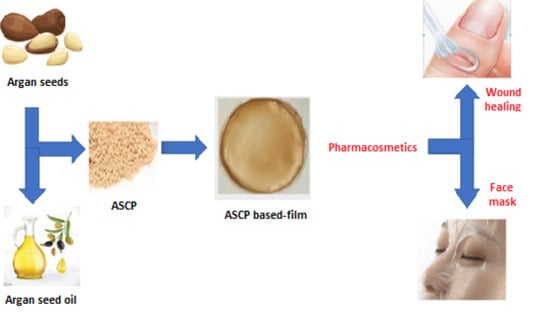
Argan (Argania spinosa L.) Seed Oil Cake as a Potential Source of Protein-Based Film Matrix for Pharmaco-Cosmetic Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Argan Seed Protein Concentrate Preparation and Characterization
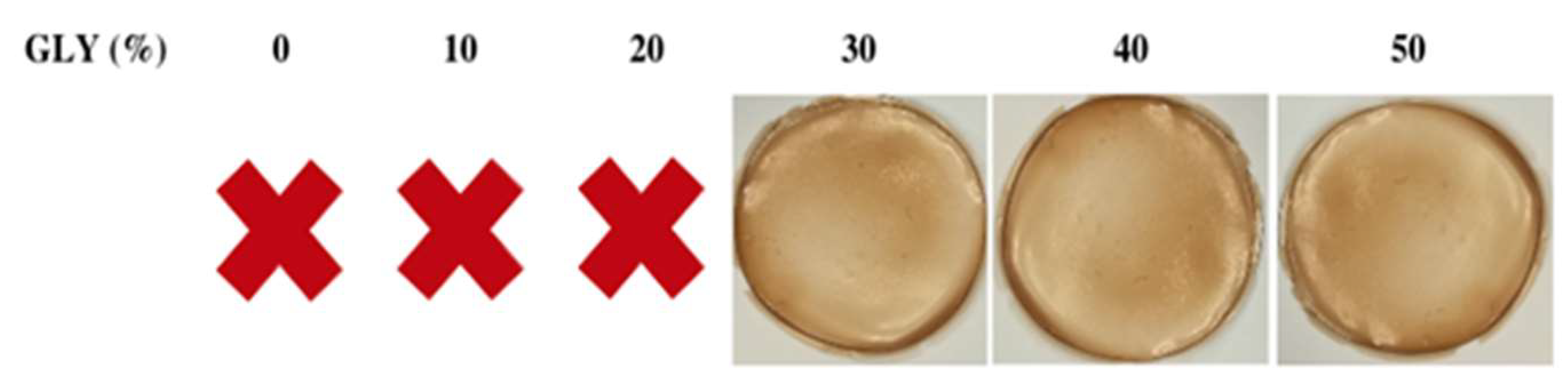
2.2. Preparation of Argan Seed Protein Concentrate Film Forming Solutions and of Derived Films
2.3. Characterization of Argan Seed Protein Concentrate-Based Films
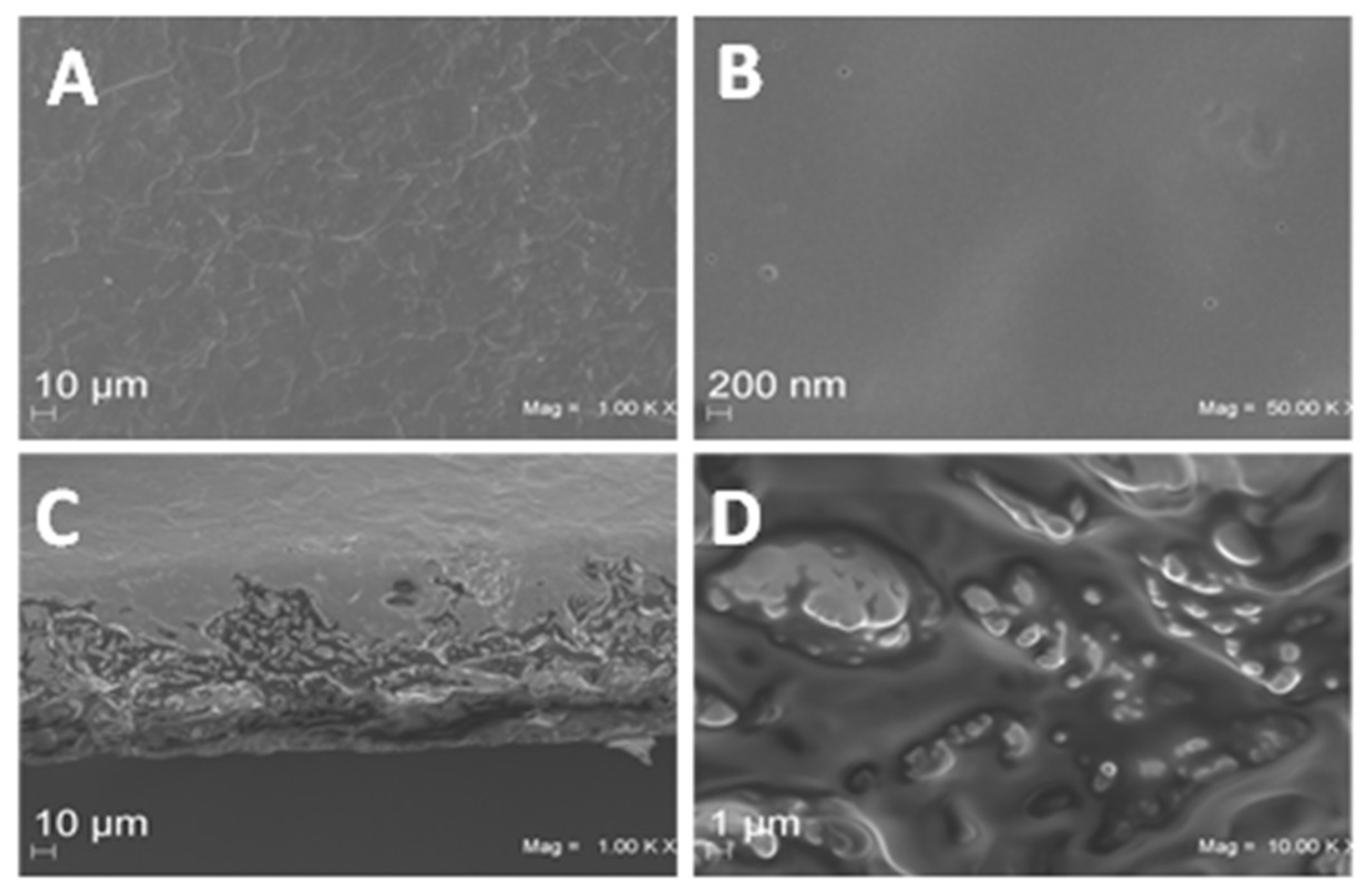
2.3.1. Film Morphology
2.3.2. Film Mechanical Properties
2.3.3. Film Moisture Content and Uptake
2.3.4. Film Barrier Properties
2.3.5. Film Contact Angle and Antioxidant Activity
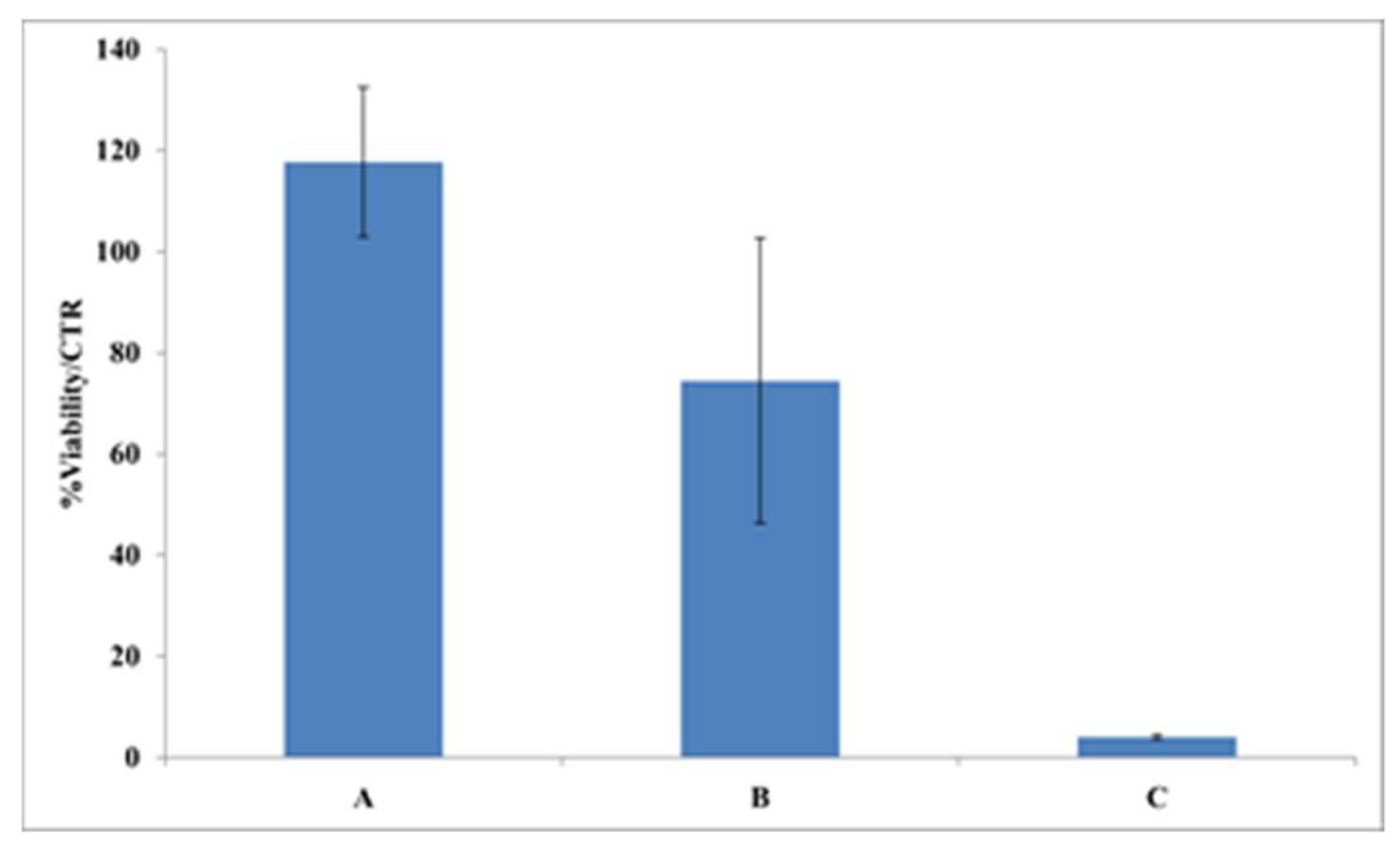
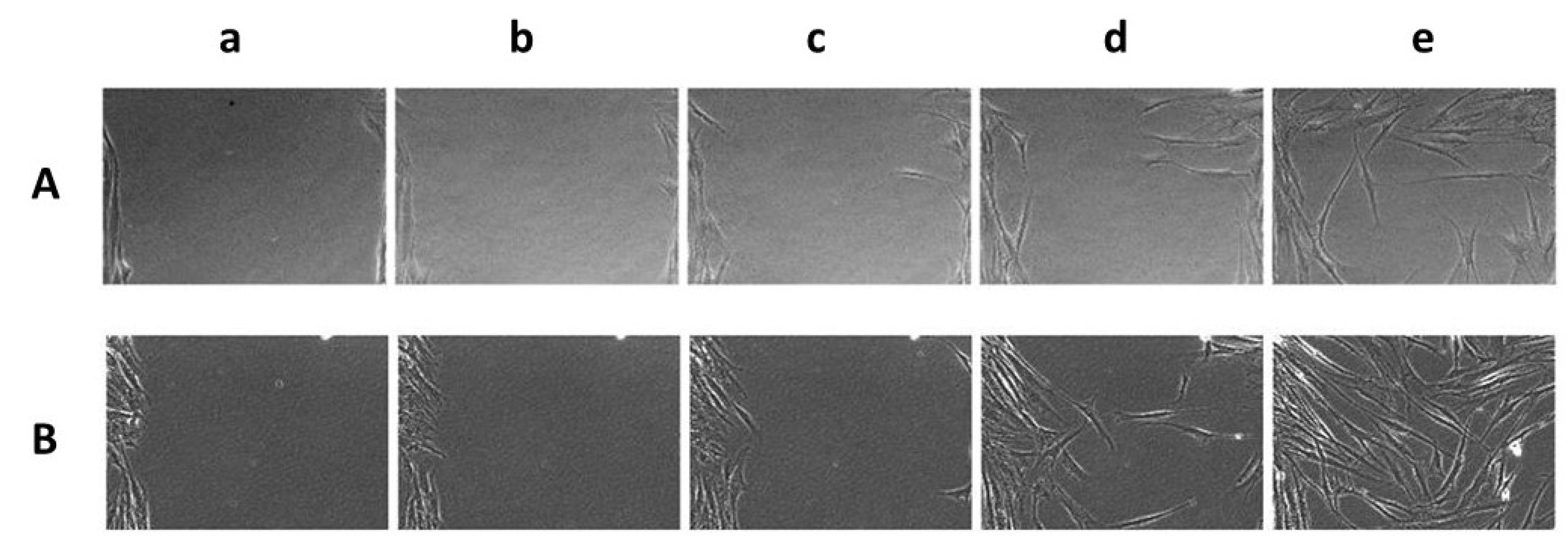
2.3.6. Film Biocompatibility and Effect on Wound Healing
3. Materials and Methods
3.1. Materials
3.2. Preparation of Argan Seed Protein Concentrate
3.3. Sodium Dodecyl Sulphate Poly Acrylamide Gel Electrophoresis Analysis of Argan Seed Protein Concentrate
3.4. Preparation of the Argan Seed Protein Concentrate-Based Film Forming Solutions and of the Derived Films
3.5. Film Characterization
3.5.1. Morphological Evaluation
3.5.2. Mechanical Properties
3.5.3. Moisture Content and Moisture Uptake
3.5.4. Permeability and Contact Angle Analyses
3.5.5. Antioxidant Activity
3.5.6. Cell Culture and Treatments
3.5.7. Cell Viability
3.5.8. Wound Healing assay and Time Lapse Video Microscopy
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abiodun, O.A. Oilseed Crops: Yield and Adaptations under Environmental Stress; John Wiley & Sons Ltd.: Chichester, NY, USA, 2017; pp. 249–263. [Google Scholar]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Porta, R. Biorefining of seed oil cakes as industrial co-streams for production of innovative bioplastics. A review. Trends Food Sci. Technol. 2021, 109, 259–270. [Google Scholar] [CrossRef]
- Ash, M.; Dohlman, E. Electronic outlook report from the Economic Research Service, United States Department of Agriculture. 2007. Available online: www.ers.usda.gov (accessed on 25 June 2022).
- Moure, A.; Sineiro, J.; Domínguez, H.; Parajó, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- Moutik, S.; Benali, A.; Bendaou, M.; Maadoudi, E.H.; Kabbour, M.R.; El Housni, A.; Es-Safi, N.E. The effect of using diet supplementation based on argan (Argania spinosa) on fattening performance, carcass characteristics and fatty acid composition of lambs. Heliyon 2021, 7, e05942. [Google Scholar] [CrossRef] [PubMed]
- Khallouki, F.; Voggel, J.; Breuer, A.; Klika, K.D.; Ulrich, C.M.; Owen, R.W. Comparison of the major polyphenols in mature Argan fruits from two regions of Morocco. Food Chem. 2017, 221, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Koufan, M.; Belkoura, I.; Mazri, M.A.; Amarraque, A.; Essatte, A.; Elhorri, H.; Zaddoug, F.; Alaoui, T. Determination of antioxidant activity, total phenolics and fatty acids in essential oils and other extracts from callus culture, seeds and leaves of Argania spinosa (L.) skeels. Plant Cell Tissue Organ. Cult. 2020, 141, 217–227. [Google Scholar] [CrossRef]
- Gharby, S.; Charrouf, Z. Argan oil: Chemical composition, extraction process, and quality control. Front. Nutr. 2022, 8, 804587. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Sustainable development in Northern Africa: The argan forest case. Sustainability. 2009, 1, 1012–1022. [Google Scholar] [CrossRef]
- Haloui, I.; Meniai, A.H. Supercritical CO2 extraction of essential oil from Algerian Argan (Argania spinosa L.) seeds and yield optimization. Int. J. Hydrogen Energy 2017, 42, 12912–12919. [Google Scholar] [CrossRef]
- Aithammou, R.; Harrouni, C.; Aboudlou, L.; Hallouti, A.; Mlouk, M.; Elasbahani, A.; Daoud, S. Effect of clones, year of harvest and geographical origin of fruits on quality and chemical composition of Argan oil. Food Chem. 2019, 297, 124749. [Google Scholar] [CrossRef] [PubMed]
- Cherki, M.; Berrougui, H.; Drissi, A.; Adlouni, A.; Khalil, A. Argan oil: Which benefits on cardiovascular diseases? Pharmacol. Res. 2006, 54, 1–5. [Google Scholar] [CrossRef] [PubMed]
- El Abbassi, A.; Khalid, N.; Zbakh, H.; Ahmad, A. Physicochemical characteristics, nutritional properties, and health benefits of argan oil: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1401–1414. [Google Scholar] [CrossRef]
- Guillaume, D.; Charrouf, Z. Argan oil and other argan products: Use in dermocosmetology. Eur. J. Lipid Sci. Technol. 2011, 113, 403–408. [Google Scholar] [CrossRef]
- Goik, U.; Goik, T.; Załęska, I. The properties and application of argan oil in cosmetology. Eur. J. Lipid Sci. Technol. 2019, 121, 1800313. [Google Scholar] [CrossRef]
- Lakram, N.; Moutik, S.; Mercha, I.; El Haj, E.M.; Kabbour, R.; Douaik, A.; Zouahri, A.; El Housni, A.; Naciri, M. Effects of the inclusion of detoxified argan press cake in the diet of dairy goats on milk production and milk quality. Turk. J. Vet. Anim. Sci. 2019, 43, 323–333. [Google Scholar] [CrossRef]
- Bourhim, T.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Depigmenting effect of argan press-cake extract through the down-regulation of Mitf and melanogenic enzymes expression in B16 murine melanoma cells. Cytotechnology 2018, 70, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Bourhim, T.; Villareal, M.O.; Gadhi, C.; Isoda, H. Elucidation of melanogenesis-associated signaling pathways regulated by argan press cake in B16 melanoma cells. Nutrients 2021, 13, 2697. [Google Scholar] [CrossRef]
- Delgado, M.; Felix, M.; Bengoechea, C. Development of bioplastic materials: From rapeseed oil industry by products to added-value biodegradable biocomposite materials. Ind. Crops Prod. 2018, 125, 401–407. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Restaino, O.F.; Schiraldi, C.; Giosafatto, C.V.L.; Ruffo, F.; Porta, R. Lignin/carbohydrate complex isolated from Posidonia oceanica sea balls (egagropili): Characterization and antioxidant reinforcement of protein-based films. Int. J. Mol. Sci. 2021, 22, 9147. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Di Girolamo, R.; Famiglietti, M.; Porta, R. Hemp (Cannabis sativa) seed oilcake as a promising by-product for developing protein-based films: Effect of transglutaminase-induced crosslinking. Food Packag. Shelf Life 2022, 31, 100779. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Varriale, S.; Porta, R.; Naviglio, D.; Spennato, M.; Gardossi, L.; Giosafatto, C.V.L.; Pezzella, C. A biorefinery approach for the conversion of Cynara cardunculus biomass to active films. Food Hydrocolloid. 2022, 122, 107099. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Physicochemical, pasting, and functional properties of amaranth seed flours: Effects of lipids removal. J. Food Sci. 2014, 79, C1271–C1277. [Google Scholar] [CrossRef] [PubMed]
- ASTM Standard Test Method for Tensile Properties of Thin Plastic Sheeting. D882–97, Philadelphia, PA, USA, 1997. Available online: https://standards.globalspec.com/std/13050669/astm-d882(accessed on 25 June 2022).
- Manrich, A.; Moreira, F.K.; Otoni, C.G.; Lorevice, M.V.; Martins, M.A.; Mattoso, L.H. Hydrophobic edible films made up of tomato cutin and pectin. Carbohydr. Polym. 2017, 164, 83–91. [Google Scholar] [CrossRef]
- ASTM Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. D3985–05, West Conshohocken, PA, USA, 2010. Available online: https://www.astm.org/d3985-17.html(accessed on 25 June 2022).
- ASTM Standard Test Method for the Determination of Carbon Dioxide Gas Transmission Rate (CO2TR) Through Barrier Materials Using An Infrared Detector F2476–13, West Conshohocken, PA, USA, 2013. Available online: https://webstore.ansi.org/Standards/ASTM/astmf247613(accessed on 25 June 2022).
- Sabbah, M.; Altamimi, M.; Di Pierro, P.; Schiraldi, C.; Cammarota, M.; Porta, R. Black edible films from protein-containing defatted cake of Nigella sativa seeds. Int. J. Mol. Sci. 2020, 21, 832. [Google Scholar] [CrossRef]
- Parveen, S.; Chaudhury, P.; Dasmahapatra, U.; Dasgupta, S. Biodegradable protein films from gallic acid and the cataractous eye protein isolate. Int. J. Biol. Macromol. 2019, 139, 12–20. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; Maritato, R.; La Gatta, A.; Fusco, A.; Reale, S.; Stellavato, A.; Virginia Adriana Pirozzi, A.; De Rosa, M.; Donnarumma, G.; Schiraldi, C. In Vitro evaluation of novel hybrid cooperative complexes in a wound healing model: A step toward improved bioreparation. Int. J. Mol. Sci. 2019, 20, 4727. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; La Gatta, A.; Stellavato, A.; Cimini, D.; Corsuto, L.; Cammarota, M.; D’Agostino, M.; Schiraldi, C. Potential of biofermentative unsulfated chondroitin and hyaluronic acid in dermal repair. Int. J. Mol. Sci. 2022, 23, 1686. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in cell biology: Viability assessment, fluorescence imaging, and labeling perspective. Acta Histochemica 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]

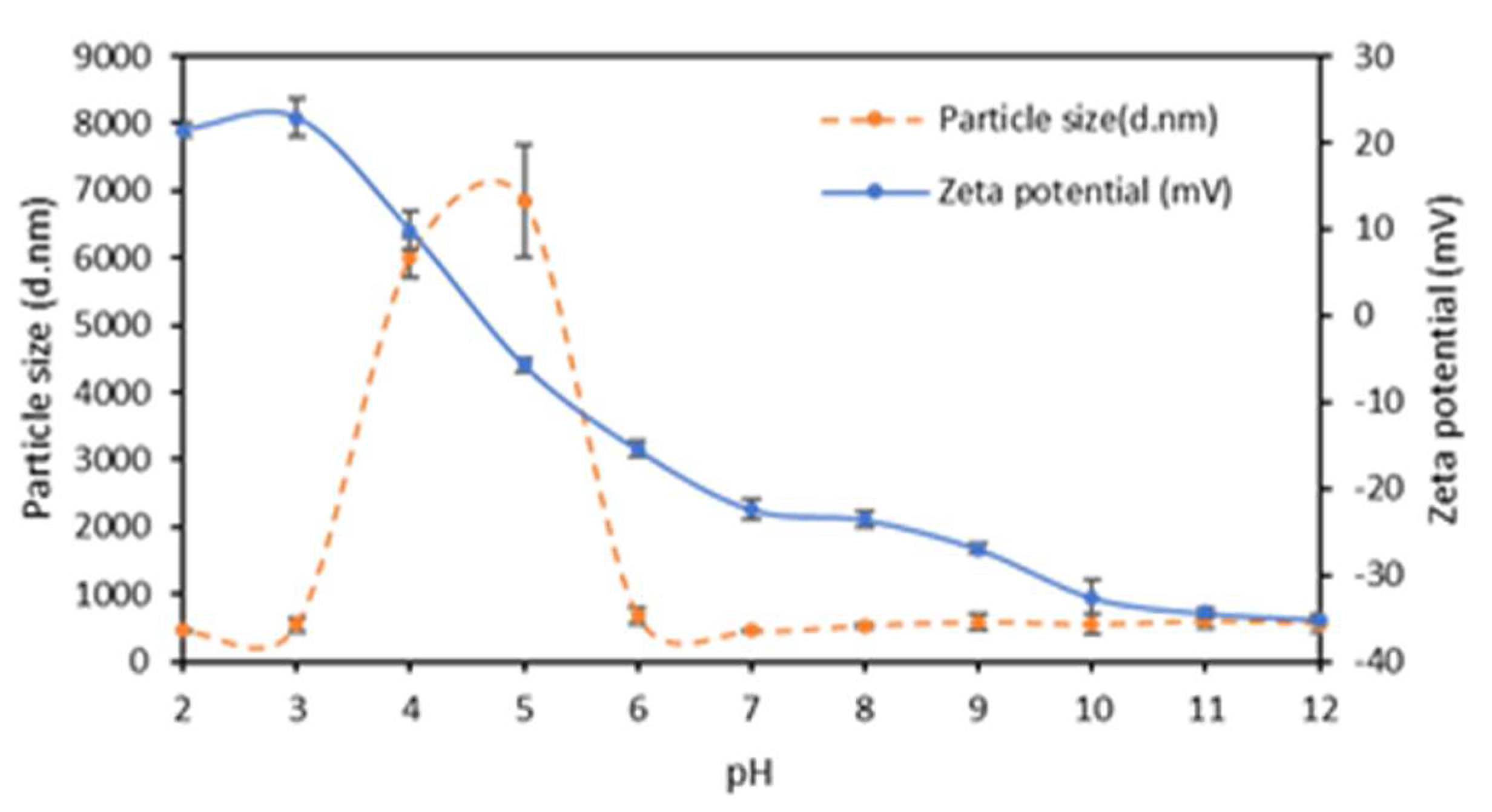

| ASPC (mg) | GLY (%) | Zeta potential (mV) | Z-Average (nm) | Polydispersity Index |
|---|---|---|---|---|
| 300 | 30 | −34.8 ± 1.9 a | 410 ± 42 d | 0.75 ± 0.02 b,c |
| 40 | −30.0 ± 2.0 a | 519 ± 45 c | 0.78 ± 0.06 b,c | |
| 50 | −34.0 ± 2.0 a | 726 ± 10 a | 0.90 ± 0.08 a | |
| 500 | 30 | −36.2 ± 4.9 a | 302 ± 11 e | 0.69 ± 0.07 d,e |
| 40 | −34.8 ± 3.7 a | 320 ± 14 e | 0.52 ± 0.02 f | |
| 50 | −35.3 ± 3.6 a | 394 ± 13 e | 0.58 ± 0.02 f,g | |
| 600 | 30 | −35.9 ± 5.8 a | 327 ± 5 e | 0.65 ± 0.02 e,f |
| 40 | −35.6 ± 5.7 a | 339 ± 15 e | 0.66 ± 0.01 d,e | |
| 50 | −34.9 ± 5.0 a | 579 ± 17 b | 0.76 ± 0.08 b,c | |
| 800 | 30 | −20.3 ± 1.3 a | 495 ± 11 c | 0.78 ± 0.02 b,c |
| 40 | −20.8 ± 0.9 a | 488 ± 26 c | 0.75 ± 0.05 b,c | |
| 50 | −20.3 ± 0.1 a | 590 ± 41 b | 0.81 ± 0.09 b |
| ASPC (mg) | GLY (%) | Moisture Content (%) | Moisture Uptake (%) |
|---|---|---|---|
| 500 | 30 | 10.03 ± 0.31 d | 9.16 ± 0.12 b |
| 40 | 10.48 ± 0.28 c,d | 9.77 ± 1.34 b | |
| 50 | 10.12 ± 2.77 c,d | 9.22 ± 1.01 b | |
| 600 | 30 | 12.11 ± 0.31 b,c | 12.74 ± 1.32 a |
| 40 | 12.62 ± 0.29 b,c | 12.02 ± 0.97 a | |
| 50 | 12.20 ± 0.70 a | 12.23 ± 1.46 a | |
| 800 | 30 | 11.43 ± 0.43 b,c,d | 12.99 ± 0.25 a |
| 40 | 12.66 ± 1.18 a,b | 13.57 ± 1.06 a | |
| 50 | 12.10 ± 0.30 b,c | 12.21 ± 0.41 a |
| Film | WV | CO2 | O2 |
|---|---|---|---|
| (cm3 mm m−2 d−1 kPa−1) | |||
| ASPC | 7.5 ± 0.6 a | 0.46 ± 0.08 a | 0.02 ± 0.01 a |
| Mater Bi® | 9.8 ± 0.6 b | 5.19 ± 0.60 b | 0.69 ± 0.09 b |
| LDPE | 0.07 ±0.01 c | 13.99 ± 1.08 c | 3.79 ± 0.80 c |
| Film | Contact angle (θ) | |
|---|---|---|
| At 0 s | After 30 s | |
| ASPC | 68.26 ± 1.16 b | 66.26 ± 1.15 b |
| HSPC | 32.15 ± 1.45 d | 30.87 ± 1.12 d |
| CSPC | 20.76 ± 1.39 e | 12.87 ± 1.89 e |
| Mater Bi® | 64.20 ± 2.03 c | 53.85 ± 0.07 c |
| LDPE | 70.66 ± 1.35 a | 69.75 ± 0.48 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirpoor, S.F.; Giosafatto, C.V.L.; Mariniello, L.; D’Agostino, A.; D’Agostino, M.; Cammarota, M.; Schiraldi, C.; Porta, R. Argan (Argania spinosa L.) Seed Oil Cake as a Potential Source of Protein-Based Film Matrix for Pharmaco-Cosmetic Applications. Int. J. Mol. Sci. 2022, 23, 8478. https://doi.org/10.3390/ijms23158478
Mirpoor SF, Giosafatto CVL, Mariniello L, D’Agostino A, D’Agostino M, Cammarota M, Schiraldi C, Porta R. Argan (Argania spinosa L.) Seed Oil Cake as a Potential Source of Protein-Based Film Matrix for Pharmaco-Cosmetic Applications. International Journal of Molecular Sciences. 2022; 23(15):8478. https://doi.org/10.3390/ijms23158478
Chicago/Turabian StyleMirpoor, Seyedeh Fatemeh, Concetta Valeria L. Giosafatto, Loredana Mariniello, Antonella D’Agostino, Maria D’Agostino, Marcella Cammarota, Chiara Schiraldi, and Raffaele Porta. 2022. "Argan (Argania spinosa L.) Seed Oil Cake as a Potential Source of Protein-Based Film Matrix for Pharmaco-Cosmetic Applications" International Journal of Molecular Sciences 23, no. 15: 8478. https://doi.org/10.3390/ijms23158478
APA StyleMirpoor, S. F., Giosafatto, C. V. L., Mariniello, L., D’Agostino, A., D’Agostino, M., Cammarota, M., Schiraldi, C., & Porta, R. (2022). Argan (Argania spinosa L.) Seed Oil Cake as a Potential Source of Protein-Based Film Matrix for Pharmaco-Cosmetic Applications. International Journal of Molecular Sciences, 23(15), 8478. https://doi.org/10.3390/ijms23158478