Reg-1α, a New Substrate of Calpain-2 Depending on Its Glycosylation Status
Abstract
1. Introduction
2. Results
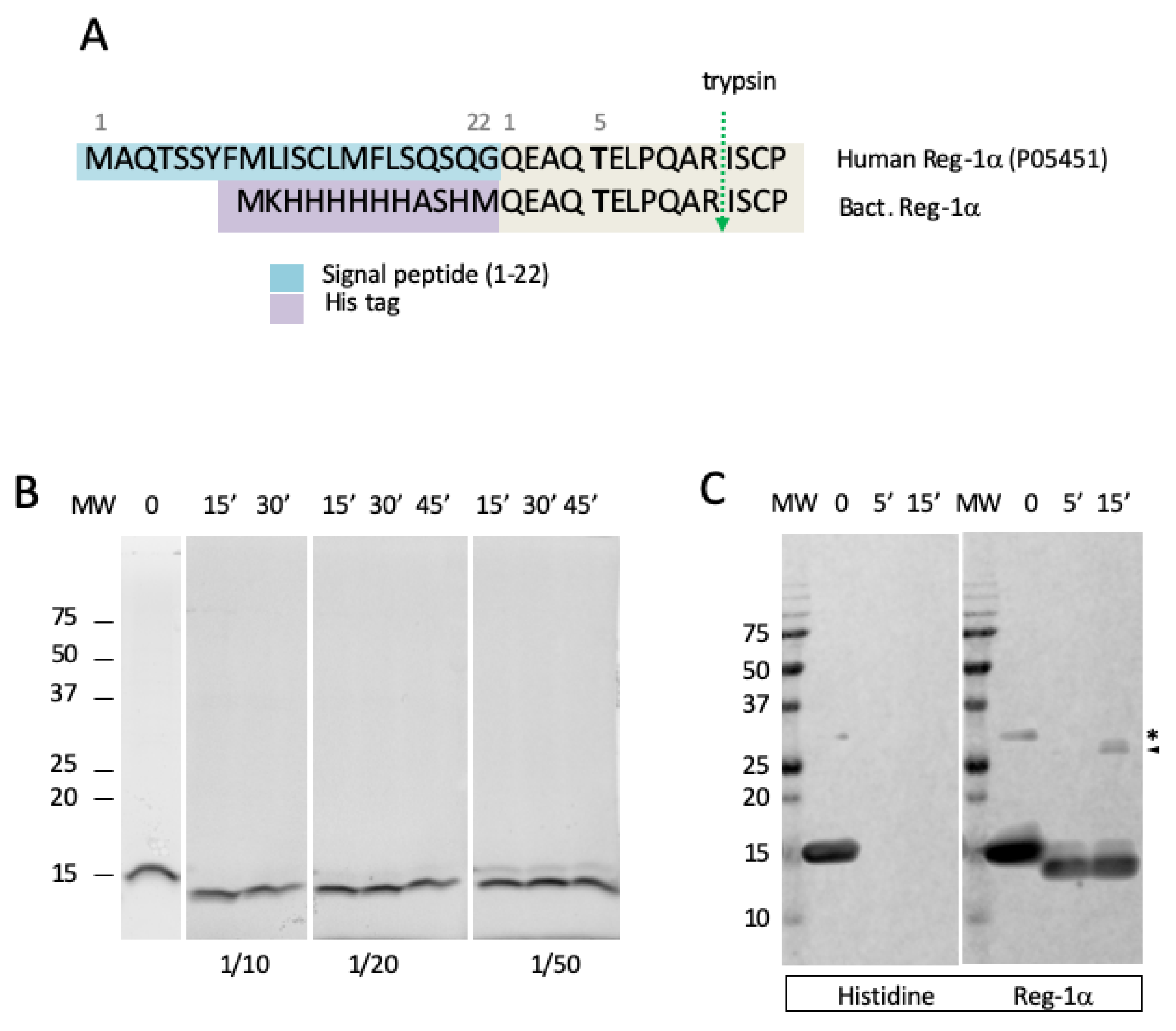
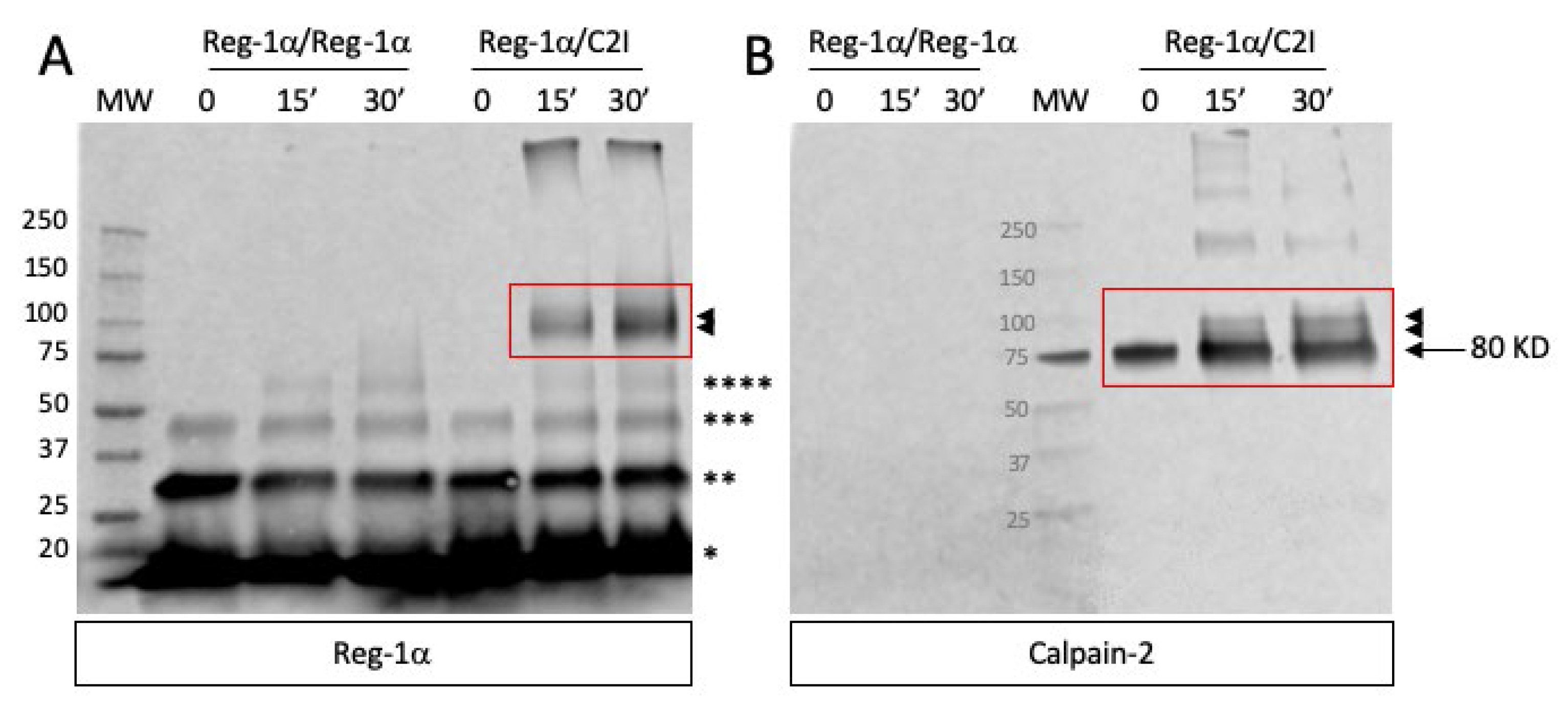
2.1. Reg-1α Is a Calpain Substrate In Vitro
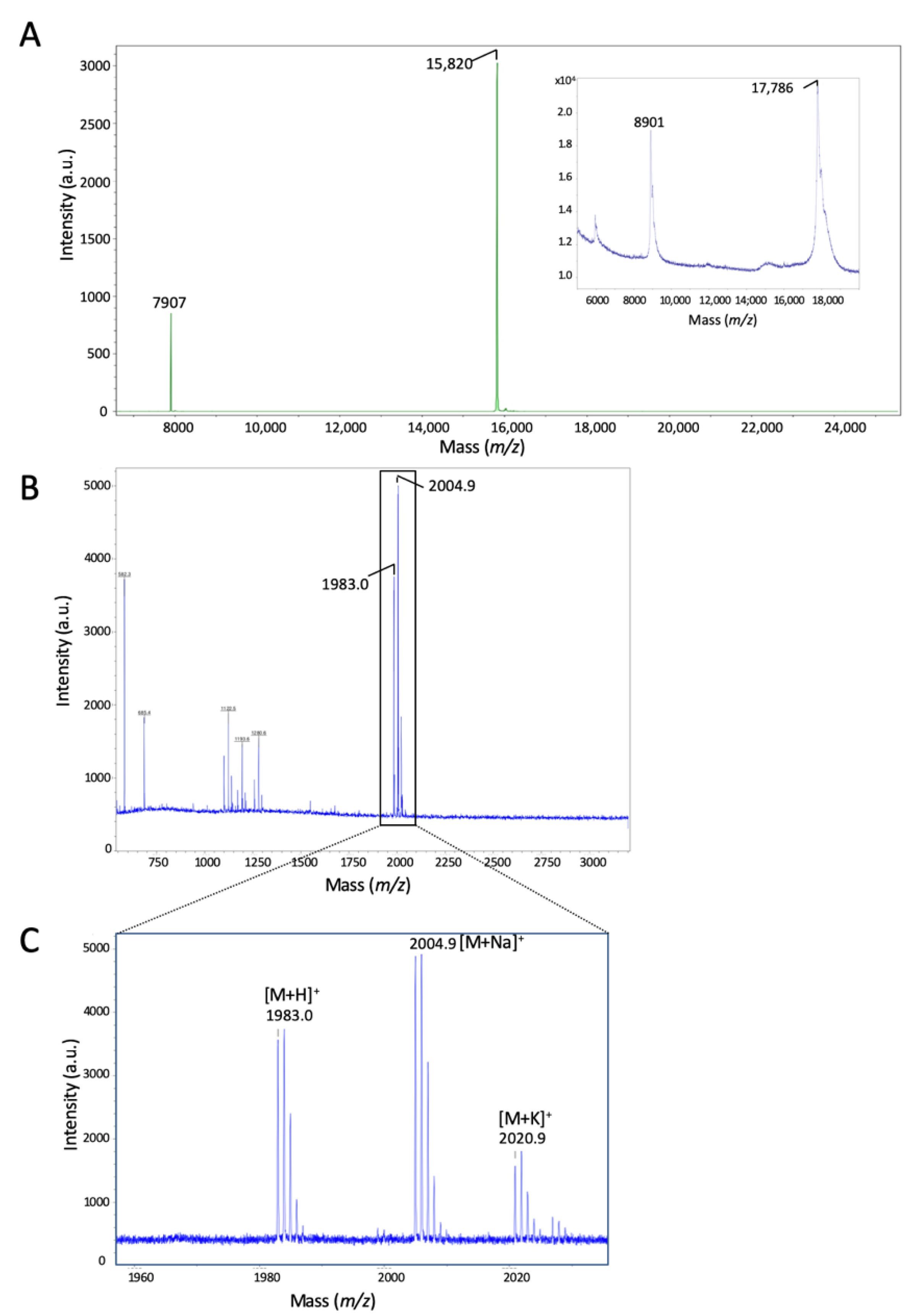
2.2. Identification of the Calpain Cleavage Site on Reg-1α
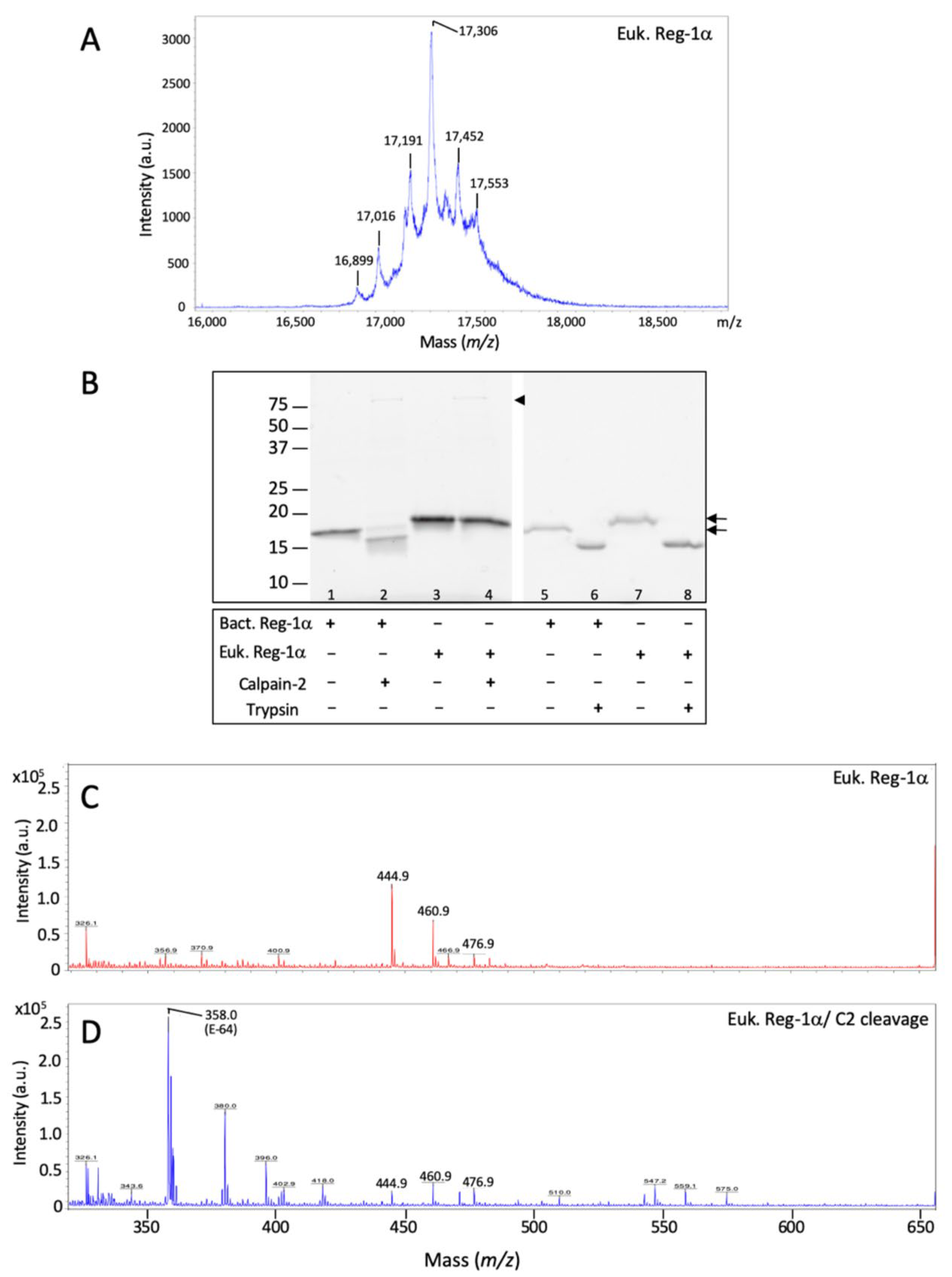
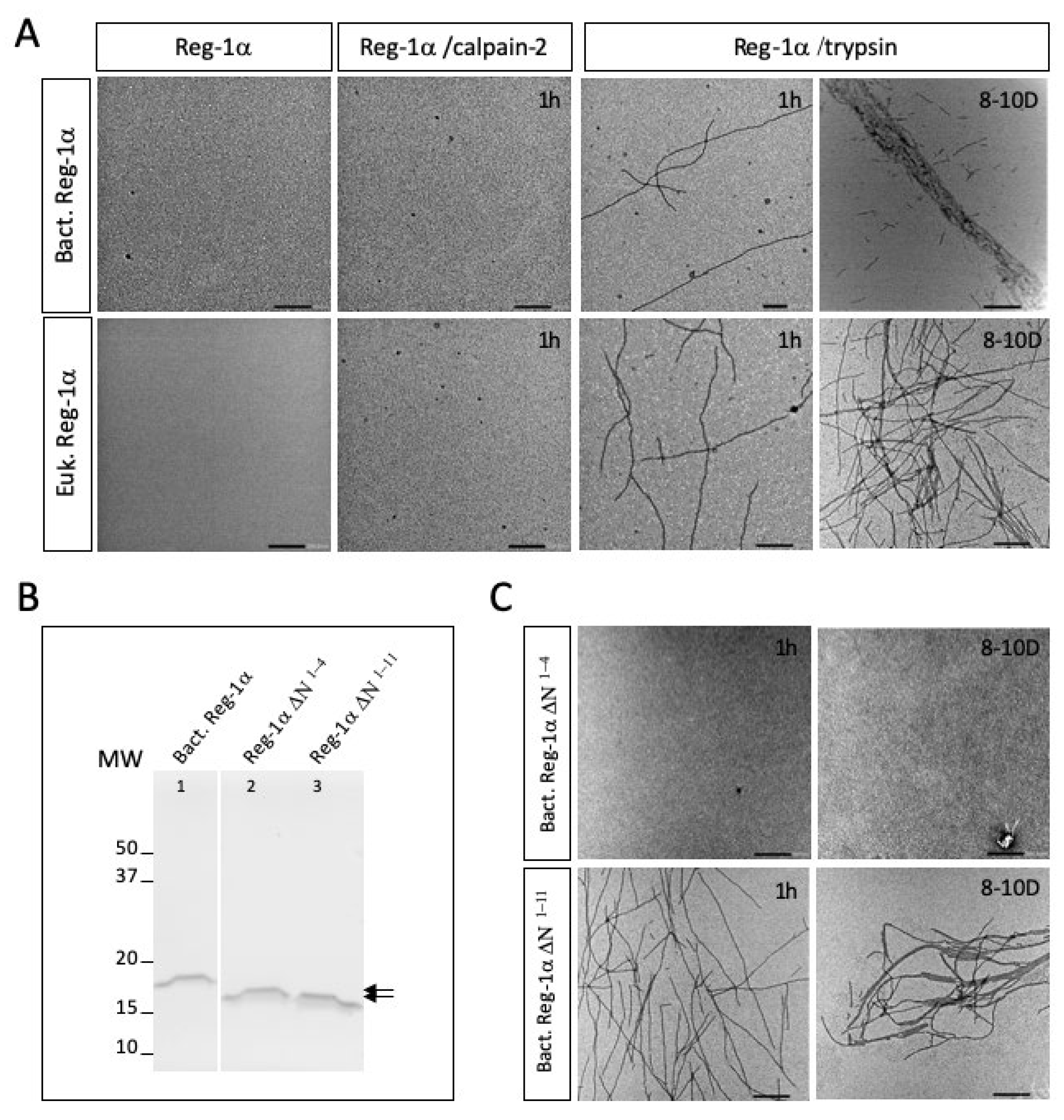
2.3. Calpain-2 Is Not Able to Cleave the Glycosylated Form of Reg-1α
2.4. Calpain2-Cleaved Reg-1α Does Not Form Fibrils
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Calpain-2 Purification (80/21kDa)
4.3. Gel Electrophoresis and Immunoblotting
4.4. Mass Spectrometry
4.5. Cross-Linking Experiments
4.6. Protein Cleavage
4.7. Preparation of the Reg-1αΔN Molecules
4.8. Transmission Electron Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Iovanna, J.L.; Dagorn, J.-C. The multifunctional family of secreted proteins containing a C-type lectin-like domain linked to a short N-terminal peptide. Biochim. Biophys. Acta 2005, 1723, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Downing, S.; Tzanakakis, E.S. Four Decades After the Discovery of Regenerating Islet-Derived (Reg) Proteins: Current Understanding and Challenges. Front. Cell Dev. Biol. 2019, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Multigner, L.; De Caro, A.; Lombardo, D.; Campese, D.; Sarles, H. Pancreatic stone protein, a phosphoprotein which inhibits calcium carbonate precipitation from human pancreatic juice. Biochem. Biophys. Res. Commun. 1983, 110, 69–74. [Google Scholar] [CrossRef]
- Geider, S.; Baronnet, A.; Cerini, C.; Nitsche, S.; Astier, J.P.; Michel, R.; Boistelle, R.; Berland, Y.; Dagorn, J.C.; Verdier, J.M. Pancreatic lithostathine as a calcite habit modifier. J. Biol. Chem. 1996, 271, 26302–26306. [Google Scholar] [CrossRef]
- Bernard, J.P.; Adrich, Z.; Montalto, G.; De Caro, A.; De Reggi, M.; Sarles, H.; Dagorn, J.C. Inhibition of nucleation and crystal growth of calcium carbonate by human lithostathine. Gastroenterology 1992, 103, 1277–1284. [Google Scholar] [CrossRef]
- Varilh, M.; Acquatella-Tran Van Ba, I.; Silhol, M.; Nieto-Lopez, F.; Moussaed, M.; Lebart, M.-C.; Bovolenta, P.; Verdier, J.-M.; Rossel, M.; Marcilhac, A.; et al. Reg-1α Promotes Differentiation of Cortical Progenitors via Its N-Terminal Active Domain. Front. Cell Dev. Biol. 2020, 8, 681. [Google Scholar] [CrossRef]
- De Caro, A.M.; Adrich, Z.; Fournet, B.; Capon, C.; Bonicel, J.J.; De Caro, J.D.; Rovery, M. N-terminal sequence extension in the glycosylated forms of human pancreatic stone protein. The 5-oxoproline N-terminal chain is O-glycosylated on the 5th amino acid residue. Biochim. Biophys. Acta 1989, 994, 281–284. [Google Scholar] [CrossRef]
- De Reggi, M.; Capon, C.; Gharib, B.; Wieruszeski, J.M.; Michel, R.; Fournet, B. The glycan moiety of human pancreatic lithostathine. Structure characterization and possible pathophysiological implications. Eur. J. Biochem. 1995, 230, 503–510. [Google Scholar] [CrossRef]
- Ozturk, M.; de la Monte, S.M.; Gross, J.; Wands, J.R. Elevated levels of an exocrine pancreatic secretory protein in Alzheimer disease brain. Proc. Natl. Acad. Sci. USA 1989, 86, 419–423. [Google Scholar] [CrossRef]
- Duplan, L.; Michel, B.; Boucraut, J.; Barthellémy, S.; Desplat-Jego, S.; Marin, V.; Gambarelli, D.; Bernard, D.; Berthézène, P.; Alescio-Lautier, B.; et al. Lithostathine and pancreatitis-associated protein are involved in the very early stages of Alzheimer’s disease. Neurobiol. Aging 2001, 22, 79–88. [Google Scholar] [CrossRef]
- Moussaed, M.; Huc-Brandt, S.; Cubedo, N.; Silhol, M.; Murat, S.; Lebart, M.-C.; Kovacs, G.; Verdier, J.-M.; Trousse, F.; Rossel, M.; et al. Regenerating islet-derived 1α (REG-1α) protein increases tau phosphorylation in cell and animal models of tauopathies. Neurobiol. Dis. 2018, 119, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Cerini, C.; Peyrot, V.; Garnier, C.; Duplan, L.; Veesler, S.; Le Caer, J.P.; Bernard, J.P.; Bouteille, H.; Michel, R.; Vazi, A.; et al. Biophysical characterization of lithostathine. Evidences for a polymeric structure at physiological pH and a proteolysis mechanism leading to the formation of fibrils. J. Biol. Chem. 1999, 274, 22266–22274. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, C.; Marco, S.; Thimonier, J.; Duplan, L.; Laurine, E.; Chauvin, J.P.; Michel, B.; Peyrot, V.; Verdier, J.M. Three-dimensional structure of the lithostathine protofibril, a protein involved in Alzheimer’s disease. EMBO J. 2001, 20, 3313–3321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laurine, E.; Grégoire, C.; Fändrich, M.; Engemann, S.; Marchal, S.; Thion, L.; Mohr, M.; Monsarrat, B.; Michel, B.; Dobson, C.M.; et al. Lithostathine quadruple-helical filaments form proteinase K-resistant deposits in Creutzfeldt-Jakob disease. J. Biol. Chem. 2003, 278, 51770–51778. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Saido, T.C.; Sorimachi, H. Calpain research for drug discovery: Challenges and potential. Nat. Rev. Drug Discov. 2016, 15, 854–876. [Google Scholar] [CrossRef]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef]
- Campbell, R.L.; Davies, P.L. Structure-function relationships in calpains. Biochem. J. 2012, 447, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Baudry, M.; Bi, X. Calpain-1 and Calpain-2: The Yin and Yang of Synaptic Plasticity and Neurodegeneration. Trends Neurosci. 2016, 39, 235–245. [Google Scholar] [CrossRef]
- Baudry, M. Calpain-1 and Calpain-2 in the Brain: Dr. Jekill and Mr Hyde? Curr. Neuropharmacol. 2019, 17, 823–829. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Bi, X.; Baudry, M. Calpain-1 and Calpain-2 in the Brain: New Evidence for a Critical Role of Calpain-2 in Neuronal Death. Cells 2020, 9, E2698. [Google Scholar] [CrossRef]
- Hosfield, C.M.; Elce, J.S.; Davies, P.L.; Jia, Z. Crystal structure of calpain reveals the structural basis for Ca(2+)-dependent protease activity and a novel mode of enzyme activation. EMBO J. 1999, 18, 6880–6889. [Google Scholar] [CrossRef]
- Strobl, S.; Fernandez-Catalan, C.; Braun, M.; Huber, R.; Masumoto, H.; Nakagawa, K.; Irie, A.; Sorimachi, H.; Bourenkow, G.; Bartunik, H.; et al. The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc. Natl. Acad. Sci. USA 2000, 97, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.A.; Campbell, R.L.; Davies, P.L. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature 2008, 456, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, T.; Gehring, K.; Green, D.R. Concerted multi-pronged attack by calpastatin to occlude the catalytic cleft of heterodimeric calpains. Nature 2008, 456, 404–408. [Google Scholar] [CrossRef]
- Saido, T.C.; Yokota, M.; Nagao, S.; Yamaura, I.; Tani, E.; Tsuchiya, T.; Suzuki, K.; Kawashima, S. Spatial resolution of fodrin proteolysis in postischemic brain. J. Biol. Chem. 1993, 268, 25239–25243. [Google Scholar] [PubMed]
- Franco, S.J.; Huttenlocher, A. Regulating cell migration: Calpains make the cut. J. Cell Sci. 2005, 118, 3829–3838. [Google Scholar] [CrossRef]
- Lebart, M.-C.; Benyamin, Y. Calpain involvement in the remodeling of cytoskeletal anchorage complexes. FEBS J. 2006, 273, 3415–3426. [Google Scholar] [CrossRef]
- Magnaghi-Jaulin, L.; Marcilhac, A.; Rossel, M.; Jaulin, C.; Benyamin, Y.; Raynaud, F. Calpain 2 is required for sister chromatid cohesion. Chromosoma 2010, 119, 267–274. [Google Scholar] [CrossRef]
- Raynaud, F.; Marcilhac, A. Implication of calpain in neuronal apoptosis. FEBS J. 2006, 273, 3437–3443. [Google Scholar] [CrossRef]
- Sorimachi, H.; Ono, Y. Regulation and physiological roles of the calpain system in muscular disorders. Cardiovasc. Res. 2012, 96, 11–22. [Google Scholar] [CrossRef]
- Mahaman, Y.A.R.; Huang, F.; Kessete Afewerky, H.; Maibouge, T.M.S.; Ghose, B.; Wang, X. Involvement of calpain in the neuropathogenesis of Alzheimer’s disease. Med. Res. Rev. 2019, 39, 608–630. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, J.; Gao, X.; Ma, Q.; Ren, J.; Xue, Y. GPS-CCD: A novel computational program for the prediction of calpain cleavage sites. PLoS ONE 2011, 6, e19001. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-X.; Yu, K.; Dong, J.; Zhao, L.; Liu, Z.; Zhang, Q.; Li, S.; Du, Y.; Cheng, H. Precise Prediction of Calpain Cleavage Sites and Their Aberrance Caused by Mutations in Cancer. Front. Genet. 2019, 10, 715. [Google Scholar] [CrossRef]
- Purwaha, P.; Silva, L.P.; Hawke, D.H.; Weinstein, J.N.; Lorenzi, P.L. An artifact in LC-MS/MS measurement of glutamine and glutamic acid: In-source cyclization to pyroglutamic acid. Anal. Chem. 2014, 86, 5633–5637. [Google Scholar] [CrossRef]
- Milhiet, P.-E.; Yamamoto, D.; Berthoumieu, O.; Dosset, P.; Le Grimellec, C.; Verdier, J.-M.; Marchal, S.; Ando, T. Deciphering the Structure, Growth and Assembly of Amyloid-Like Fibrils Using High-Speed Atomic Force Microscopy. PLoS ONE 2010, 5, e13240. [Google Scholar] [CrossRef]
- De Reggi, M.; Gharib, B. Protein-X, Pancreatic Stone-, Pancreatic thread-, reg-protein, P19, lithostathine, and now what? Characterization, structural analysis and putative function(s) of the major non-enzymatic protein of pancreatic secretions. Curr. Protein Pept. Sci. 2001, 2, 19–42. [Google Scholar] [CrossRef]
- Shinkai-Ouchi, F.; Koyama, S.; Ono, Y.; Hata, S.; Ojima, K.; Shindo, M.; duVerle, D.; Ueno, M.; Kitamura, F.; Doi, N.; et al. Predictions of Cleavability of Calpain Proteolysis by Quantitative Structure-Activity Relationship Analysis Using Newly Determined Cleavage Sites and Catalytic Efficiencies of an Oligopeptide Array. Mol. Cell. Proteomics. 2016, 15, 1262–1280. [Google Scholar] [CrossRef]
- Tompa, P.; Buzder-Lantos, P.; Tantos, A.; Farkas, A.; Szilágyi, A.; Bánóczi, Z.; Hudecz, F.; Friedrich, P. On the sequential determinants of calpain cleavage. J. Biol. Chem. 2004, 279, 20775–20785. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Pignol, D.; Bernard, J.P.; Verdier, J.M.; Dagorn, J.C.; Fontecilla-Camps, J.C. Crystal structure of human lithostathine, the pancreatic inhibitor of stone formation. EMBO J. 1996, 15, 2678–2684. [Google Scholar] [CrossRef]
- duVerle, D.; Takigawa, I.; Ono, Y.; Sorimachi, H.; Mamitsuka, H. CaMPDB: A Resource for Calpain and Modulatory Proteolysis. In Genome Informatics 2009; Imperial College Press: London, UK, 2010; pp. 202–213. [Google Scholar]
- Jentoft, N. Why are proteins O-glycosylated? Trends Biochem. Sci. 1990, 15, 291–294. [Google Scholar] [CrossRef]
- King, S.L.; Goth, C.K.; Eckhard, U.; Joshi, H.J.; Haue, A.D.; Vakhrushev, S.Y.; Schjoldager, K.T.; Overall, C.M.; Wandall, H.H. TAILS N-terminomics and proteomics reveal complex regulation of proteolytic cleavage by O-glycosylation. J. Biol. Chem. 2018, 293, 7629–7644. [Google Scholar] [CrossRef] [PubMed]
- Gram Schjoldager, K.T.-B.; Vester-Christensen, M.B.; Goth, C.K.; Petersen, T.N.; Brunak, S.; Bennett, E.P.; Levery, S.B.; Clausen, H. A Systematic Study of Site-specific GalNAc-type O-Glycosylation Modulating Proprotein Convertase Processing. J. Biol. Chem. 2011, 286, 40122–40132. [Google Scholar] [CrossRef] [PubMed]
- Goth, C.K.; Halim, A.; Khetarpal, S.A.; Rader, D.J.; Clausen, H.; Schjoldager, K.T.-B.G. A systematic study of modulation of ADAM-mediated ectodomain shedding by site-specific O-glycosylation. Proc. Natl. Acad. Sci. USA 2015, 112, 14623–14628. [Google Scholar] [CrossRef]
- Park, M.; Reddy, G.R.; Wallukat, G.; Xiang, Y.K.; Steinberg, S.F. β1-adrenergic receptor O-glycosylation regulates N-terminal cleavage and signaling responses in cardiomyocytes. Sci. Rep. 2017, 7, 7890. [Google Scholar] [CrossRef]
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1999, 1473, 4–8. [Google Scholar] [CrossRef]
- Thanka Christlet, T.H.; Veluraja, K. Database analysis of O-glycosylation sites in proteins. Biophys. J. 2001, 80, 952–960. [Google Scholar] [CrossRef]
- Acquatella-Tran Van Ba, I.; Marchal, S.; François, F.; Silhol, M.; Lleres, C.; Michel, B.; Benyamin, Y.; Verdier, J.-M.; Trousse, F.; Marcilhac, A. Regenerating islet-derived 1α (Reg-1α) protein is new neuronal secreted factor that stimulates neurite outgrowth via exostosin Tumor-like 3 (EXTL3) receptor. J. Biol. Chem. 2012, 287, 4726–4739. [Google Scholar] [CrossRef]
- Hood, J.L.; Brooks, W.H.; Roszman, T.L. Differential compartmentalization of the calpain/calpastatin network with the endoplasmic reticulum and Golgi apparatus. J. Biol. Chem. 2004, 279, 43126–43135. [Google Scholar] [CrossRef]
- Graham-Siegenthaler, K.; Gauthier, S.; Davies, P.L.; Elce, J.S. Active recombinant rat calpain II. Bacterially produced large and small subunits associate both in vivo and in vitro. J. Biol. Chem. 1994, 269, 30457–30460. [Google Scholar] [CrossRef]
- McCartney, C.-S.E.; Davies, P.L. Bacterial Expression and Purification of Calpains. Methods Mol. Biol. 2019, 1915, 13–27. [Google Scholar]

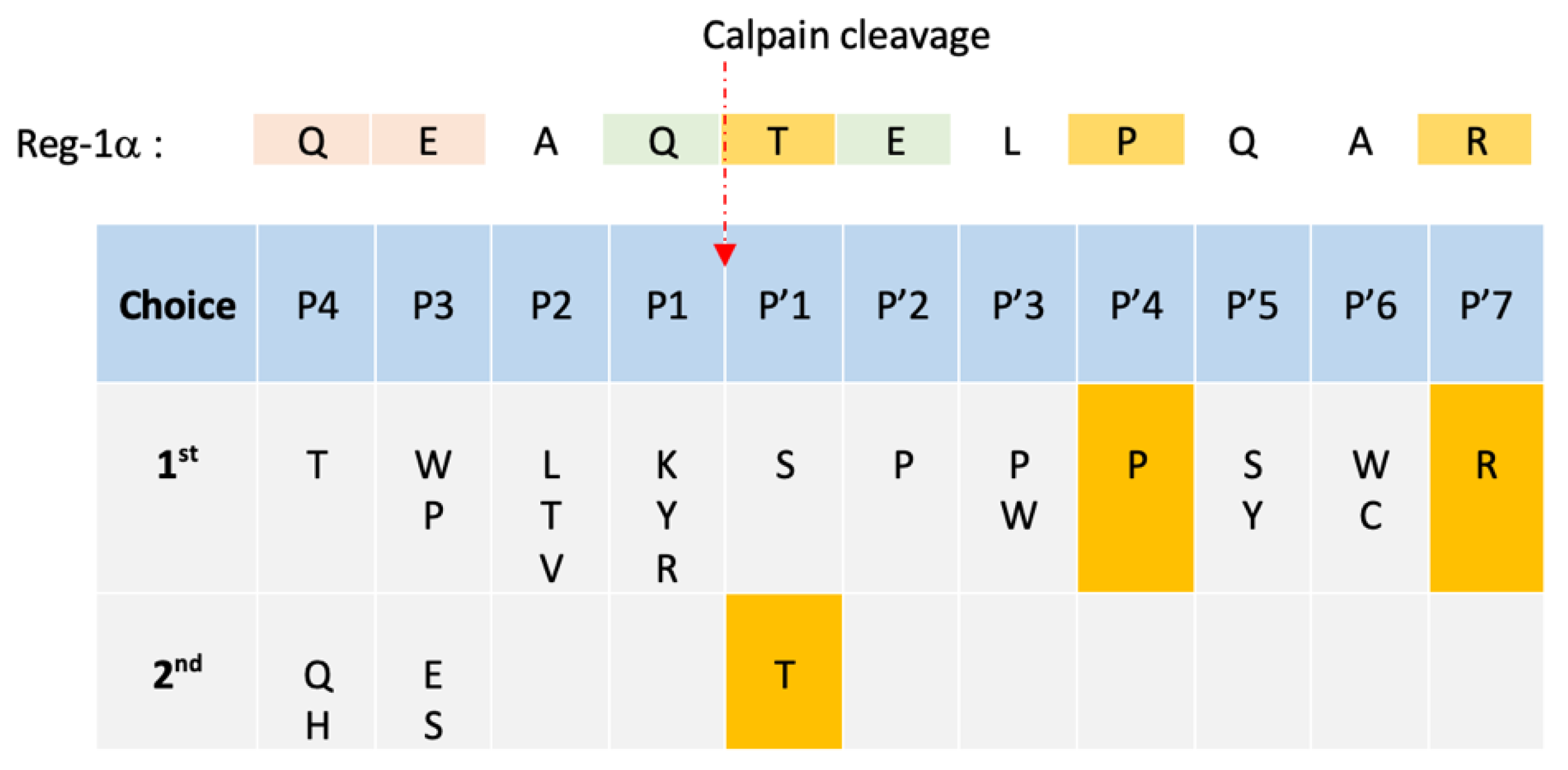 | |
| Amino acid corresponding to the consensus Calpastatin inhibitory segment in positions P’1–P’7 | |
| 2nd choice of amino acid preference | |
| Xth choice of amino acid preference according to [33,37,38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebart, M.-C.; Trousse, F.; Valette, G.; Torrent, J.; Denus, M.; Mestre-Frances, N.; Marcilhac, A. Reg-1α, a New Substrate of Calpain-2 Depending on Its Glycosylation Status. Int. J. Mol. Sci. 2022, 23, 8591. https://doi.org/10.3390/ijms23158591
Lebart M-C, Trousse F, Valette G, Torrent J, Denus M, Mestre-Frances N, Marcilhac A. Reg-1α, a New Substrate of Calpain-2 Depending on Its Glycosylation Status. International Journal of Molecular Sciences. 2022; 23(15):8591. https://doi.org/10.3390/ijms23158591
Chicago/Turabian StyleLebart, Marie-Christine, Françoise Trousse, Gilles Valette, Joan Torrent, Morgane Denus, Nadine Mestre-Frances, and Anne Marcilhac. 2022. "Reg-1α, a New Substrate of Calpain-2 Depending on Its Glycosylation Status" International Journal of Molecular Sciences 23, no. 15: 8591. https://doi.org/10.3390/ijms23158591
APA StyleLebart, M.-C., Trousse, F., Valette, G., Torrent, J., Denus, M., Mestre-Frances, N., & Marcilhac, A. (2022). Reg-1α, a New Substrate of Calpain-2 Depending on Its Glycosylation Status. International Journal of Molecular Sciences, 23(15), 8591. https://doi.org/10.3390/ijms23158591






