High-Efficiency and High-Quality Extraction of Hemicellulose of Bamboo by Freeze-Thaw Assisted Two-Step Alkali Treatment
Abstract
:1. Introduction
2. Results and Discussion
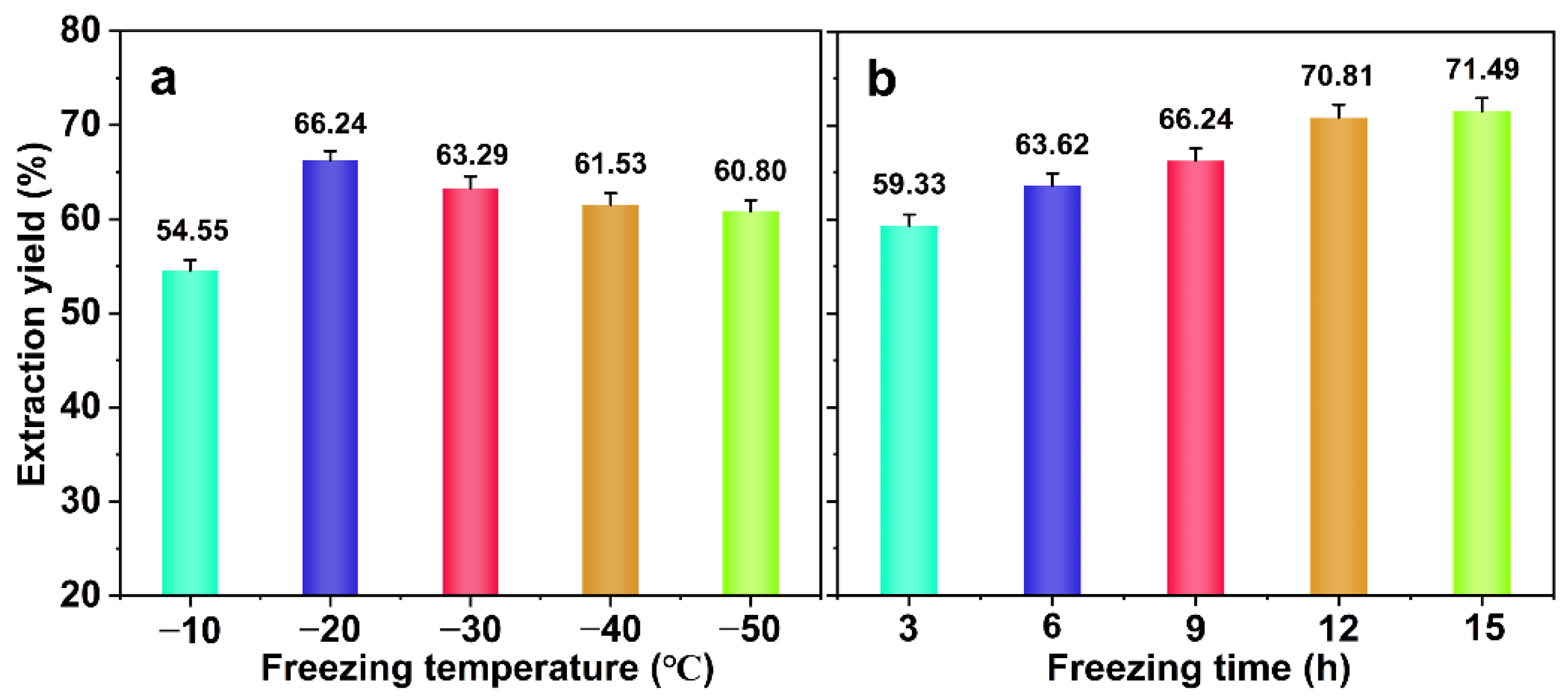
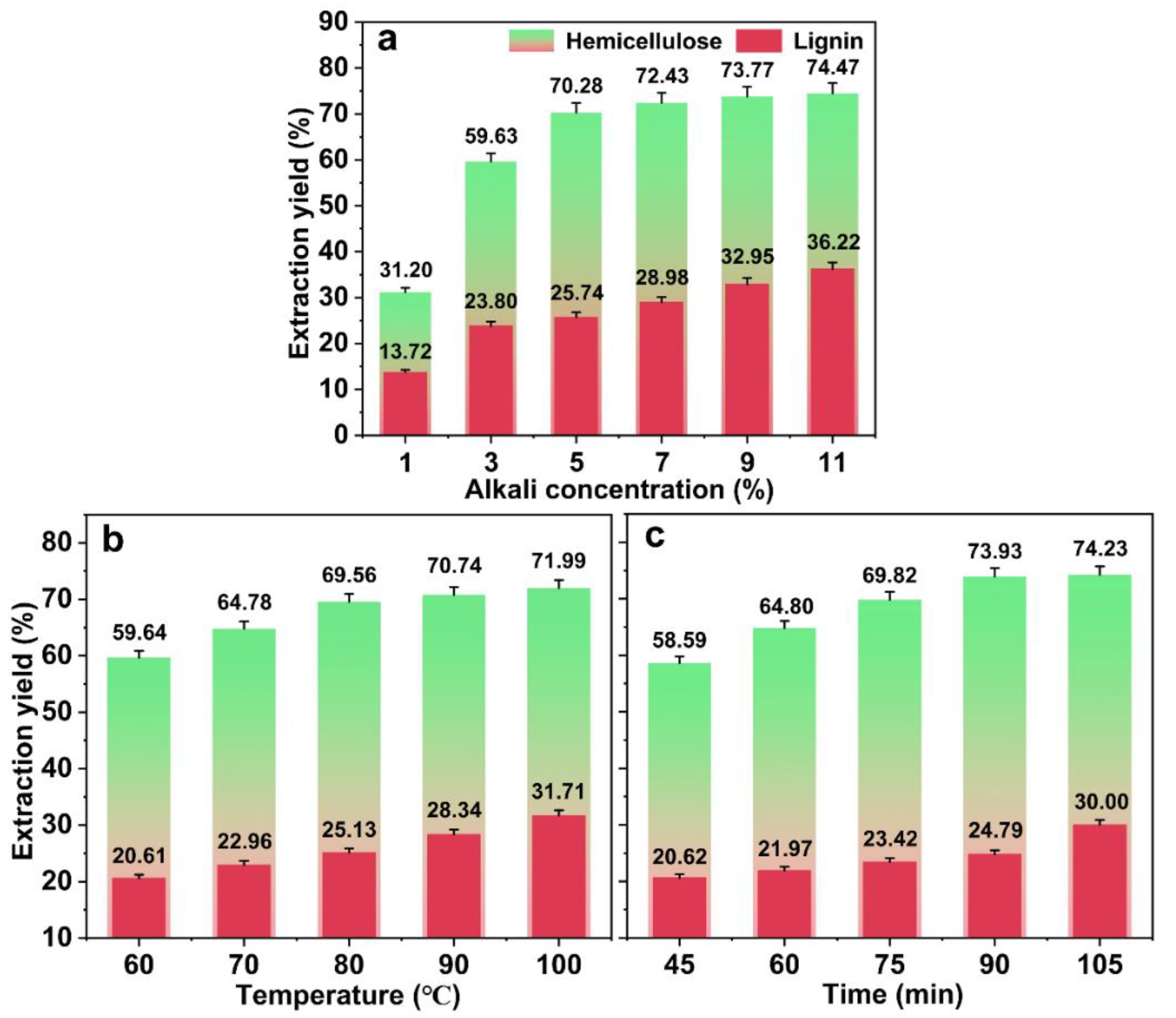
2.1. Effects of Freezing Treatment and Alkaline Treatment on Hemicellulose Extraction
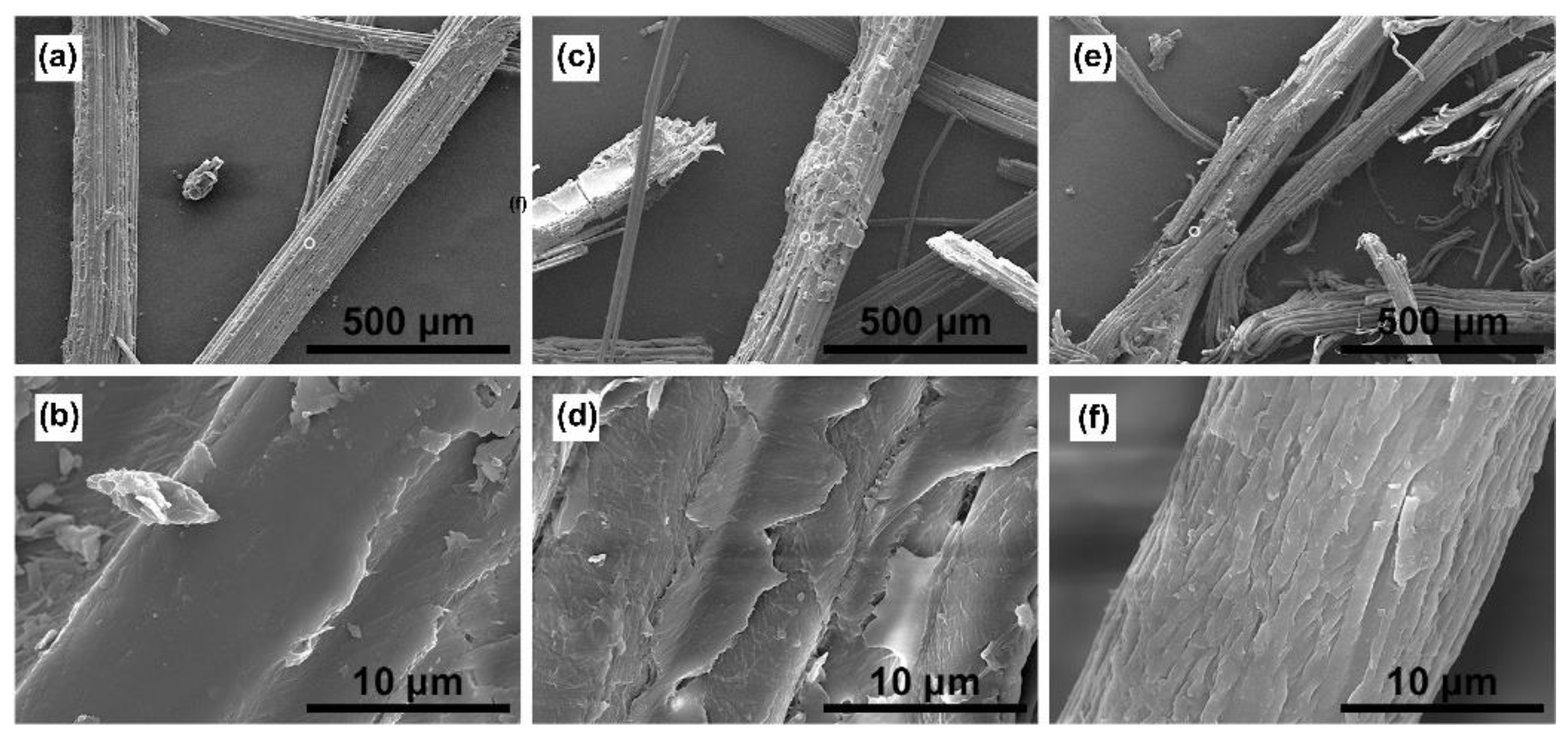
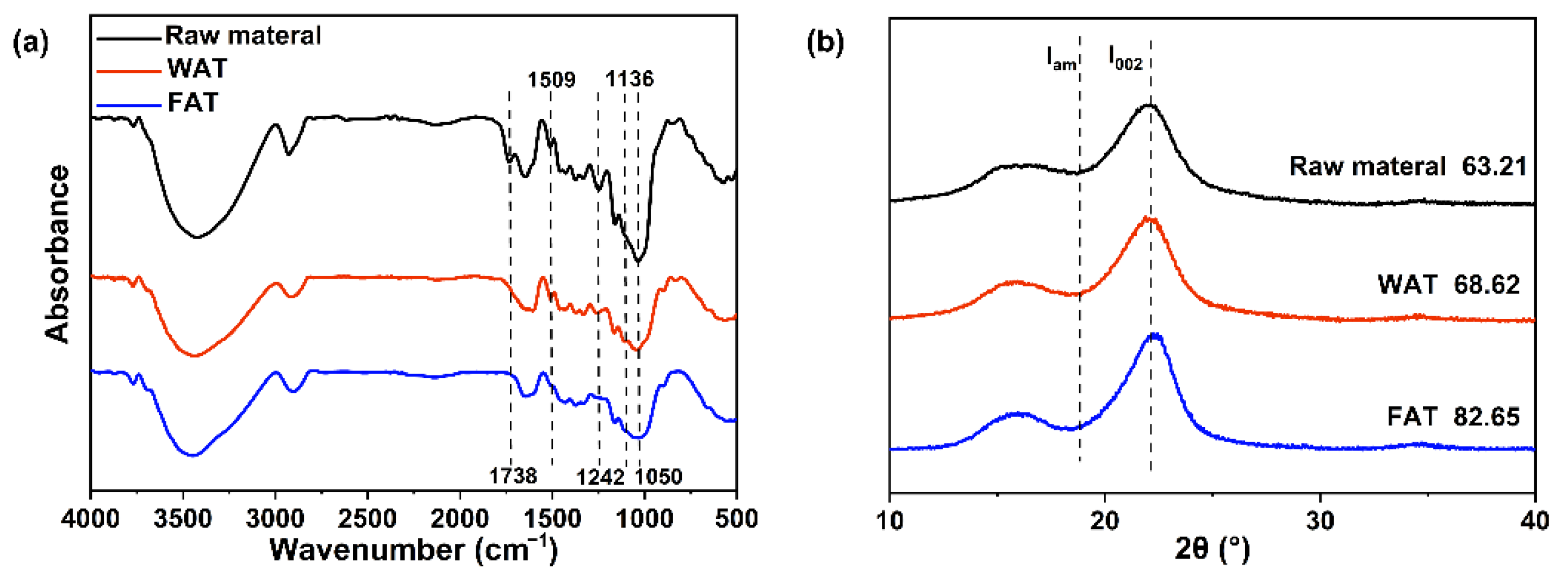
2.2. Characterization of the Residual Solids
2.3. Characterization of the Hemicellulose
3. Materials and Methods
3.1. Materials and Reagents
3.2. Chemical Composition Analysis of the Bamboos
3.3. Weak-Alkali Treatment
3.4. Freeze–Thaw Alkali Treatment of the Residual of Bamboo Powder
3.5. Characterization of the Structures of Solid Residue
3.6. Detection of Lignin Content
3.7. Analysis of Hemicellulose
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Giummarella, N.; Pu, Y.Q.; Ragauskas, A.J.; Lawoko, M. A critical review on the analysis of lignin carbohydrate bonds. Green Chem. 2019, 21, 1573–1595. [Google Scholar] [CrossRef]
- Dev, A.; Srivastava, A.K.; Karmakar, S. Nanomaterial toxicity for plants. Environ. Chem. Lett. 2018, 16, 85–100. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.H.; Zhang, C.H.; Lin, Q.X.; Cheng, B.G.; Kong, F.G.; Li, H.L.; Ren, J.L. Solid acid-induced hydrothermal treatment of bagasse for production of furfural and levulinic acid by a two-step process. Ind. Crop. Prod. 2018, 123, 118–127. [Google Scholar] [CrossRef]
- Yao, S.Q.; Nie, S.X.; Yuan, Y.; Wang, S.F.; Qin, C.R. Efficient extraction of bagasse hemicelluloses and characterization of solid remainder. Bioresour. Technol. 2015, 185, 21–27. [Google Scholar] [CrossRef]
- Methacanon, P.; Chaikumpollert, O.; Thavorniti, P.; Suchiva, K. Hemicellulosic polymer from Vetiver grass and its physicochemical properties. Carbohydr. Polym. 2003, 54, 335–342. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Saake, B.; Kruse, T.; Puls, J. Investigation on molar mass, solubility and enzymatic fragmentation of xylans by multi-detected SEC chromatography. Bioresour. Technol. 2001, 80, 195–204. [Google Scholar] [CrossRef]
- Svard, A.; Brannvall, E.; Edlund, U. Rapeseed straw polymeric hemicelluloses obtained by extraction methods based on severity factor. Ind. Crop. Prod. 2017, 95, 305–315. [Google Scholar] [CrossRef]
- Sun, R.C.; Tomkinson, J.; Ma, P.L.; Liang, S.F. Comparative study of hemicelluloses from rice straw by alkali and hydrogen peroxide treatments. Carbohydr. Polym. 2000, 42, 111–122. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.X.; Liu, C.F.; Sun, R.C. Comparative study of alkali- and acidic organic solvent-soluble hemicellulosic polysaccharides from sugarcane bagasse. Carbohydr. Res. 2006, 341, 253–261. [Google Scholar] [CrossRef]
- Peng, P.; Peng, F.; Bian, J.; Xu, F.; Sun, R.C.; Kennedy, J.F. Isolation and structural characterization of hemicelluloses from the bamboo species Phyllostachys incarnata Wen. Carbohydr. Polym. 2011, 86, 883–890. [Google Scholar] [CrossRef]
- Sun, S.L.; Wen, J.L.; Ma, M.G.; Sun, R.C. Successive alkali extraction and structural characterization of hemicelluloses from sweet sorghum stem. Carbohydr. Polym. 2013, 92, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Jnawali, P.; Kumar, V.; Tanwar, B.; Hirdyani, H.; Gupta, P. Enzymatic Production of Xylooligosaccharides from Brown Coconut Husk Treated with Sodium Hydroxide. Waste Biomass Valorization 2018, 9, 1757–1766. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.M.; Feng, C.Q.; Liu, X.Y.; Qin, F.Y.; Liang, C.; Bian, H.Y.; Qin, C.R.; Yao, S.Q. Green, efficient extraction of bamboo hemicellulose using freeze-thaw assisted alkali treatment. Bioresour. Technol. 2021, 333, 125107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.X.; Ma, Q.L.; Sheng, J.; Yang, R.D. Freeze-thaw repetition as an auxiliary method to promote efficient separation of hemicellulose from poplar. Green Chem. 2020, 22, 942–949. [Google Scholar] [CrossRef]
- Hallet, B. Geology. Why do freezing rocks break? Science 2006, 314, 1092–1093. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Pettersson, T.; Ingverud, T.; Granberg, H.; Larsson, P.A.; Malkoch, M.; Wagberg, L. On the mechanism behind freezing-induced chemical crosslinking in ice-templated cellulose nanofibril aerogels. J. Mater. Chem. A 2018, 6, 19371–19380. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.X.; Wang, L.L.; Wang, Z.J.; Li, J.J.; Wang, J.C. Single Ice Crystal Growth with Controlled Orientation during Directional Freezing. J. Phys. Chem. B 2021, 125, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Hagiwara, S.; Nabetani, H.; Okunishi, T.; Okadome, H. Impact of ice crystal development on electrical impedance characteristics and mechanical property of green asparagus stems. J. Food Eng. 2019, 256, 46–52. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Xu, S.Y.; He, T.; Dong, R.; Ren, T.; Wang, X.L.; Hu, X.Z. Effect of quick-freezing temperature on starch retrogradation and ice crystals properties of steamed oat roll. J. Cereal Sci. 2020, 96, 103109. [Google Scholar] [CrossRef]
- Lappalainen, J.; Baudouin, D.; Hornung, U.; Schuler, J.; Melin, K.; Bjelic, S.; Vogel, F.; Konttinen, J.; Joronen, T. Sub- and Supercritical Water Liquefaction of Kraft Lignin and Black Liquor Derived Lignin. Energies 2020, 13, 3309. [Google Scholar] [CrossRef]
- El Hage, R.; Chrusciel, L.; Desharnais, L.; Brosse, N. Effect of autohydrolysis of Miscanthus x giganteus on lignin structure and organosolv delignification. Bioresour. Technol. 2010, 101, 9321–9329. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Hu, H.C.; Li, H.L.; Huang, L.L.; Chen, L.H.; Ni, Y.H. Kinetics and mechanism of hemicelluloses removal from cellulosic fibers during the cold caustic extraction process. Bioresour. Technol. 2017, 234, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Ray, M.J.; To, T.Q.; Leak, D.J.; Murphy, R.J.; Welton, T. Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid-water mixtures. Green Chem. 2011, 13, 2489–2499. [Google Scholar] [CrossRef]
- Sun, X.F.; Xu, F.; Zhao, H.; Sun, R.C.; Fowler, P.; Baird, M.S. Physicochemical characterisation of residual hemicelluloses isolated with cyanamide-activated hydrogen peroxide from organosolv pre-treated wheat straw. Bioresour. Technol. 2005, 96, 1342–1349. [Google Scholar] [CrossRef]
- Liu, X.; Wei, W.Q.; Wu, S.B. Synergism of organic acid and deep eutectic solvents pretreatment for the co-production of oligosaccharides and enhancing enzymatic saccharification. Bioresour. Technol. 2019, 290, 121775. [Google Scholar] [CrossRef]
- Liu, C.F.; Sun, R.C.; Zhang, A.P.; Ren, J.L. Preparation of sugarcane bagasse cellulosic phthalate using an ionic liquid as reaction medium. Carbohydr. Polym. 2007, 68, 17–25. [Google Scholar] [CrossRef]
- Luo, Y.D.; Li, Y.; Cao, L.M.; Zhu, J.T.; Deng, B.J.; Hou, Y.J.; Liang, C.; Huang, C.X.; Qin, C.R.; Yao, S.Q. High efficiency and clean separation of eucalyptus components by glycolic acid pretreatment. Bioresour. Technol. 2021, 341, 125757. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Peng, F.; Bian, J.; Xu, F.; Sun, R.C. Studies on the Starch and Hemicelluloses Fractionated by Graded Ethanol Precipitation from Bamboo Phyllostachys bambusoides f. shouzhu Yi. J. Agric. Food Chem. 2011, 59, 2680–2688. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.Y.; Wu, Y.T.; Han, Y.S.; Qin, C.R.; Nie, S.X.; Liu, S.J.; Wang, S.F.; Yao, S.Q. Effect of hydrothermal pretreatment on the demineralization and thermal degradation behavior of eucalyptus. Bioresour. Technol. 2020, 307, 123246. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.C.; Hu, Z.H.; Wu, S.F.; Song, T.; Yue, F.X.; Xiang, Z.Y. Promoting the material properties of xylan-type hemicelluloses from the extraction step. Carbohydr. Polym. 2019, 215, 235–245. [Google Scholar] [CrossRef]
| Raw Material | Extraction Method | Extraction Yield (%) |
|---|---|---|
| Poplar [15] | Steam explosion, alkali and alkali/ethanol were treated at 75 °C for 3.0 h with 0.3%, 0.6%, 1.0%,1.5% and 2.5% KOH. | 76.40 |
| Coconut shell [16] | Combined treatment with 20% NaOH and steam for 1.0 h. | 93.0 |
| Bamboo [14] | Distilled water, alkali and organic solvents were treated with 1.0%, 3.0%, 5.0% and 8.0% NaOH for 3.0 h at 60 °C. | 80.10 |
| Bamboo [17] | −30 °C for 12.0 h, 7.0% NaOH treatment for 1.5 h at 75 °C. | 64.71 |
| Bamboo | Weak alkali treatment, −20 °C for 12.0 h, 5.0% NaOH treatment for 1.5 h at 80 °C. | 90.54 |
| Samples | Extraction (%) | Purity (%) | Xylose (%) | Glucose (%) | Arabinose (%) | Galactose (%) |
|---|---|---|---|---|---|---|
| WAT | 16.61 ± 0.66 | 77.94 ± 3.12 | 47.01 ± 1.88 | 37.52 ± 1.50 | 11.48 ± 0.46 | 3.99 ± 0.16 |
| FAT | 73.93 ± 2.96 | 89.60 ± 3.58 | 60.18 ± 2.41 | 33.97 ± 1.36 | 4.39 ± 0.17 | 1.46 ± 0.06 |
| Samples | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Raw material | 49.50 ± 2.47 | 17.19 ± 0.86 | 25.25 ± 1.26 |
| WAT | 47.75 ± 2.12 ** | 14.33 ± 0.72 ** | 24.00 ± 1.15 ** |
| FAT | 43.41 ± 2.03 ** | 4.47 ± 0.16 ** | 18.99 ± 0.89 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; He, J.; Pang, S.; Yao, S.; Zhu, C.; Zhao, J.; Liu, Y.; Liang, C.; Qin, C. High-Efficiency and High-Quality Extraction of Hemicellulose of Bamboo by Freeze-Thaw Assisted Two-Step Alkali Treatment. Int. J. Mol. Sci. 2022, 23, 8612. https://doi.org/10.3390/ijms23158612
Wang X, He J, Pang S, Yao S, Zhu C, Zhao J, Liu Y, Liang C, Qin C. High-Efficiency and High-Quality Extraction of Hemicellulose of Bamboo by Freeze-Thaw Assisted Two-Step Alkali Treatment. International Journal of Molecular Sciences. 2022; 23(15):8612. https://doi.org/10.3390/ijms23158612
Chicago/Turabian StyleWang, Xin, Jiahao He, Shuyu Pang, Shuangquan Yao, Chunxia Zhu, Jinwei Zhao, Yang Liu, Chen Liang, and Chengrong Qin. 2022. "High-Efficiency and High-Quality Extraction of Hemicellulose of Bamboo by Freeze-Thaw Assisted Two-Step Alkali Treatment" International Journal of Molecular Sciences 23, no. 15: 8612. https://doi.org/10.3390/ijms23158612
APA StyleWang, X., He, J., Pang, S., Yao, S., Zhu, C., Zhao, J., Liu, Y., Liang, C., & Qin, C. (2022). High-Efficiency and High-Quality Extraction of Hemicellulose of Bamboo by Freeze-Thaw Assisted Two-Step Alkali Treatment. International Journal of Molecular Sciences, 23(15), 8612. https://doi.org/10.3390/ijms23158612








