Toll-like Receptor 7 (TLR7) Is Expressed in Adipocytes and the Pharmacological TLR7 Agonist Imiquimod and Adipocyte-Derived Cell-Free Nucleic Acids (cfDNA) Regulate Adipocyte Function
Abstract
:1. Introduction
2. Results
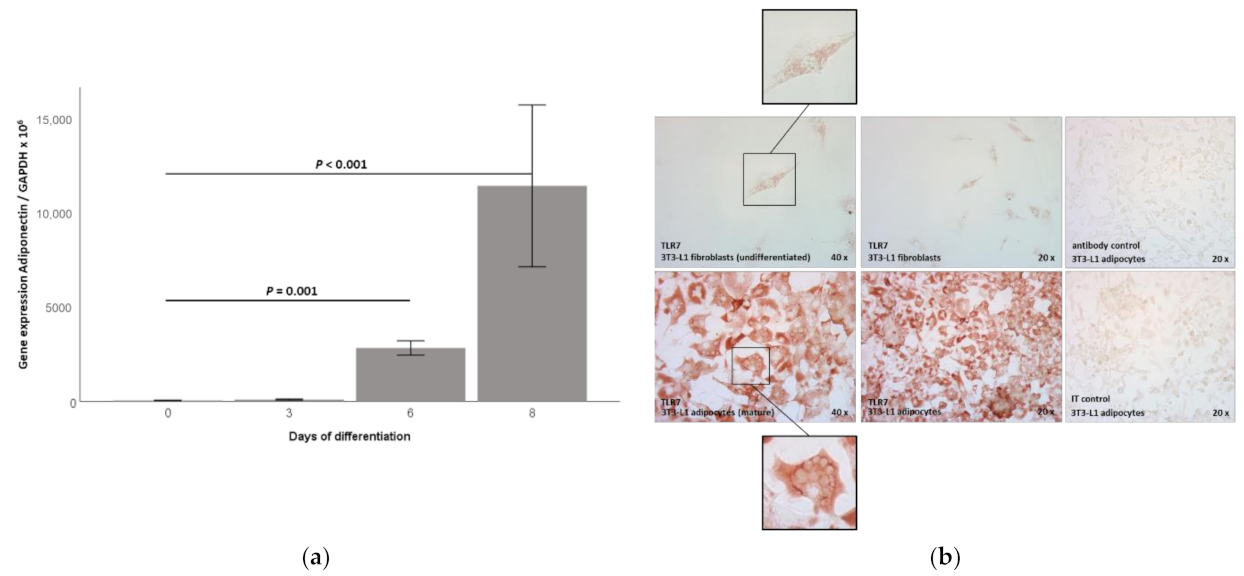
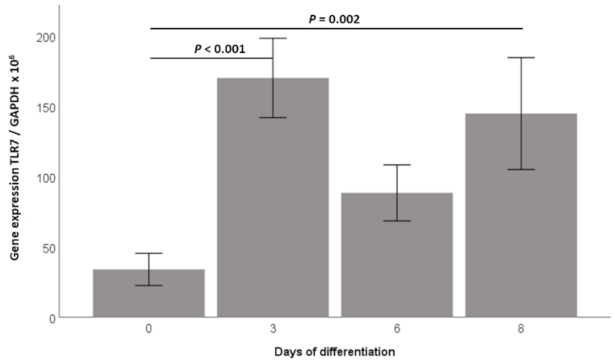
2.1. TLR7 Gene Expression Is Induced during 3T3-L1 Adipocyte Differentiation and TLR7 Protein Is Expressed in 3T3-L1 Adipocytes
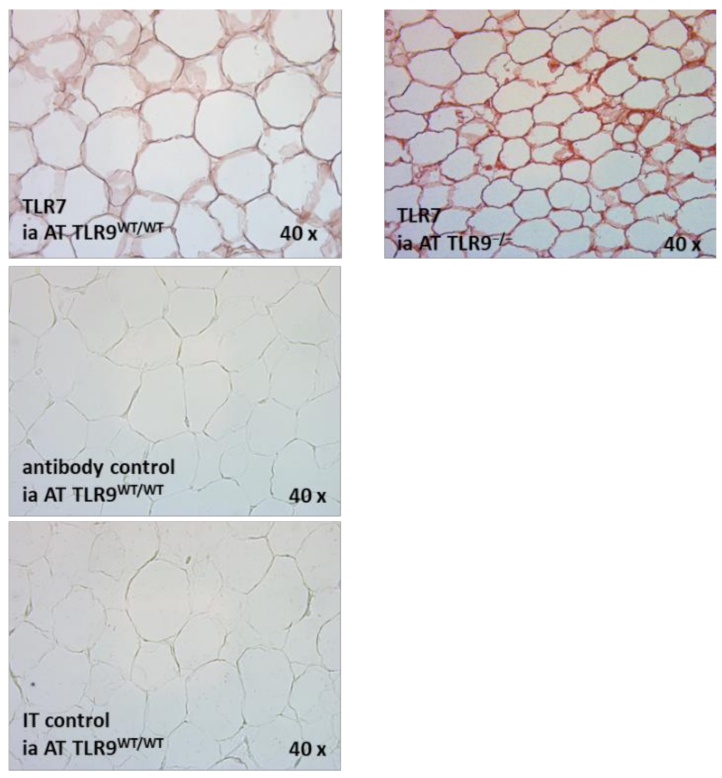
2.2. TLR7 mRNA Is Expressed in the SVC and the Adipocyte Fraction of Intra-Abdominal Adipose Tissue (AT) in TLR9wt/wt and in TLR9−/− Mice
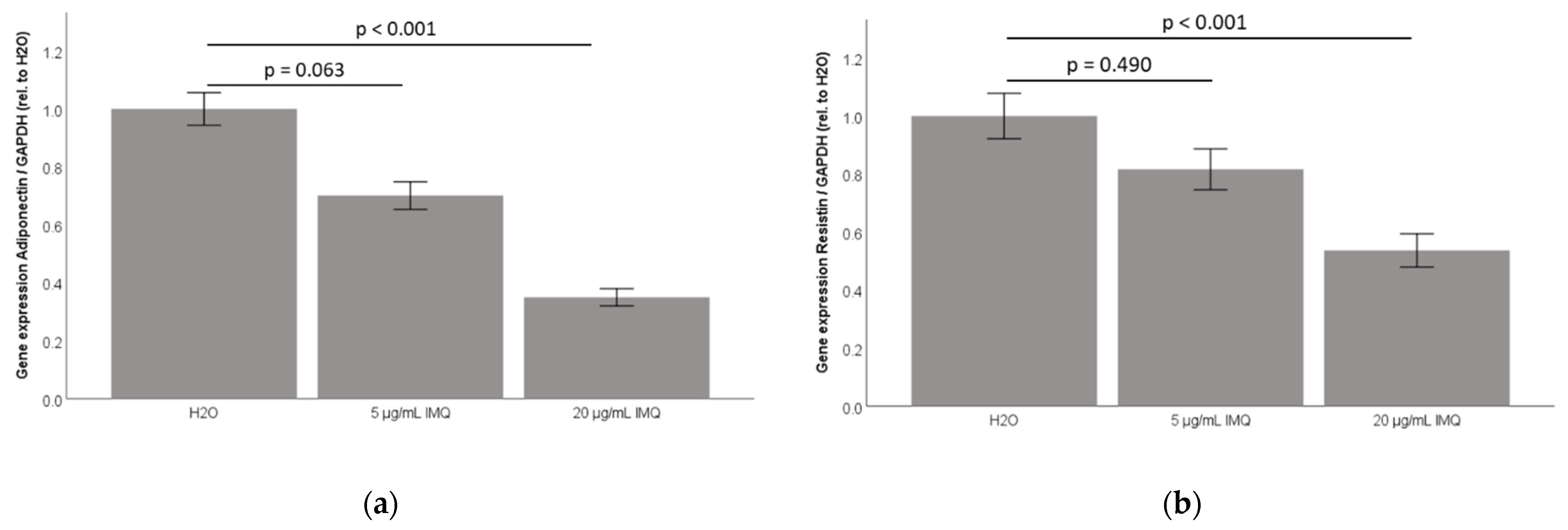
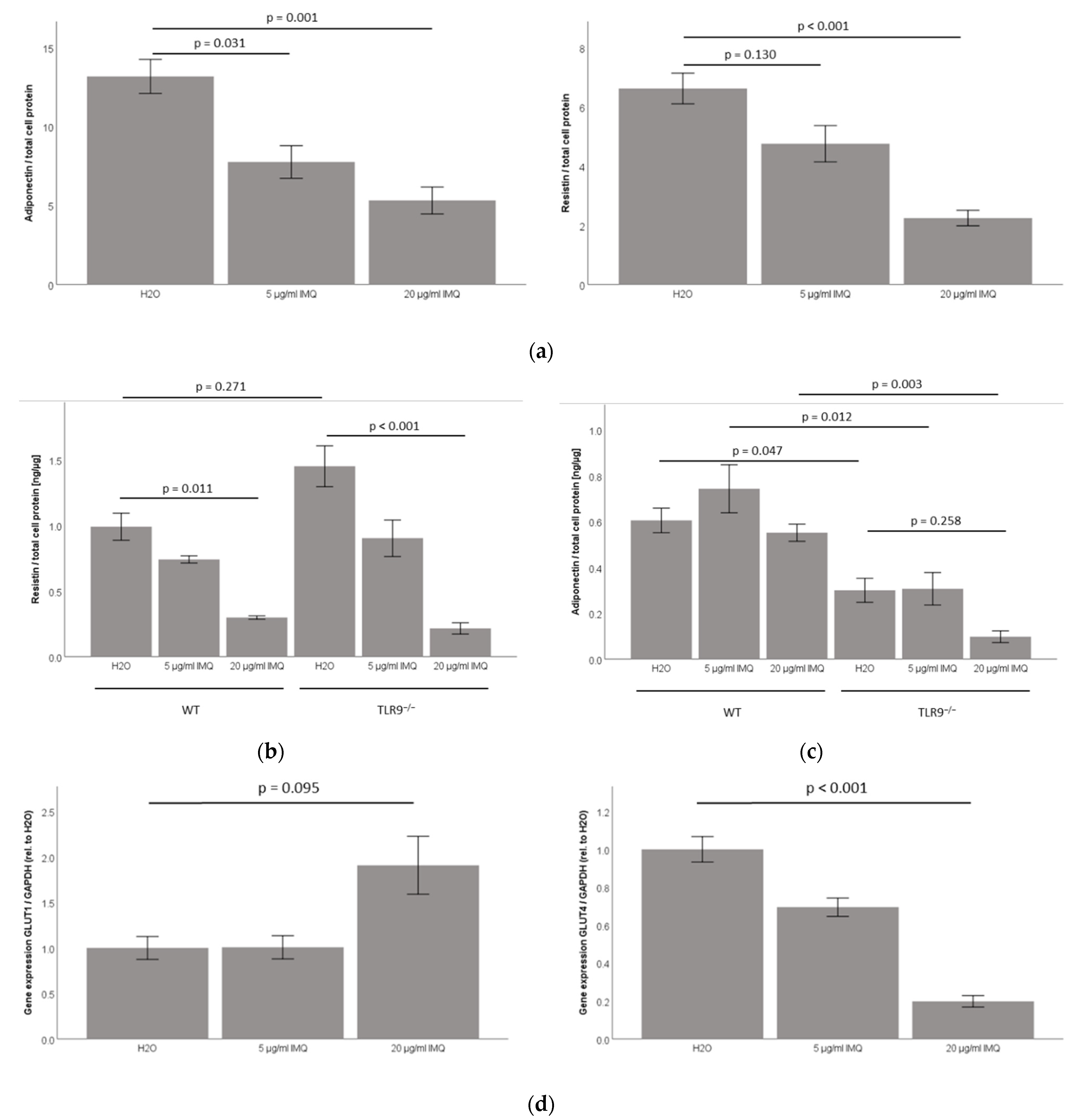
2.3. TLR7 Agonist Imiquimod Inhibits Adiponectin and Resistin Accumulation in 3T3-L1 Adipocyte Cell-Culture Supernatants
2.4. TLR7 Agonist Imiquimod Inhibits Resistin Accumulation in Primary Murine TLR9wt/wt and TLR9−/− Subcutaneous Adipocyte Cell-Culture Supernatants, While Leaving Adiponectin Unchanged in Both TLR9−/− and TLR9wt/wt Adipocyte Cell-Culture Supernatants
2.5. TLR7 Agonist Imiquimod Inhibits Glut4 mRNA Expression in 3T3-L1 Adipocytes
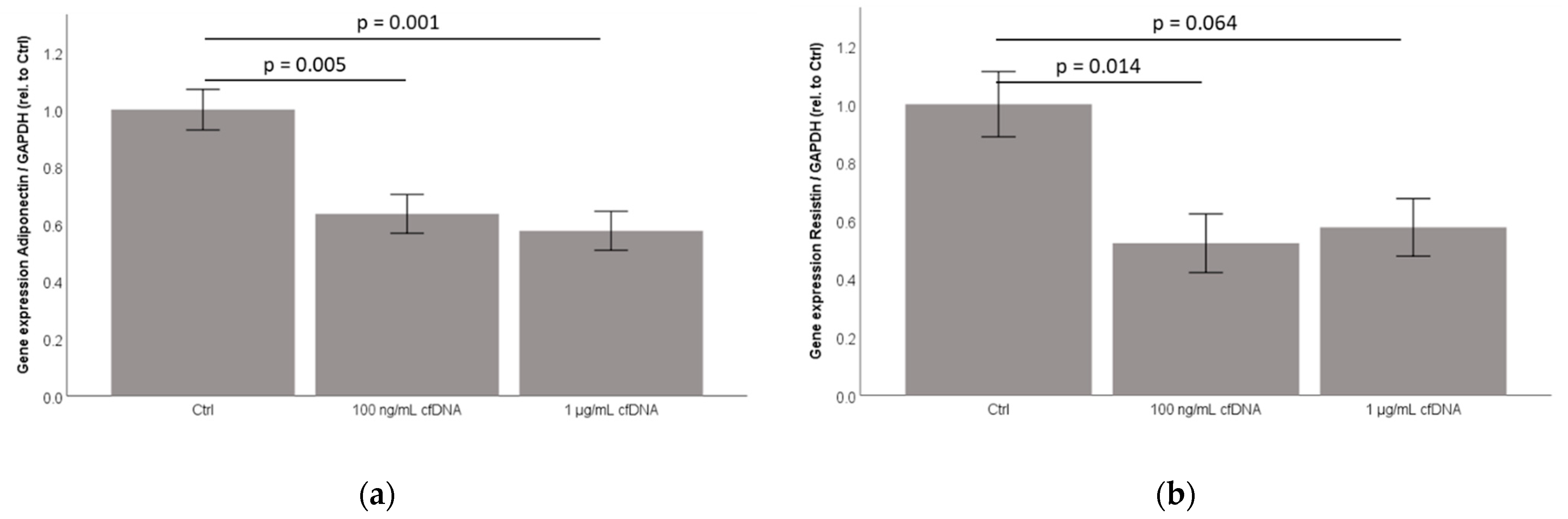
2.6. Cell-Free Nucleic Acids (cfDNA) from 3T3-L1 Adipocytes Treated by TNFα Dose-Dependently Inhibit Adiponectin and Resistin Concentration in Cell-Culture Supernatants
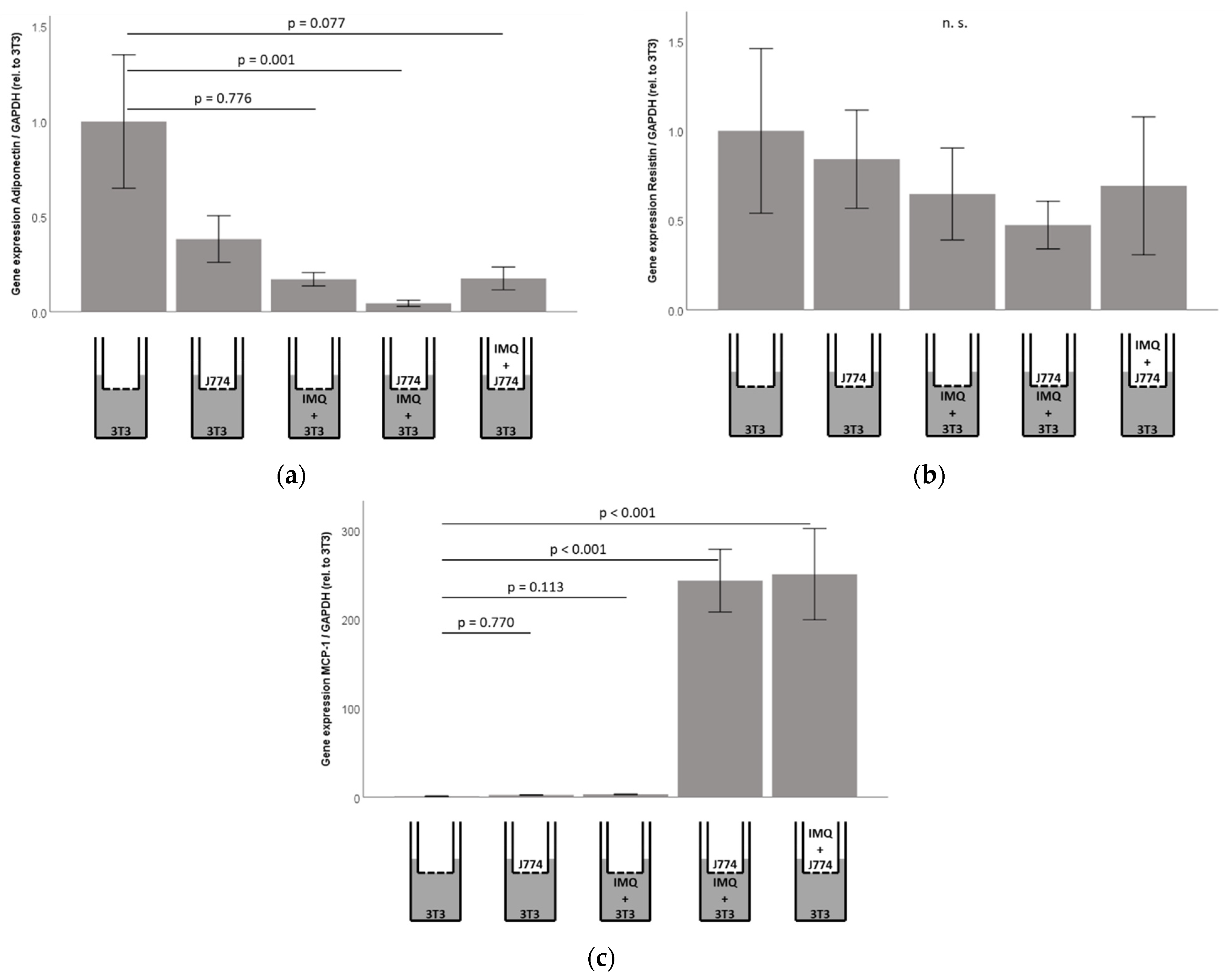
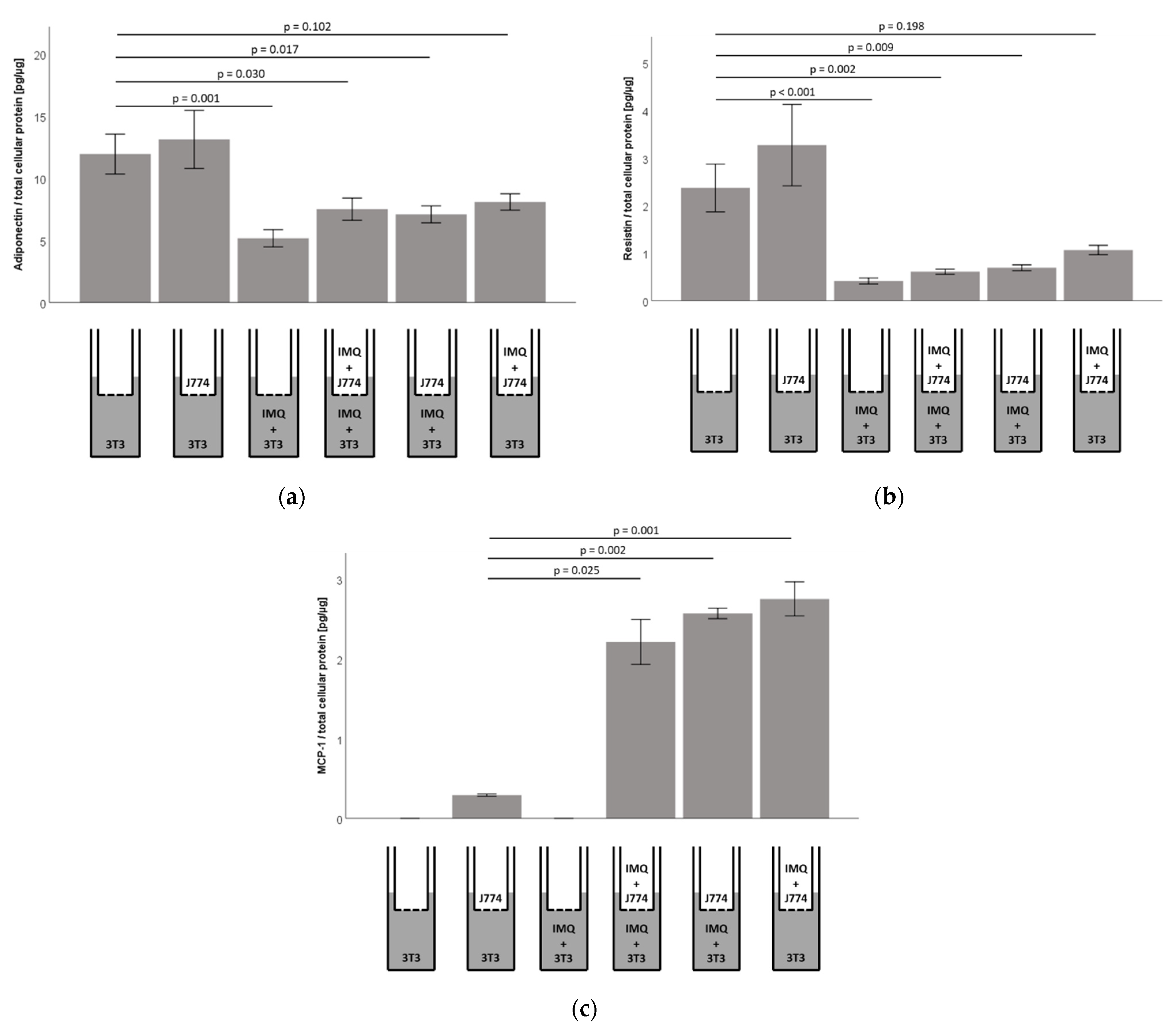
2.7. Responsiveness of Murine 3T3-L1 Adipocytes to Imiquimod Is Preserved in Co-Culture with Murine J774A.1 Monocytes, However, Co-Cultures Show Significantly Increased Concentrations of MCP1/CCL2 in 3T3-L1 Adipocyte Cell-Culture Supernatants
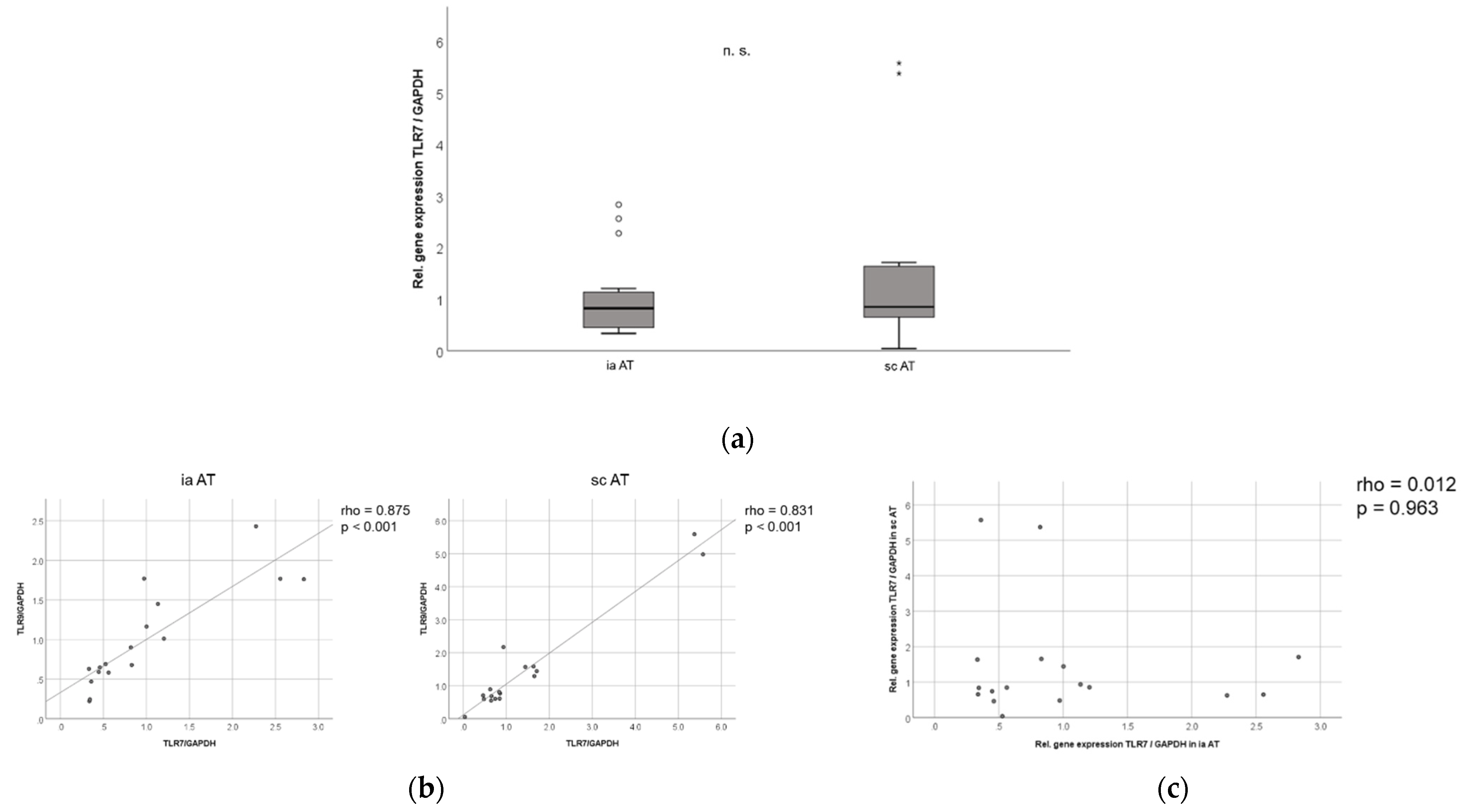
2.8. TLR7 mRNA Is Expressed in Similar Levels in Murine Subcutaneous and Intra-Abdominal Adipose Tissue, and TLR7 and TLR9 mRNA Expression in Murine Adipose Tissues Are Strongly Correlated to Each Other
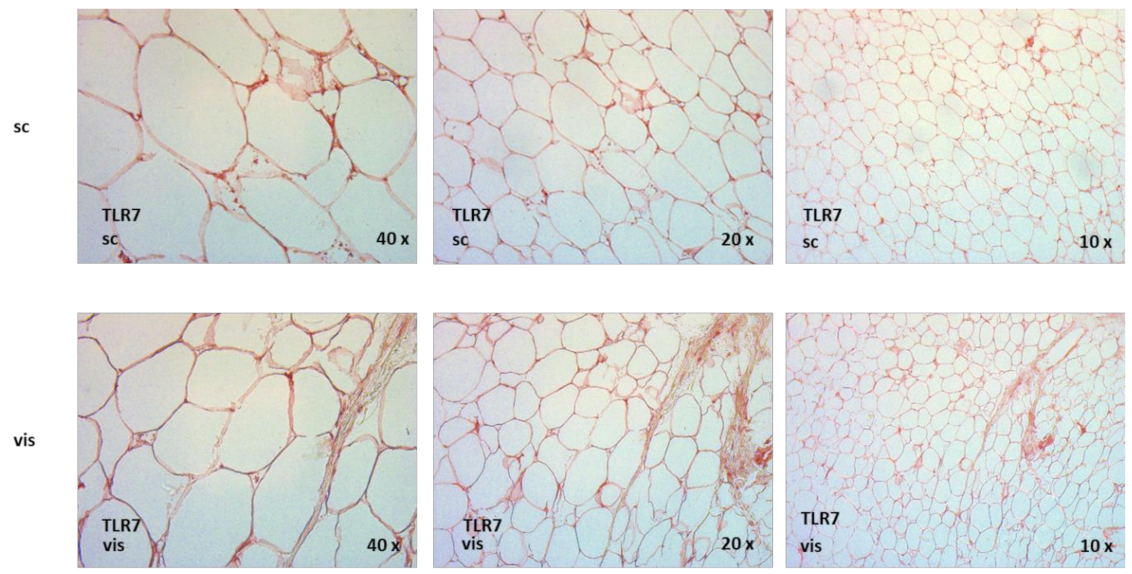
2.9. TLR7 Is Expressed in Human Adipose Tissue Compartments, and TLR7 Is Significantly Increased in Visceral as Compared to Subcutaneous Adipose Tissue in Non-Diabetic Obese Patients Undergoing Bariatric Surgery
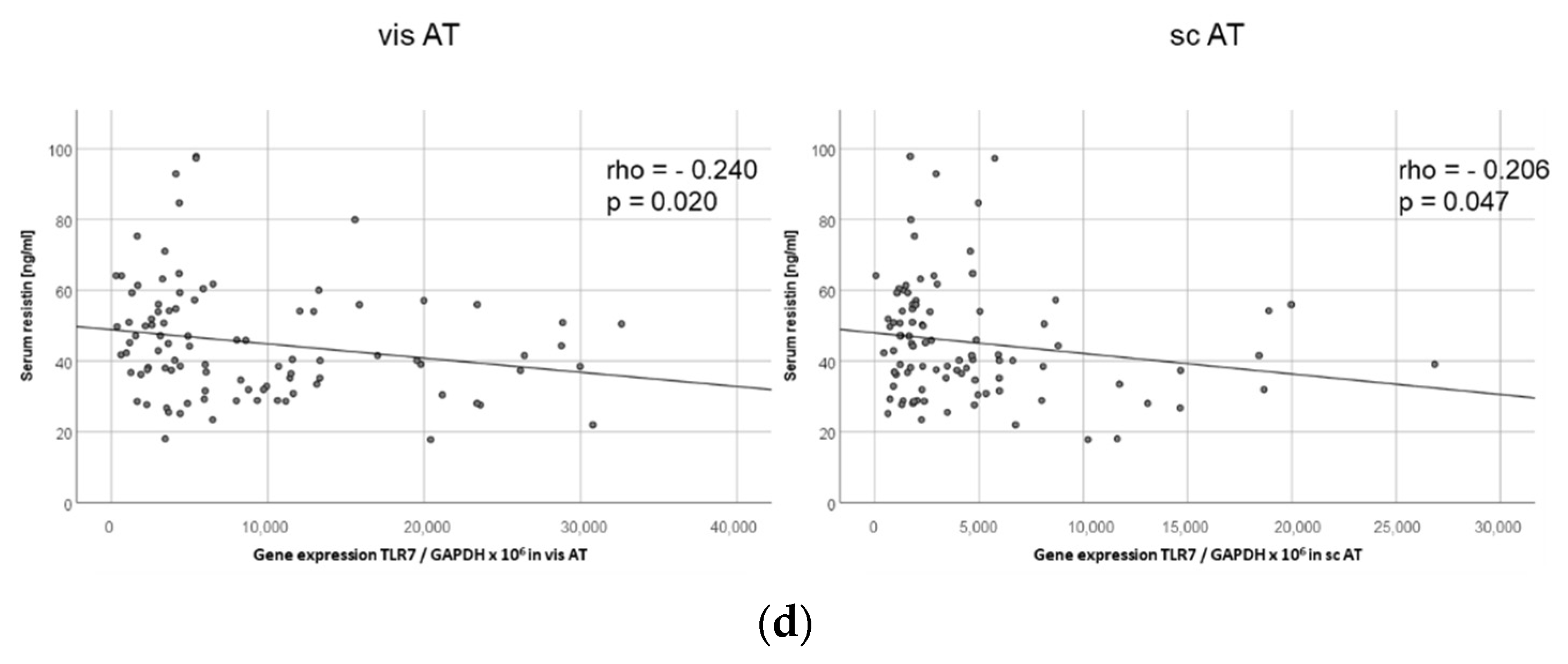
2.10. TLR7 Expression Negatively Correlates with Resistin Serum Levels in Both Visceral and Subcutaneous Adipose Tissue in Human Obese Patients
3. Discussion
4. Materials and Methods
4.1. Adipocyte Cell Culture and Stimulation Experiments
4.2. Co-Culture of Adipocytes and Monocytes
4.3. Isolation of Adipocytes and SVCs from Mice
4.4. Preparation of Human and Murine Tissues for mRNA Isolation
4.5. Quantification of mRNA Expression in Murine 3T3-L1 Adipocytes In Vitro and Ex Vivo in Murine and Human Adipose Tissues
| Murine adiponectin: | 5′-AGGGAGAGAAAGGAGATGCAG-3′/5′-CAGACTTGGGCTCCCACCTC-3′ |
| Murine GLUT1: | 5′-AGCAGAGGCTTGCTTGTAGA-3′/5′-AACTCCTCAATAACCTTCTGGGG-3′ |
| Murine GLUT4: | 5′-TGGTTCATTGTGGCAGAGC-3′/5′-CGTAAGGACCCATAGCATCC-3′ |
| Murine resistin: | 5′-TGCTAAGTCCTCTGCCACGTA-3′/5′-TCAACTGACCGACATCAG GA-3′ |
| Murine TLR7: | 5′-GGCATTCCCACTAACACCAC-3′/5′-TTGGACCCCAGTAGAACAGG-3′ |
| Murine TLR9: | 5′-CATCTCCCAACATGGTTCTCC-3′/5′-GCAGAGAAACGGGGTACAGA-3′ |
| Murine CD45: | 5′-TGACCATGGGTTTGTGGCTC-3′/5′-TCGTTGTGGTAGCATCACTGG-3′ |
| Human TLR7: | 5′-TCAAGAAAGTTGATGCTATTGGGC-3′/5′-CTGTGCAGTCCACGATCACA-3′ |
| Human TLR9: | 5′-CCCCCAGCATGGGTTTCT-3′/5′-TGGAGCTCACAGGGTAGGAA-3′ |
4.6. Quantification of Adiponectin and Resistin Protein Concentrations in 3T3-L1 and pmAd Cell Culture Supernatants
4.7. Immunocytochemistry (ICC)
4.8. Immunohistochemistry (IHC)
4.9. Animals
4.10. Patients and Study Cohort
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| rho | p | |
|---|---|---|
| TLR7 mRNA (visceral AT) | +0.479 | <0.001 |
| Age | −0.024 | 0.818 |
| Body weight | −0.015 | 0.888 |
| BMI | −0.024 | 0.820 |
| Body fat percentage | −0.110 | 0.322 |
| WHR | +0.030 | 0.796 |
| Excessive weight | −0.026 | 0.804 |
| Total cholesterol | −0.069 | 0.528 |
| LDL cholesterol | +0.078 | 0.477 |
| HDL cholesterol | −0.226 | 0.038 |
| Triglycerides | +0.107 | 0.328 |
| CRP | +0.026 | 0.799 |
| Adiponectin | −0.159 | 0.125 |
| Leptin | −0.140 | 0.179 |
| Resistin | −0.206 | 0.047 |
| CTRP3 | −0.166 | 0.112 |
| Progranulin | −0.215 | 0.043 |
| rho | p | |
|---|---|---|
| TLR7 mRNA (subcutaneous AT) | +0.479 | <0.001 |
| Age | −0.101 | 0.331 |
| Body weight | −0.103 | 0.320 |
| BMI | −0.038 | 0.714 |
| Body fat percentage | −0.142 | 0.200 |
| WHR | +0.016 | 0.886 |
| Excessive weight | −0.091 | 0.382 |
| Total cholesterol | −0.027 | 0.808 |
| LDL cholesterol | +0.045 | 0.680 |
| HDL cholesterol | −0.016 | 0.884 |
| Triglycerides | −0.001 | 0.996 |
| CRP | +0.187 | 0.070 |
| Adiponectin | −0.049 | 0.636 |
| Leptin | −0.107 | 0.305 |
| Resistin | −0.240 | 0.020 |
| CTRP3 | −0.185 | 0.075 |
| Progranulin | −0.202 | 0.057 |
References
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Antony, V.; Sun, H.; Liang, G. Metabolism-Associated Molecular Patterns (MAMPs). Trends Endocrinol. Metab. 2020, 31, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, A.; Schölmerich, J. Innate immunity and adipose tissue biology. Trends Immunol. 2010, 31, 228–235. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, Y.; Choi, Y.H.; Park, T. Obesity Activates Toll-Like Receptor-Mediated Proinflammatory Signaling Cascades in the Adipose Tissue of Mice. J. Nutr. Biochem. 2012, 23, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-P.; Yun, C.H.; Lee, G.-W.; Park, A.; Kim, Y.-M.; Jang, M.H. TLR9 regulates adipose tissue inflammation and obesity-related metabolic disorders. Obesity 2015, 23, 2199–2206. [Google Scholar] [CrossRef] [Green Version]
- Nishimoto, S.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Murata, C.; Kim-Kaneyama, J.-R.; Sato, F.; Bando, M.; Yagi, S.; et al. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci. Adv. 2016, 2, e1501332. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.R.; Bhattacharya, R.; Bhattacharya, S.; Nargis, T.; Rahaman, O.; Duttagupta, P.; Raychaudhuri, D.; Liu, C.S.C.; Roy, S.; Ghosh, P.; et al. Adipose Recruitment and Activation of Plasmacytoid Dendritic Cells Fuel Metaflammation. Diabetes 2016, 65, 3440–3452. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A.J.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef]
- Revelo, X.S.; Ghazarian, M.; Chng, M.H.Y.; Luck, H.; Kim, J.H.; Zeng, K.; Shi, S.Y.; Tsai, S.; Lei, H.; Kenkel, J.; et al. Nucleic Acid-Targeting Pathways Promote Inflammation in Obesity-Related Insulin Resistance. Cell Rep. 2016, 16, 717–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latorre, J.; Moreno-Navarrete, J.M.; Sabater, M.; Buxo, M.; Rodriguez-Hermosa, J.I.; Girones, J.; Fort, J.M.; Vilallonga, R.; Ricart, W.; Simo, R.; et al. Decreased TLR3 in Hyperplastic Adipose Tissue, Blood and Inflamed Adipocytes is Related to Metabolic Inflammation. Cell. Physiol. Biochem. 2018, 51, 1051–1068. [Google Scholar] [CrossRef]
- Maeda, K.; Akira, S. TLR7 Structure: Cut in Z-Loop. Immunity 2016, 45, 705–707. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Ohto, U.; Shibata, T.; Krayukhina, E.; Taoka, M.; Yamauchi, Y.; Tanji, H.; Isobe, T.; Uchiyama, S.; Miyake, K.; et al. Structural Analysis Reveals that Toll-like Receptor 7 Is a Dual Receptor for Guanosine and Single-Stranded RNA. Immunity 2016, 45, 737–748. [Google Scholar] [CrossRef] [Green Version]
- Thomalla, M.; Schmid, A.; Neumann, E.; Pfefferle, P.I.; Muller-Ladner, U.; Schaffler, A.; Karrasch, T. Evidence of an Anti-Inflammatory Toll-Like Receptor 9 (Tlr 9) Pathway in Adipocytes. J. Endocrinol. 2019, 240, 325–343. [Google Scholar] [CrossRef] [Green Version]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What Causes the Insulin Resistance Underlying Obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Porro, S.; Genchi, V.A.; Cignarelli, A.; Natalicchio, A.; Laviola, L.; Giorgino, F.; Perrini, S. Dysmetabolic Adipose Tissue in Obesity: Morphological and Functional Characteristics of Adipose Stem Cells and Mature Adipocytes in Healthy and Unhealthy Obese Subjects. J. Endocrinol. Investig. 2021, 44, 921–941. [Google Scholar] [CrossRef]
- Cariou, B. The Metabolic Triad of Non-Alcoholic Fatty Liver Disease, Visceral Adiposity and Type 2 Diabetes: Implications for Treatment. Diabetes Obes. Metab. 2022, 24 (Suppl. S2), 15–27. [Google Scholar] [CrossRef]
- Fillatreau, S.; Manfroi, B.; Dörner, T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020, 17, 98–108. [Google Scholar] [CrossRef]
- Abate, N.; Sallam, H.S.; Rizzo, M.; Nikolic, D.; Obradovic, M.; Bjelogrlic, P.; Isenovic, E.R. Resistin: An Inflammatory Cytokine. Role in Cardiovascular Diseases, Diabetes and the Metabolic Syndrome. Curr. Pharm. Des. 2014, 20, 4961–4969. [Google Scholar] [CrossRef] [PubMed]
- Denou, E.; Lolmede, K.; Garidou, L.; Pomie, C.; Chabo, C.; Lau, T.C.; Fullerton, M.D.; Nigro, G.; Zakaroff-Girard, A.; Luche, E.; et al. Defective Nod2 Peptidoglycan Sensing Promotes Diet-Induced Inflammation, Dysbiosis, and Insulin Resistance. EMBO Mol. Med. 2015, 7, 259–274. [Google Scholar] [CrossRef]
- Cavallari, J.F.; Fullerton, M.D.; Duggan, B.M.; Foley, K.P.; Denou, E.; Smith, B.K.; Desjardins, E.M.; Henriksbo, B.D.; Kim, K.J.; Tuinema, B.R.; et al. Muramyl Dipeptide-Based Postbiotics Mitigate Obesity-Induced Insulin Resistance via IRF4. Cell Metab. 2017, 25, 1063–1074.e3. [Google Scholar] [CrossRef]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef] [Green Version]
- Stienstra, R.; Joosten, L.A.; Koenen, T.; van Tits, B.; van Diepen, J.A.; van den Berg, S.A.; Rensen, P.C.N.; Voshol, P.J.; Fantuzzi, G.; Hijmans, A.; et al. The Inflammasome-Mediated Caspase-1 Activation Controls Adipocyte Differentiation and Insulin Sensitivity. Cell Metab. 2010, 12, 593–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govers, R. Molecular mechanisms of GLUT4 regulation in adipocytes. Diabetes Metab. 2014, 40, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Obesity Lipotoxicity 2017, 960, 327–343. [Google Scholar]
- Bai, Y.; Sun, Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015, 16, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Suganami, T.; Nishida, J.; Ogawa, Y. A Paracrine Loop between Adipocytes and Macrophages Aggravates Inflammatory Changes: Role of Free Fatty Acids and Tumor Necrosis Factor Alpha. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.S.; Impreso, S.; Lui, A.; Vidyarthi, G.; Albear, P.; Patel, N.A. Long Noncoding Rna Gas5 Contained in Exosomes Derived from Human Adipose Stem Cells Promotes Repair and Modulates Inflammation in a Chronic Dermal Wound Healing Model. Biology 2022, 11, 426. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. Mcp-1 Contributes to Macrophage Infiltration into Adipose Tissue, Insulin Resistance, and Hepatic Steatosis in Obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Wu, N.; Morsey, B.M.; Emanuel, K.M.; Fox, H.S. Sequence-specific extracellular microRNAs activate TLR7 and induce cytokine secretion and leukocyte migration. Mol. Cell. Biochem. 2021, 476, 4139–4151. [Google Scholar] [CrossRef]
- Green, H.; Kehinde, O. An Established Preadipose Cell Line and Its Differentiation in Culture. Ii. Factors Affecting the Adipose Conversion. Cell 1975, 5, 19–27. [Google Scholar] [CrossRef]
- Zaitsu, H.; Serrero, G. Pedersen fetuin contains three adipogenic factors with distinct biochemical characteristics. J. Cell. Physiol. 1990, 144, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, M.; Löffler, G. Adipogenic Activities in Commercial Preparations of Fetuin. Horm. Metab. Res. 1994, 26, 92–96. [Google Scholar] [CrossRef] [PubMed]
- MacDougald, O.A.; Lane, M.D. Transcriptional Regulation of Gene Expression During Adipocyte Differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J. Cell. Physiol. 1979, 101, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef]
- Cornelius, P.; MacDougald, O.A.; Lane, M.D. Regulation of Adipocyte Development. Annu. Rev. Nutr. 1994, 14, 99–129. [Google Scholar] [CrossRef]
- Hochberg, A.; Patz, M.; Karrasch, T.; Schäffler, A.; Schmid, A. Serum Levels and Adipose Tissue Gene Expression of Cathelicidin Antimicrobial Peptide (CAMP) in Obesity and During Weight Loss. Horm. Metab. Res. 2021, 53, 169–177. [Google Scholar] [CrossRef]
- Brock, J.; Schmid, A.; Karrasch, T.; Pfefferle, P.; Schlegel, J.; Busse, I.; Hauenschild, A.; Schmidt, B.; Koukou, M.; Arapogianni, E.; et al. Progranulin serum levels and gene expression in subcutaneous vs visceral adipose tissue of severely obese patients undergoing bariatric surgery. Clin. Endocrinol. 2019, 91, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Gehl, J.; Thomalla, M.; Hochberg, A.; Kreiß, A.; Patz, M.; Karrasch, T.; Schäffler, A. Downregulation of CTRP-3 by Weight Loss In Vivo and by Bile Acids and Incretins in Adipocytes In Vitro. Int. J. Mol. Sci. 2020, 21, 8168. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomalla, M.; Schmid, A.; Hehner, J.; Koehler, S.; Neumann, E.; Müller-Ladner, U.; Schäffler, A.; Karrasch, T. Toll-like Receptor 7 (TLR7) Is Expressed in Adipocytes and the Pharmacological TLR7 Agonist Imiquimod and Adipocyte-Derived Cell-Free Nucleic Acids (cfDNA) Regulate Adipocyte Function. Int. J. Mol. Sci. 2022, 23, 8475. https://doi.org/10.3390/ijms23158475
Thomalla M, Schmid A, Hehner J, Koehler S, Neumann E, Müller-Ladner U, Schäffler A, Karrasch T. Toll-like Receptor 7 (TLR7) Is Expressed in Adipocytes and the Pharmacological TLR7 Agonist Imiquimod and Adipocyte-Derived Cell-Free Nucleic Acids (cfDNA) Regulate Adipocyte Function. International Journal of Molecular Sciences. 2022; 23(15):8475. https://doi.org/10.3390/ijms23158475
Chicago/Turabian StyleThomalla, Miriam, Andreas Schmid, Julia Hehner, Sebastian Koehler, Elena Neumann, Ulf Müller-Ladner, Andreas Schäffler, and Thomas Karrasch. 2022. "Toll-like Receptor 7 (TLR7) Is Expressed in Adipocytes and the Pharmacological TLR7 Agonist Imiquimod and Adipocyte-Derived Cell-Free Nucleic Acids (cfDNA) Regulate Adipocyte Function" International Journal of Molecular Sciences 23, no. 15: 8475. https://doi.org/10.3390/ijms23158475
APA StyleThomalla, M., Schmid, A., Hehner, J., Koehler, S., Neumann, E., Müller-Ladner, U., Schäffler, A., & Karrasch, T. (2022). Toll-like Receptor 7 (TLR7) Is Expressed in Adipocytes and the Pharmacological TLR7 Agonist Imiquimod and Adipocyte-Derived Cell-Free Nucleic Acids (cfDNA) Regulate Adipocyte Function. International Journal of Molecular Sciences, 23(15), 8475. https://doi.org/10.3390/ijms23158475






