 1818, 2252–2259. [Google Scholar] [CrossRef] [Green Version]
- Car, Ž.; Kodrin, I.; Požar, J.; Ribić, R.; Kovačević, D.; Peroković, V.P. Experimental and Computational Study of the Complexation of Adamantyl Glycosides with β-Cyclodextrin. Tetrahedron 2013, 69, 8051–8063. [Google Scholar] [CrossRef]
- Ribić, R.; Manček-Keber, M.; Chain, F.; Sinnaeve, D.; Martins, J.C.; Jerala, R.; Tomić, S.; Fehér, K. Targeted Delivery of Adamantylated Peptidoglycan Immunomodulators in Lipid Nanocarriers: NMR Shows That Cargo Fragments Are Available on the Surface. J. Phys. Chem. B 2020, 124, 4132–4145. [Google Scholar] [CrossRef]
- Manček-Keber, M.; Ribić, R.; Chain, F.; Sinnaeve, D.; Martins, J.C.; Jerala, R.; Tomić, S.; Fehér, K. Adamantane Containing Peptidoglycan Fragments Enhance RANTES and IL-6 Production in Lipopolysaccharide-Induced Macrophages. Molecules 2020, 25, 3707. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Milošev, M.Z.; Jakovljević, K.; Joksović, M.D.; Stanojković, T.; Matić, I.Z.; Perović, M.; Tešić, V.; Kanazir, S.; Mladenović, M.; Rodić, M.V.; et al. Mannich Bases of 1,2,4-Triazole-3-Thione Containing Adamantane Moiety: Synthesis, Preliminary Anticancer Evaluation, and Molecular Modeling Studies. Chem. Biol. Drug Des. 2017, 89, 943–952. [Google Scholar] [CrossRef]
- Al-Abdullah, E.S.; Asiri, H.H.; Lahsasni, S.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, Antimicrobial, and Anti-Inflammatory Activity, of Novel S-Substituted and N-Substituted 5-(1-Adamantyl)-1,2,4-Triazole-3-Thiols. Drug Des. Devel. Ther. 2014, 8, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, J.G.; Fritz, J.H.; Bourhis, L.L.; Sellge, G.; Travassos, L.H.; Selvanantham, T.; Girardin, S.E.; Gommerman, J.L.; Philpott, D.J. Nod2-Dependent Th2 Polarization of Antigen-Specific Immunity. J. Immunol. 2008, 181, 7925–7935. [Google Scholar] [CrossRef] [Green Version]
- Tretiakova, D.S.; Vodovozova, E.L. Liposomes as Adjuvants and Vaccine Delivery Systems. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2022, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Habjanec, L.; Frkanec, R.; Halassy, B.; Tomasić, J. Effect of Liposomal Formulations and Immunostimulating Peptidoglycan Monomer (PGM) on the Immune Reaction to Ovalbumin in Mice. J. Liposome Res. 2006, 16, 1–16. [Google Scholar] [CrossRef]
- Phillips, N.C.; Moras, M.L.; Chedid, L.; Lefrancier, P.; Bernard, J.M. Activation of Alveolar Macrophage Tumoricidal Activity and Eradication of Experimental Métastasesby Freeze-Dried Liposomes Containing a New Lipophilic Muramyl Dipeptide Derivative. Cancer Res. 1985, 45, 7. [Google Scholar]
- Maharana, J.; Patra, M.C.; De, B.C.; Sahoo, B.R.; Behera, B.K.; De, S.; Pradhan, S.K. Structural Insights into the MDP Binding and CARD–CARD Interaction in Zebrafish (Danio Rerio) NOD2: A Molecular Dynamics Approach. J. Mol. Recognit. 2014, 27, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, C.; Kobayashi, Y.-J.L.; Kobayashi, K.S. Muramyl Dipeptide and Its Derivatives: Peptide Adjuvant in Immunological Disorders and Cancer Therapy. Available online: https://www.eurekaselect.com/74827/article (accessed on 10 February 2021).
- Jakopin, Ž. Nucleotide-Binding Oligomerization Domain (NOD) Inhibitors: A Rational Approach toward Inhibition of NOD Signaling Pathway. J. Med. Chem. 2014, 57, 6897–6918. [Google Scholar] [CrossRef]
- Lauro, M.L.; D’Ambrosio, E.A.; Bahnson, B.J.; Grimes, C.L. Molecular Recognition of Muramyl Dipeptide Occurs in the Leucine-Rich Repeat Domain of Nod2. ACS Infect. Dis. 2017, 3, 264–270. [Google Scholar] [CrossRef]
- Tada, H.; Aiba, S.; Shibata, K.-I.; Ohteki, T.; Takada, H. Synergistic Effect of Nod1 and Nod2 Agonists with Toll-Like Receptor Agonists on Human Dendritic Cells to Generate Interleukin-12 and T Helper Type 1 Cells. Infect. Immun. 2005, 73, 7967–7976. [Google Scholar] [CrossRef] [Green Version]
- Tukhvatulin, A.I.; Dzharullaeva, A.S.; Tukhvatulina, N.M.; Shcheblyakov, D.V.; Shmarov, M.M.; Dolzhikova, I.V.; Stanhope-Baker, P.; Naroditsky, B.S.; Gudkov, A.V.; Logunov, D.Y.; et al. Powerful Complex Immunoadjuvant Based on Synergistic Effect of Combined TLR4 and NOD2 Activation Significantly Enhances Magnitude of Humoral and Cellular Adaptive Immune Responses. PLoS ONE 2016, 11, e0155650. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, A.; Wolfert, M.A.; Boons, G.-J. Synthesis and Proinflammatory Properties of Muramyl Tripeptides Containing Lysine and Diaminopimelic Acid Moieties. ChemBioChem 2005, 6, 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Tom, J.K.; Albin, T.J.; Manna, S.; Moser, B.A.; Steinhardt, R.C.; Esser-Kahn, A.P. Applications of Immunomodulatory Immune Synergies to Adjuvant Discovery and Vaccine Development. Trends Biotechnol. 2019, 37, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Du, J.; Liu, R.; Dong, S.; Cai, J.; Gao, F.; Liu, C. Novel Chimeric TLR2/NOD2 Agonist CL429 Exhibited Significant Radioprotective Effects in Mice. J. Cell. Mol. Med. 2021, 25, 3785–3792. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLano , W. The PyMOL Molecular Graphics System; Version 2.0; Schrödinger, LLC: New York, NY, USA, 2015. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović Peroković, V.; Car, Ž.; Bušljeta, M.; Mihelec, D.; Paurević, M.; Ivanković, S.; Stojković, R.; Ribić, R. Synthesis and Immunological Evaluation of Mannosylated Desmuramyl Dipeptides Modified by Lipophilic Triazole Substituents. Int. J. Mol. Sci. 2022, 23, 8628. https://doi.org/10.3390/ijms23158628
Petrović Peroković V, Car Ž, Bušljeta M, Mihelec D, Paurević M, Ivanković S, Stojković R, Ribić R. Synthesis and Immunological Evaluation of Mannosylated Desmuramyl Dipeptides Modified by Lipophilic Triazole Substituents. International Journal of Molecular Sciences. 2022; 23(15):8628. https://doi.org/10.3390/ijms23158628
Chicago/Turabian StylePetrović Peroković, Vesna, Željka Car, Mia Bušljeta, Danijela Mihelec, Marija Paurević, Siniša Ivanković, Ranko Stojković, and Rosana Ribić. 2022. "Synthesis and Immunological Evaluation of Mannosylated Desmuramyl Dipeptides Modified by Lipophilic Triazole Substituents" International Journal of Molecular Sciences 23, no. 15: 8628. https://doi.org/10.3390/ijms23158628
APA StylePetrović Peroković, V., Car, Ž., Bušljeta, M., Mihelec, D., Paurević, M., Ivanković, S., Stojković, R., & Ribić, R. (2022). Synthesis and Immunological Evaluation of Mannosylated Desmuramyl Dipeptides Modified by Lipophilic Triazole Substituents. International Journal of Molecular Sciences, 23(15), 8628. https://doi.org/10.3390/ijms23158628






