Relaxin Affects Airway Remodeling Genes Expression through Various Signal Pathways Connected with Transcription Factors
Abstract
1. Introduction
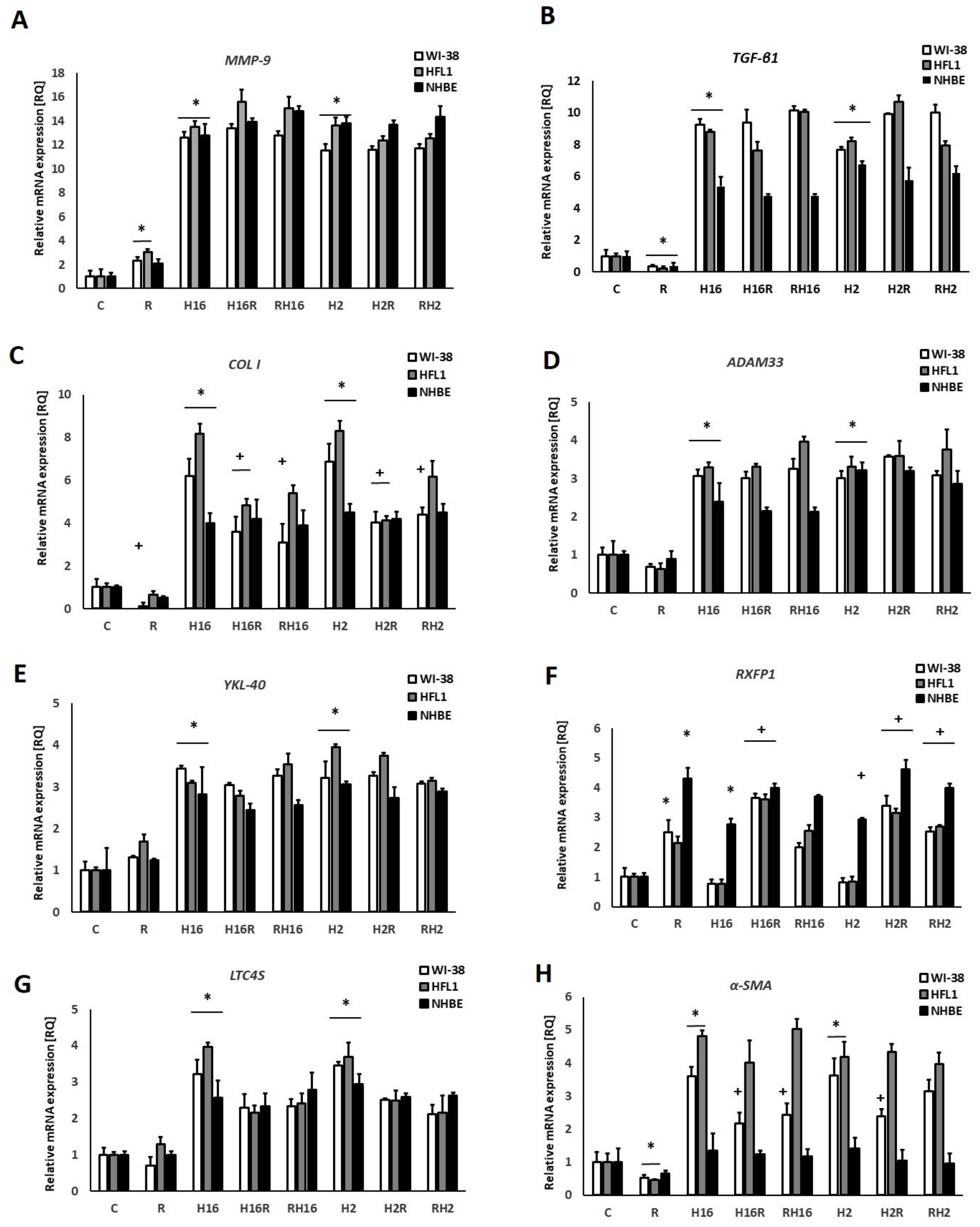
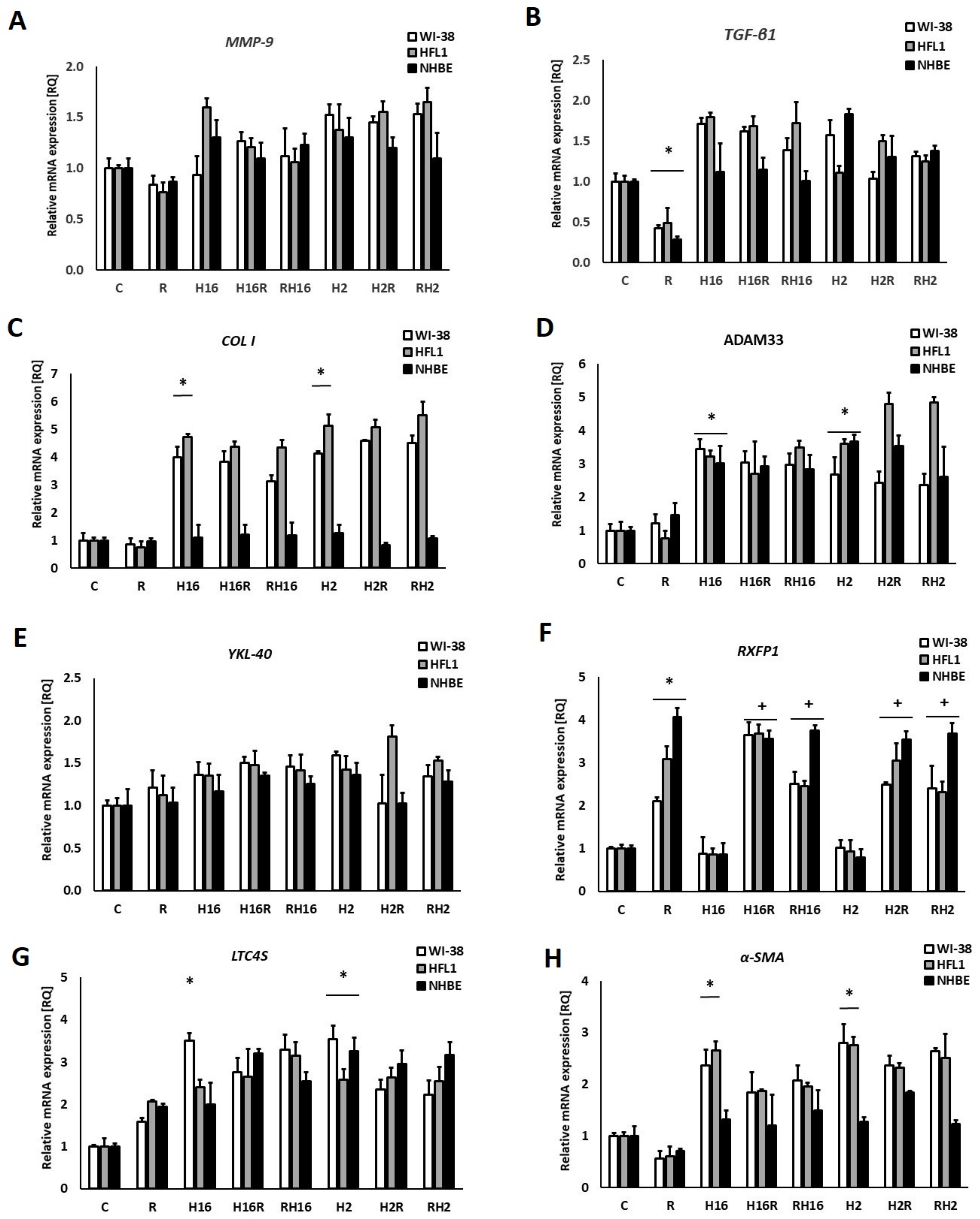
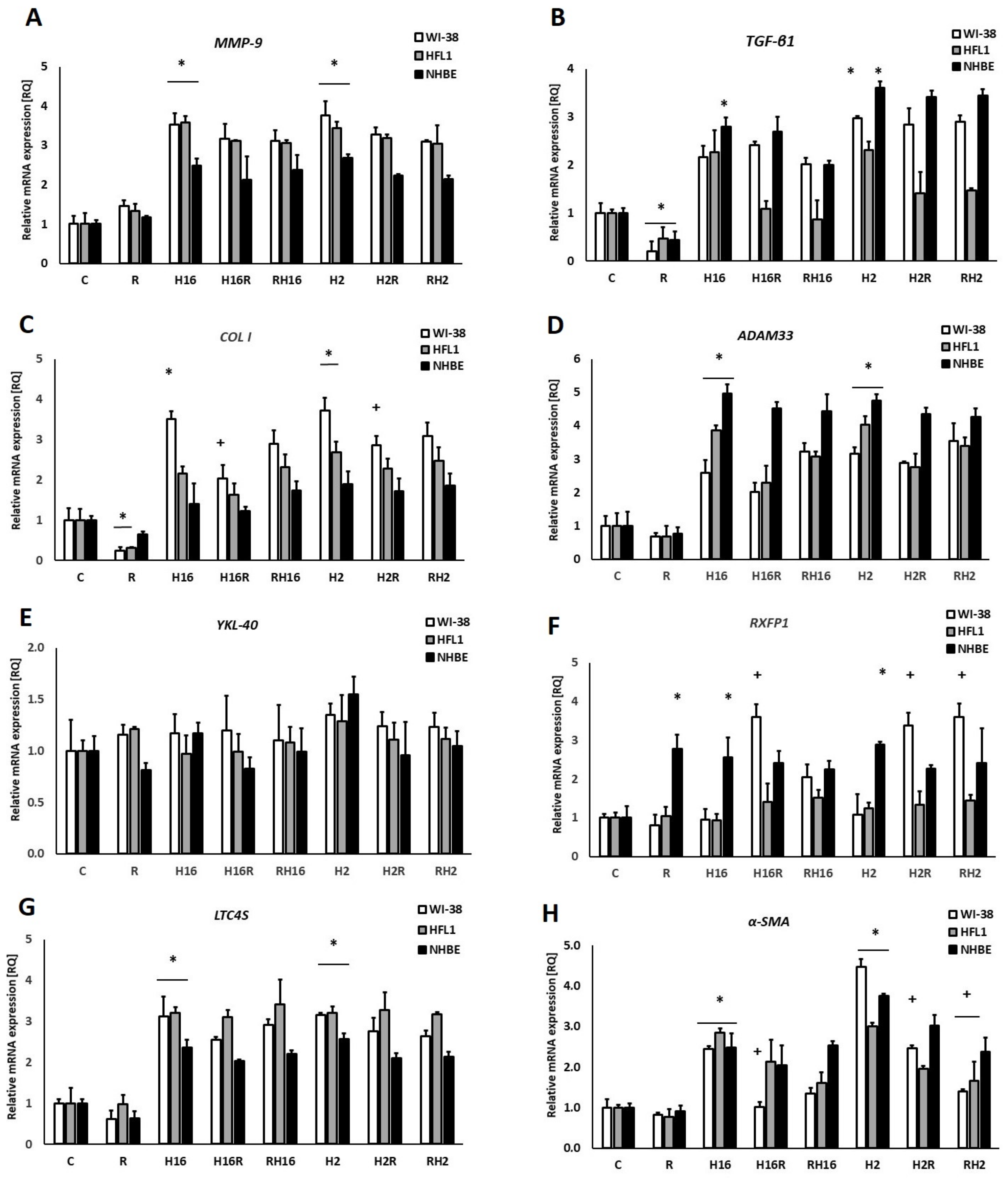
2. Results
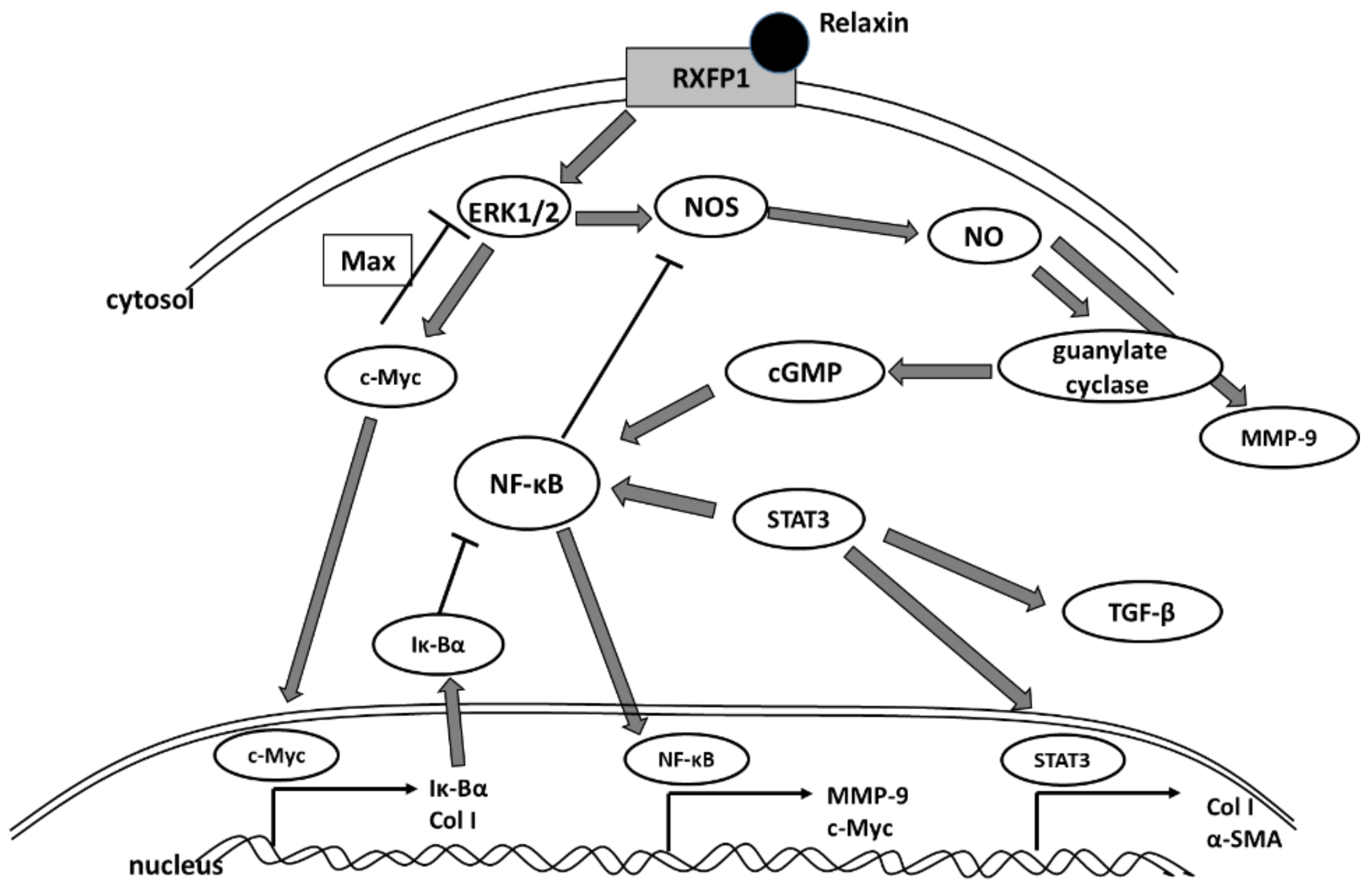
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Virus Preparation and Cell Infection
4.3. Experimental Procedure
4.4. RNA Isolation and cDNA Synthesis
4.5. Gene Expression Analysis
4.6. Protein Isolation and Immunoblotting
4.7. siRNA Silencing of Transcription Factors
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Derseh, H.B.; Dewage, S.N.V.; Perera, U.E.; Koumoundouros, E.; Pagel, C.N.; Organ, L.; Snibson, K.J. Small airway remodeling in a sheep model of bleomycin-induced pulmonary fibrosis. Exp. Lung. Res. 2020, 46, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, H.; Shimokata, K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr. Drug Targets Inflamm. Allergy 2005, 4, 177–181. [Google Scholar] [CrossRef] [PubMed]
- XuChen, X.; Weinstock, J.; Arroyo, M.; Salka, K.; Chorvinsky, E.; Abutaleb, K.; Aguilar, H.; Kahanowitch, R.; Rodríguez-Martínez, C.E.; Perez, G.F.; et al. Airway Remodeling Factors during Early-Life Rhinovirus Infection and the Effect of Premature Birth. Front. Pediatr. 2021, 9, 610478. [Google Scholar] [CrossRef] [PubMed]
- Royce, S.G.; Miao, Y.R.; Lee, M.; Samuel, C.S.; Tregear, G.W.; Tang, M.L.K. Relaxin reverses airway remodeling and airway dysfunction in allergic airways disease. Endocrinology 2009, 150, 2692–2699. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Ishioka, T.; Noda, M.; Kozawa, K.; Kimura, H. Molecular epidemiology of respiratory viruses in virus-induced asthma. Front. Microbiol. 2013, 4, 278. [Google Scholar] [CrossRef]
- Wark, P.A.; Johnston, S.L.; Moric, I.; Simpson, J.; Hensley, M.; Gibson, P. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur. Respir. J. 2002, 19, 68–75. [Google Scholar] [CrossRef]
- Heymann, P.W.; Carper, H.T.; Murphy, D.D.; Platts-Mills, T.A.; Patrie, J.; McLaughlin, A.P.; Erwin, E.A.; Shaker, M.S.; Hellems, M.; Peerzada, J.; et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J. Allergy Clin. Immunol. 2004, 114, 239–247. [Google Scholar] [CrossRef]
- Bochynska, A.I.; Hannink, G.; Verhoeven, R.; Grijpma, D.W.; Buma, P. The effect of tissue surface modification with collagenase and addition of TGF-beta3 on the healing potential of meniscal tears repaired with tissue glues in vitro. J. Mater. Sci. Mater. Med. 2017, 28, 22. [Google Scholar] [CrossRef]
- Kuo, C.; Lim, S.; King, N.J.; Bartlett, N.W.; Walton, R.P.; Zhu, J.; Glanville, N.; Aniscenko, J.; Johnston, S.L.; Burgess, J.K.; et al. Rhinovirus infection induces expression of airway remodelling factors in vitro and in vivo. Respirology 2011, 16, 367–377. [Google Scholar] [CrossRef]
- Skevaki, C.L.; Psarras, S.; Volonaki, E.; Pratsinis, H.; Spyridaki, I.S.; Gaga, M.; Georgiou, V.; Vittorakis, S.; Telcian, A.G.; Maggina, P.; et al. Rhinovirus-induced basic fibroblast growth factor release mediates airway remodeling features. Clin. Transl. Allergy 2012, 2, 14. [Google Scholar] [CrossRef]
- Du, X.J.; Bathgate, R.A.D.; Samuel, C.S.; Dart, A.M.; Summers, R.J. Cardiovascular effects of relaxin: From basic science to clinical therapy. Nat. Rev. Cardiol. 2010, 7, 48–58. [Google Scholar] [CrossRef]
- Samuel, C.S.; Cendrawan, S.; Gao, X.-M.; Ming, Z.; Zhao, C.; Kiriazis, H.; Xu, Q.; Tregear, G.W.; Bathgate, R.; Du, X.-J. Relaxin remodels fibrotic healing following myocardial infarction. Lab. Investig. 2011, 91, 675–690. [Google Scholar] [CrossRef]
- Mookerjee, I.; Solly, N.R.; Royce, S.G.; Tregear, G.W.; Samuel, C.S.; Tang, M.L.K. Endogenous relaxin regulates collagen deposition in an animal model of allergic airway disease. Endocrinology 2006, 147, 754–761. [Google Scholar] [CrossRef][Green Version]
- Kenyon, N.J.; Ward, R.W.; Last, J.A. Airway fibrosis in a mouse model of airway inflammation. Toxicol. Appl. Pharmacol. 2003, 186, 90–100. [Google Scholar] [CrossRef]
- Samuel, C.S.; Hewitson, T.D.; Unemori, E.N.; Tang, M.L.-K. Drugs of the future: The hormone relaxin. Cell. Mol. Life Sci. 2007, 64, 1539–1557. [Google Scholar] [CrossRef]
- Bathgate, R.; Halls, M.; Van Der Westhuizen, E.T.; Callander, G.E.; Kocan, M.; Summers, R. Relaxin family peptides and their receptors. Physiol. Rev. 2013, 93, 405–480. [Google Scholar] [CrossRef]
- Tan, J.; Tedrow, J.R.; Dutta, J.A.; Juan-Guardela, B.; Nouraie, M.; Chu, Y.; Bittar, H.T.; Ramani, K.; Biswas, P.S.; Veraldi, K.L.; et al. Expression of RXFP1 Is Decreased in Idiopathic Pulmonary Fibrosis. Implications for Relaxin-based Therapies. Am. J. Respir. Crit. Care Med. 2016, 194, 1392–1402. [Google Scholar] [CrossRef]
- Kanai, A.J.; Konieczko, E.M.; Bennett, R.G.; Samuel, C.S.; Royce, S.G. Relaxin and fibrosis: Emerging targets, challenges, and future directions. Mol. Cell. Endocrinol. 2019, 487, 66–74. [Google Scholar] [CrossRef]
- Samuel, C.S.; Bennett, R.G. Relaxin as an anti-fibrotic treatment: Perspectives, challenges and future directions. Biochem. Pharmacol. 2022, 197, 114884. [Google Scholar] [CrossRef]
- Pinar, A.A.; Yuferov, A.; Gaspari, T.A.; Samuel, C.S. Relaxin Can Mediate Its Anti-Fibrotic Effects by Targeting the Myofibroblast NLRP3 Inflammasome at the Level of Caspase-1. Front. Pharmacol. 2020, 11, 1201. [Google Scholar] [CrossRef]
- Tully, J.E.; Hoffman, S.M.; Lahue, K.G.; Nolin, J.D.; Anathy, V.; Lundblad, L.K.; Daphtary, N.; Aliyeva, M.; Black, K.E.; Dixon, A.E.; et al. Epithelial NF-kappaB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling. J. Immunol. 2013, 191, 5811–5821. [Google Scholar] [CrossRef]
- Sun, Z.; Ji, N.; Ma, Q.; Zhu, R.; Chen, Z.; Wang, Z.; Qian, Y.; Wu, C.; Hu, F.; Huang, M.; et al. Epithelial-Mesenchymal Transition in Asthma Airway Remodeling Is Regulated by the IL-33/CD146 Axis. Front. Immunol. 2020, 11, 1598. [Google Scholar] [CrossRef]
- Cao, L.; Liu, F.; Liu, Y.; Liu, T.; Wu, J.; Zhao, J.; Wang, J.; Li, S.; Xu, J.; Dong, L. TSLP promotes asthmatic airway remodeling via p38-STAT3 signaling pathway in human lung fibroblast. Exp. Lung. Res. 2018, 44, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Frey, U. Airway remodeling: Shifting the trigger point for exacerbations in asthma. J. Allergy Clin. Immunol. 2021, 148, 710–712. [Google Scholar] [CrossRef]
- Yamauchi, K.; Inoue, H. Airway remodeling in asthma and irreversible airflow limitation-ECM deposition in airway and possible therapy for remodeling. Allergol. Int. 2007, 56, 321–329. [Google Scholar] [CrossRef]
- Wieczfinska, J.; Sitarek, P.; Kowalczyk, T.; Rieske, P.; Pawliczak, R. Curcumin modulates airway remodelling-contributing genes-the significance of transcription factors. J. Cell. Mol. Med. 2022, 26, 736–749. [Google Scholar] [CrossRef]
- Wieczfinska, J.; Pawliczak, R. Thymic stromal lymphopoietin and apocynin alter the expression of airway remodeling factors in human rhinovirus-infected cells. Immunobiology 2017, 222, 892–899. [Google Scholar] [CrossRef]
- Wieczfinska, J.; Sitarek, P.; Kowalczyk, T.; Pawliczak, R. Leonurus sibiricus root extracts decrease airway remodeling markers expression in fibroblasts. Clin. Exp. Immunol. 2020, 202, 28–46. [Google Scholar] [CrossRef]
- Mehta, A.K.; Doherty, T.; Broide, D.; Croft, M. Tumor necrosis factor family member LIGHT acts with IL-1beta and TGF-beta to promote airway remodeling during rhinovirus infection. Allergy 2018, 73, 1415–1424. [Google Scholar] [CrossRef]
- Yu, F.; Sun, Y.; Yu, J.; Ding, Z.; Wang, J.; Zhang, L.; Zhang, T.; Bai, Y.; Wang, Y. ORMDL3 is associated with airway remodeling in asthma via the ERK/MMP-9 pathway. Mol. Med. Rep. 2017, 15, 2969–2976. [Google Scholar] [CrossRef]
- Liu, P.; Wilson, M.J. miR-520c and miR-373 upregulate MMP9 expression by targeting mTOR and SIRT1, and activate the Ras/Raf/MEK/Erk signaling pathway and NF-kappaB factor in human fibrosarcoma cells. J. Cell. Physiol. 2012, 227, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tang, W.; Gwaltney, J.M., Jr.; Wu, Y.; Elias, J.A. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: Role of NF-kappaB. Am. J. Physiol. 1997, 273, L814–L824. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.Y.; Yan, W.; Bagnell, C.A. Relaxin-induced matrix metalloproteinase-9 expression is associated with activation of the NF-kappaB pathway in human THP-1 cells. J. Leukoc. Biol. 2007, 81, 1303–1310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karin, M.; Greten, F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Wiehler, S.; Proud, D. Interferon regulatory factor 7 regulates airway epithelial cell responses to human rhinovirus infection. BMC Genom. 2016, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Han, F.; Gong, L.; Lv, Y.; Wan, Z.; Liu, H.; Ren, L.; Yang, S.; Zhang, W.; Li, T.; et al. Ceramide induces MMP-9 expression through JAK2/STAT3 pathway in airway epithelium. Lipids Health Dis. 2020, 19, 196. [Google Scholar] [CrossRef]
- Zheng, L.; Dong, H.; Zhao, W.; Zhang, X.; Duan, X.; Zhang, H.; Liu, S.; Sui, G. An Air-Liquid Interface Organ-Level Lung Microfluidics Platform for Analysis on Molecular Mechanisms of Cytotoxicity Induced by Cancer-Causing Fine Particles. ACS Sens. 2019, 4, 907–917. [Google Scholar] [CrossRef]
- Moore-Smith, L.D.; Isayeva, T.; Lee, J.H.; Frost, A.; Ponnazhagan, S. Silencing of TGF-beta1 in tumor cells impacts MMP-9 in tumor microenvironment. Sci. Rep. 2017, 7, 8678. [Google Scholar] [CrossRef]
- Lin, S.C.; Chou, H.C.; Chen, C.M.; Chiang, B.L. Anti-thymic stromal lymphopoietin antibody suppresses airway remodeling in asthma through reduction of MMP and CTGF. Pediatr. Res. 2019, 86, 181–187. [Google Scholar] [CrossRef]
- Perng, D.W.; Chang, K.T.; Su, K.C.; Wu, Y.C.; Chen, C.S.; Hsu, W.H.; Tsai, C.M.; Lee, Y.C. Matrix metalloprotease-9 induces transforming growth factor-beta(1) production in airway epithelium via activation of epidermal growth factor receptors. Life Sci. 2011, 89, 204–212. [Google Scholar] [CrossRef]
- Pedroza, M.; To, S.; Assassi, S.; Wu, M.; Tweardy, D.; Agarwal, S.K. Role of STAT3 in skin fibrosis and transforming growth factor beta signalling. Rheumatology 2018, 57, 1838–1850. [Google Scholar] [CrossRef]
- Kasembeli, M.M.; Bharadwaj, U.; Robinson, P.; Tweardy, D.J. Contribution of STAT3 to Inflammatory and Fibrotic Diseases and Prospects for its Targeting for Treatment. Int. J. Mol. Sci. 2018, 19, 2299. [Google Scholar] [CrossRef]
- Prêle, C.M.; Yao, E.; O’Donoghue, R.J.; Mutsaers, S.E.; Knight, D.A. STAT3, a central mediator of pulmonary fibrosis? Proc. Am. Thorac. Soc. 2012, 9, 177–182. [Google Scholar] [CrossRef]
- Unemori, E.N.; Pickford, L.B.; Salles, A.L.; Piercy, C.E.; Grove, B.H.; Erikson, M.E.; Amento, E.P. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J. Clin. Investig. 1996, 98, 2739–2745. [Google Scholar] [CrossRef]
- Ko, J.H.; Kang, Y.M.; Yang, J.-H.; Kim, J.S.; Lee, W.J.; Kim, S.H.; Yang, I.H.; Moon, S.H. Regulation of MMP and TIMP expression in synovial fibroblasts from knee osteoarthritis with flexion contracture using adenovirus-mediated relaxin gene therapy. Knee 2019, 26, 317–329. [Google Scholar] [CrossRef]
- Ahmad, N.; Wang, W.; Nair, R.; Kapila, S. Relaxin induces matrix-metalloproteinases-9 and -13 via RXFP1, induction of MMP-9 involves the PI3K, ERK, Akt and PKC-zeta pathways. Mol. Cell. Endocrinol. 2012, 363, 46–61. [Google Scholar] [CrossRef]
- Ng, H.H.; Shen, M.; Samuel, C.S.; Schlossmann, J.; Bennett, R.G. Relaxin and extracellular matrix remodeling: Mechanisms and signaling pathways. Mol. Cell. Endocrinol. 2019, 487, 59–65. [Google Scholar] [CrossRef]
- Michalik, M.; Wójcik-Pszczoła, K.; Paw, M.; Wnuk, D.; Koczurkiewicz, P.; Sanak, M.; Pękala, E.; Madeja, Z. Fibroblast-to-myofibroblast transition in bronchial asthma. Cell. Mol. Life Sci. 2018, 75, 3943–3961. [Google Scholar] [CrossRef]
- Unemori, E.N.; Amento, E.P. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J. Biol. Chem. 1990, 265, 10681–10685. [Google Scholar] [CrossRef]
- Bennett, R.G.; Kharbanda, K.K.; Tuma, D.J. Inhibition of markers of hepatic stellate cell activation by the hormone relaxin. Biochem. Pharmacol. 2003, 66, 867–874. [Google Scholar] [CrossRef][Green Version]
- Naqvi, T.; Duong, T.T.; Hashem, G.; Shiga, M.; Zhang, Q.; Kapila, S. Relaxin’s induction of metalloproteinases is associated with the loss of collagen and glycosaminoglycans in synovial joint fibrocartilaginous explants. Arthritis Res. Ther. 2005, 7, R1–R11. [Google Scholar] [CrossRef]
- Williams, E.J.; Benyon, R.C.; Trim, N.; Hadwin, R.; Grove, B.H.; Arthur, M.J.P.; Unemori, E.N.; Iredale, J.P. Relaxin inhibits effective collagen deposition by cultured hepatic stellate cells and decreases rat liver fibrosis in vivo. Gut 2001, 49, 577–583. [Google Scholar] [CrossRef][Green Version]
- Unemori, E.N.; Beck, L.S.; Lee, W.P.; Xu, Y.; Siegel, M.; Keller, G.; Liggitt, H.D.; Bauer, E.A.; Amento, E.P. Human relaxin decreases collagen accumulation in vivo in two rodent models of fibrosis. J. Investig. Dermatol. 1993, 101, 280–285. [Google Scholar] [CrossRef]
- Papaioannou, I.; Xu, S.; Denton, C.P.; Abraham, D.J.; Ponticos, M. STAT3 controls COL1A2 enhancer activation cooperatively with JunB, regulates type I collagen synthesis posttranscriptionally, and is essential for lung myofibroblast differentiation. Mol. Biol. Cell. 2018, 29, 84–95. [Google Scholar] [CrossRef]
- Jin, A.; Tang, X.; Zhai, W.; Li, Y.; Sun, Q.; Liu, L.; Yang, X.; Ren, H.; Lu, S. TSLP-induced collagen type-I synthesis through STAT3 and PRMT1 is sensitive to calcitriol in human lung fibroblasts. Biochim. Biophys. Acta Mol. Cell. Res. 2021, 1868, 119083. [Google Scholar] [CrossRef]
- Han, M.; Bentley, J.K.; Rajput, C.; Lei, J.; Ishikawa, T.; Jarman, C.R.; Lee, J.; Goldsmith, A.M.; Jackson, W.T.; Hoenerhoff, M.J.; et al. Inflammasome activation is required for human rhinovirus-induced airway inflammation in naive and allergen-sensitized mice. Mucosal. Immunol. 2019, 12, 958–968. [Google Scholar] [CrossRef]
- Gasse, P.; Riteau, N.; Pétrilli, V.; Tschopp, J.; Lagente, V.; Quesniaux, V.F.J.; Ryffel, B.; Couillin, I. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2009, 179, 903–913. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NFk B Family of Transcription Factors and Its Regulation. Cold Spring Harb Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Kuang, J.; Xie, M.; Wei, X. The NALP3 inflammasome is required for collagen synthesis via the NFkappaB pathway. Int. J. Mol. Med. 2018, 41, 2279–2287. [Google Scholar]
- Martin, B.; Gabris-Weber, B.A.; Reddy, R.; Romero, G.; Chattopadhyay, A.; Salama, G. Relaxin reverses inflammatory and immune signals in aged hearts. PLoS ONE 2018, 13, e0190935. [Google Scholar] [CrossRef]
- Valle Raleigh, J.; Mauro, A.G.; Devarakonda, T.; Marchetti, C.; He, J.; Kim, E.; Filippone, S.; Das, A.; Toldo, S.; Abbate, A.; et al. Reperfusion therapy with recombinant human relaxin-2 (Serelaxin) attenuates myocardial infarct size and NLRP3 inflammasome following ischemia/reperfusion injury via eNOS-dependent mechanism. Cardiovasc. Res. 2017, 113, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdewegh, P.; Little, R.D.; Dupuis, J.; Del Mastro, R.G.; Falls, K.; Simon, J.; Torrey, D.; Pandit, S.; McKenny, J.; Braunschweiger, K.; et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature 2002, 418, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.D.; Postma, D.S.; Jongepier, H.; Moore, W.C.; Koppelman, G.H.; Zheng, S.L.; Xu, J.; Bleecker, E.R.; Meyers, D.A. Association of a disintegrin and metalloprotease 33 (ADAM33) gene with asthma in ethnically diverse populations. J. Allergy Clin. Immunol. 2003, 112, 717–722. [Google Scholar] [CrossRef]
- Konradsen, J.R.; James, A.; Nordlund, B.; Reinius, L.E.; Söderhäll, C.; Melén, E.; Wheelock, A.M.; Lödrup Carlsen, K.C.; Lidegran, M.; Verhoek, M.; et al. The chitinase-like protein YKL-40, a possible biomarker of inflammation and airway remodeling in severe pediatric asthma. J. Allergy Clin. Immunol. 2013, 132, 328–335.e5. [Google Scholar] [CrossRef]
- Bara, I.; Ozier, A.; Girodet, P.-O.; Carvalho, G.; Cattiaux, J.; Begueret, H.; Thumerel, M.; Ousova, O.; Kolbeck, R.; Coyle, A.J.; et al. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. Am. J. Respir. Crit. Care Med. 2012, 185, 715–722. [Google Scholar] [CrossRef]
- Furuhashi, K.; Suda, T.; Nakamura, Y.; Inui, N.; Hashimoto, D.; Miwa, S.; Hayakawa, H.; Kusagaya, H.; Nakano, Y.; Nakamura, H.; et al. Increased expression of YKL-40, a chitinase-like protein, in serum and lung of patients with idiopathic pulmonary fibrosis. Respir. Med. 2010, 104, 1204–1210. [Google Scholar] [CrossRef]
- Corallo, C.; Pinto, A.M.; Renieri, A.; Cheleschi, S.; Fioravanti, A.; Cutolo, M.; Soldano, S.; Nuti, R.; Giordano, N. Altered expression of RXFP1 receptor contributes to the inefficacy of relaxin-based anti-fibrotic treatments in systemic sclerosis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 119), 69–75. [Google Scholar]
- Chen, T.Y.; Li, X.; Goobie, G.C.; Hung, C.-H.; Hung, T.-K.; Hamilton, K.; Bahudhanapati, H.; Tan, J.; Kass, D.J.; Zhang, Y. Identification of a distal RXFP1 gene enhancer with differential activity in fibrotic lung fibroblasts involving AP-1. PLoS ONE 2021, 16, e0254466. [Google Scholar] [CrossRef]
- Chen, T.X.; Li, X.; Hung, C.; Bahudhanapati, H.; Tan, J.; Kass, D.J.; Zhang, Y. The relaxin family peptide receptor 1 (RXFP1): An emerging player in human health and disease. Mol. Genet. Genom. Med. 2020, 8, e1194. [Google Scholar] [CrossRef]
- Chow, B.S.; Chew, E.G.; Zhao, C.; Bathgate, R.A.; Hewitson, T.D.; Samuel, C.S. Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: The additional involvement of iNOS. PLoS ONE 2012, 7, e42714. [Google Scholar] [CrossRef]
- Mookerjee, I.; Hewitson, T.D.; Halls, M.L.; Summers, R.J.; Mathai, M.L.; Bathgate, R.A.; Tregear, G.W.; Samuel, C.S. Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2. FASEB J. 2009, 23, 1219–1229. [Google Scholar] [CrossRef]
- Wilhelmi, T.; Xu, X.; Tan, X.; Hulshoff, M.S.; Maamari, S.; Sossalla, S.; Zeisberg, M.; Zeisberg, E.M. Serelaxin alleviates cardiac fibrosis through inhibiting endothelial-to-mesenchymal transition via RXFP1. Theranostics 2020, 10, 3905–3924. [Google Scholar] [CrossRef]
- Royce, S.G.; Bathgate, R.A.D.; Samuel, C.S. Promise and Limitations of Relaxin-based Therapies in Chronic Fibrotic Lung Diseases. Am. J. Respir. Crit. Care Med. 2016, 194, 1434–1435. [Google Scholar] [CrossRef]
- Royce, S.G.; Sedjahtera, A.; Samuel, C.S.; Tang, M.L.K. Combination therapy with relaxin and methylprednisolone augments the effects of either treatment alone in inhibiting subepithelial fibrosis in an experimental model of allergic airways disease. Clin. Sci. 2013, 124, 41–51. [Google Scholar] [CrossRef]
- Tang, M.L.K.; Samuel, C.S.; Royce, S. Role of relaxin in regulation of fibrosis in the lung. Ann. N. Y. Acad. Sci. 2009, 1160, 342–347. [Google Scholar] [CrossRef]
- Herro, R.; Miki, H.; Sethi, G.S.; Mills, D.; Mehta, A.K.; Nguyen, X.-X.; Feghali-Bostwick, C.; Miller, M.; Broide, D.H.; Soloff, R.; et al. TL1A Promotes Lung Tissue Fibrosis and Airway Remodeling. J. Immunol. 2020, 205, 2414–2422. [Google Scholar] [CrossRef]
- O’Dwyer, D.N.; Moore, B.B. The role of periostin in lung fibrosis and airway remodeling. Cell. Mol. Life Sci. 2017, 74, 4305–4314. [Google Scholar] [CrossRef]
- Royce, S.G.; Cheng, V.; Samuel, C.S.; Tang, M.L. The regulation of fibrosis in airway remodeling in asthma. Mol. Cell. Endocrinol. 2012, 351, 167–175. [Google Scholar] [CrossRef]
- Lam, M.; Royce, S.G.; Samuel, C.S.; Bourke, J.E. Serelaxin as a novel therapeutic opposing fibrosis and contraction in lung diseases. Pharmacol. Ther. 2018, 187, 61–70. [Google Scholar] [CrossRef]
- Masterson, R.; Hewitson, T.D.; Kelynack, K.; Martic, M.; Parry, L.; Bathgate, R.; Darby, I.; Becker, G. Relaxin down-regulates renal fibroblast function and promotes matrix remodelling in vitro. Nephrol. Dial. Transplant. 2004, 19, 544–552. [Google Scholar] [CrossRef]
- Nielsen, S.H.; Willumsen, N.; Leeming, D.J.; Daniels, S.J.; Brix, S.; Karsdal, M.A.; Genovese, F.; Nielsen, M.J. Serological Assessment of Activated Fibroblasts by alpha-Smooth Muscle Actin (alpha-SMA): A Noninvasive Biomarker of Activated Fibroblasts in Lung Disorders. Transl. Oncol. 2019, 12, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Gharaee-Kermani, M.; Zhang, K.; Karmiol, S.; Phan, S.H. Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 1996, 148, 527–537. [Google Scholar] [PubMed]
- Ina, K.; Kitamura, H.; Tatsukawa, S.; Fujikura, Y. Significance of alpha-SMA in myofibroblasts emerging in renal tubulointerstitial fibrosis. Histol. Histopathol. 2011, 26, 855–866. [Google Scholar] [PubMed]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell. 2001, 12, 2730–2741. [Google Scholar] [CrossRef]
- Shen, Y.; Miao, N.; Wang, B.; Xu, J.; Gan, X.; Xu, D.; Zhou, L.; Xue, H.; Zhang, W.; Yang, L.; et al. c-Myc promotes renal fibrosis by inducing integrin alphav-mediated transforming growth factor-beta signaling. Kidney Int. 2017, 92, 888–899. [Google Scholar] [CrossRef]
- Rosch, R.; Binnebösel, M.; Junge, K.; Lynen-Jansen, P.; Mertens, P.R.; Klinge, U.; Schumpelick, V. Analysis of c-myc, PAI-1 and uPAR in patients with incisional hernias. Hernia 2008, 12, 285–288. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Shariff, S.; Shelfoon, C.; Holden, N.S.; Traves, S.L.; Wiehler, S.; Kooi, C.; Proud, D.; Leigh, R. Human Rhinovirus Infection of Epithelial Cells Modulates Airway Smooth Muscle Migration. Am. J. Respir. Cell Mol. Biol. 2017, 56, 796–803. [Google Scholar] [CrossRef]
- Bennett, R.G. Relaxin and its role in the development and treatment of fibrosis. Transl. Res. 2009, 154, 1–6. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, L.; Wang, M.; Carr, B.I. Phosphorylation regulates Myc expression via prolonged activation of the mitogen-activated protein kinase pathway. J. Cell. Physiol. 2006, 208, 133–140. [Google Scholar] [CrossRef]
- Yoshida, G.J. Emerging roles of Myc in stem cell biology and novel tumor therapies. J. Exp. Clin. Cancer Res. 2018, 37, 173. [Google Scholar] [CrossRef]
- De las Heras, B.; Navarro, A.; Díaz-Guerra, M.J.; Bermejo, P.; Castrillo, A.; Boscá, L.; Villar, A. Inhibition of NOS-2 expression in macrophages through the inactivation of NF-kappaB by andalusol. Br. J. Pharmacol. 1999, 128, 605–612. [Google Scholar] [CrossRef]
- Tang, L.Y.; Heller, M.; Meng, Z.; Yu, L.R.; Tang, Y.; Zhou, M.; Zhang, Y.E. Transforming Growth Factor-beta (TGF-beta) Directly Activates the JAK1-STAT3 Axis to Induce Hepatic Fibrosis in Coordination with the SMAD Pathway. J. Biol. Chem. 2017, 292, 4302–4312. [Google Scholar] [CrossRef]
- Liu, R.Y.; Zeng, Y.; Lei, Z.; Wang, L.; Yang, H.; Liu, Z.; Zhao, J.; Zhang, H.T. JAK/STAT3 signaling is required for TGF-beta-induced epithelial-mesenchymal transition in lung cancer cells. Int. J. Oncol. 2014, 44, 1643–1651. [Google Scholar] [CrossRef]
- Kanoh, H.; Iwashita, S.; Kuraishi, T.; Goto, A.; Fuse, N.; Ueno, H.; Nimura, M.; Oyama, T.; Tang, C.; Watanabe, R.; et al. cGMP signaling pathway that modulates NF-kappaB activation in innate immune responses. iScience 2021, 24, 103473. [Google Scholar] [CrossRef]
- Luu, V.V.; Hanatate, K.; Tanihara, F.; Sato, Y.; Do, L.T.; Taniguchi, M.; Otoi, T. The effect of relaxin supplementation of in vitro maturation medium on the development of cat oocytes obtained from ovaries stored at 4 degrees C. Reprod. Biol. 2013, 13, 122–126. [Google Scholar] [CrossRef]
- Tanaka, M.; Osanai, T.; Murakami, R.; Sasaki, S.; Tomita, H.; Maeda, N.; Satoh, K.; Magota, K.; Okumura, K. Effect of vasoconstrictor coupling factor 6 on gene expression profile in human vascular endothelial cells: Enhanced release of asymmetric dimethylarginine. J. Hypertens. 2006, 24, 489–497. [Google Scholar] [CrossRef]
- Nistri, S.; Mannelli, L.D.C.; Ghelardini, C.; Zanardelli, M.; Bani, D.; Failli, P. Pretreatment with Relaxin Does Not Restore NO-Mediated Modulation of Calcium Signal in Coronary Endothelial Cells Isolated from Spontaneously Hypertensive Rats. Molecules 2015, 20, 9524–9535. [Google Scholar] [CrossRef]
- Feiteng, C.; Lei, C.; Deng, L.; Chaoliang, X.; Zijie, X.; Yi, S.; Minglei, S. Relaxin inhibits renal fibrosis and the epithelial-to-mesenchymal transition via the Wnt/beta-catenin signaling pathway. Ren Fail. 2022, 44, 513–524. [Google Scholar] [CrossRef]
- Radestock, Y.; Hoang-Vu, C.; Hombach-Klonisch, S. Relaxin reduces xenograft tumour growth of human MDA-MB-231 breast cancer cells. Breast Cancer Res. 2008, 10, R71. [Google Scholar] [CrossRef]
- Tian, B.; Patrikeev, I.; Ochoa, L.; Vargas, G.; Belanger, K.K.; Litvinov, J.; Boldogh, I.; Ameredes, B.T.; Motamedi, M.; Brasier, A.R. NF-kappaB Mediates Mesenchymal Transition, Remodeling, and Pulmonary Fibrosis in Response to Chronic Inflammation by Viral RNA Patterns. Am. J. Respir. Cell. Mol. Biol. 2017, 56, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Schuliga, M. NF-kappaB Signaling in Chronic Inflammatory Airway Disease. Biomolecules 2015, 5, 1266–1283. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, G.; Chen, F.; Ding, Y.; Wang, T.; Liu, S.; Lu, W.; Xu, W.; Flores, J.; Ocak, U.; et al. Rh-relaxin-2 attenuates degranulation of mast cells by inhibiting NF-kappaB through PI3K-AKT/TNFAIP3 pathway in an experimental germinal matrix hemorrhage rat model. J. Neuroinflamm. 2020, 17, 250. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.Y.; Santora, K.; Chen, J.C.; Frankshun, A.L.; Bagnell, C.A. Effects of relaxin and estrogens on bone remodeling markers, receptor activator of NF-kB ligand (RANKL) and osteoprotegerin (OPG), in rat adjuvant-induced arthritis. Bone 2011, 48, 1346–1353. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Han, X.; Li, Y.; Zhao, X.; Sheng, L.; Li, Y. Relaxin alleviates TGFbeta1-induced cardiac fibrosis via inhibition of Stat3-dependent autophagy. Biochem. Biophys. Res. Commun. 2017, 493, 1601–1607. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczfinska, J.; Pawliczak, R. Relaxin Affects Airway Remodeling Genes Expression through Various Signal Pathways Connected with Transcription Factors. Int. J. Mol. Sci. 2022, 23, 8413. https://doi.org/10.3390/ijms23158413
Wieczfinska J, Pawliczak R. Relaxin Affects Airway Remodeling Genes Expression through Various Signal Pathways Connected with Transcription Factors. International Journal of Molecular Sciences. 2022; 23(15):8413. https://doi.org/10.3390/ijms23158413
Chicago/Turabian StyleWieczfinska, Joanna, and Rafal Pawliczak. 2022. "Relaxin Affects Airway Remodeling Genes Expression through Various Signal Pathways Connected with Transcription Factors" International Journal of Molecular Sciences 23, no. 15: 8413. https://doi.org/10.3390/ijms23158413
APA StyleWieczfinska, J., & Pawliczak, R. (2022). Relaxin Affects Airway Remodeling Genes Expression through Various Signal Pathways Connected with Transcription Factors. International Journal of Molecular Sciences, 23(15), 8413. https://doi.org/10.3390/ijms23158413






