An Association between Insulin Resistance and Neurodegeneration in Zebrafish Larval Model (Danio rerio)
Abstract
1. Introduction
2. Results
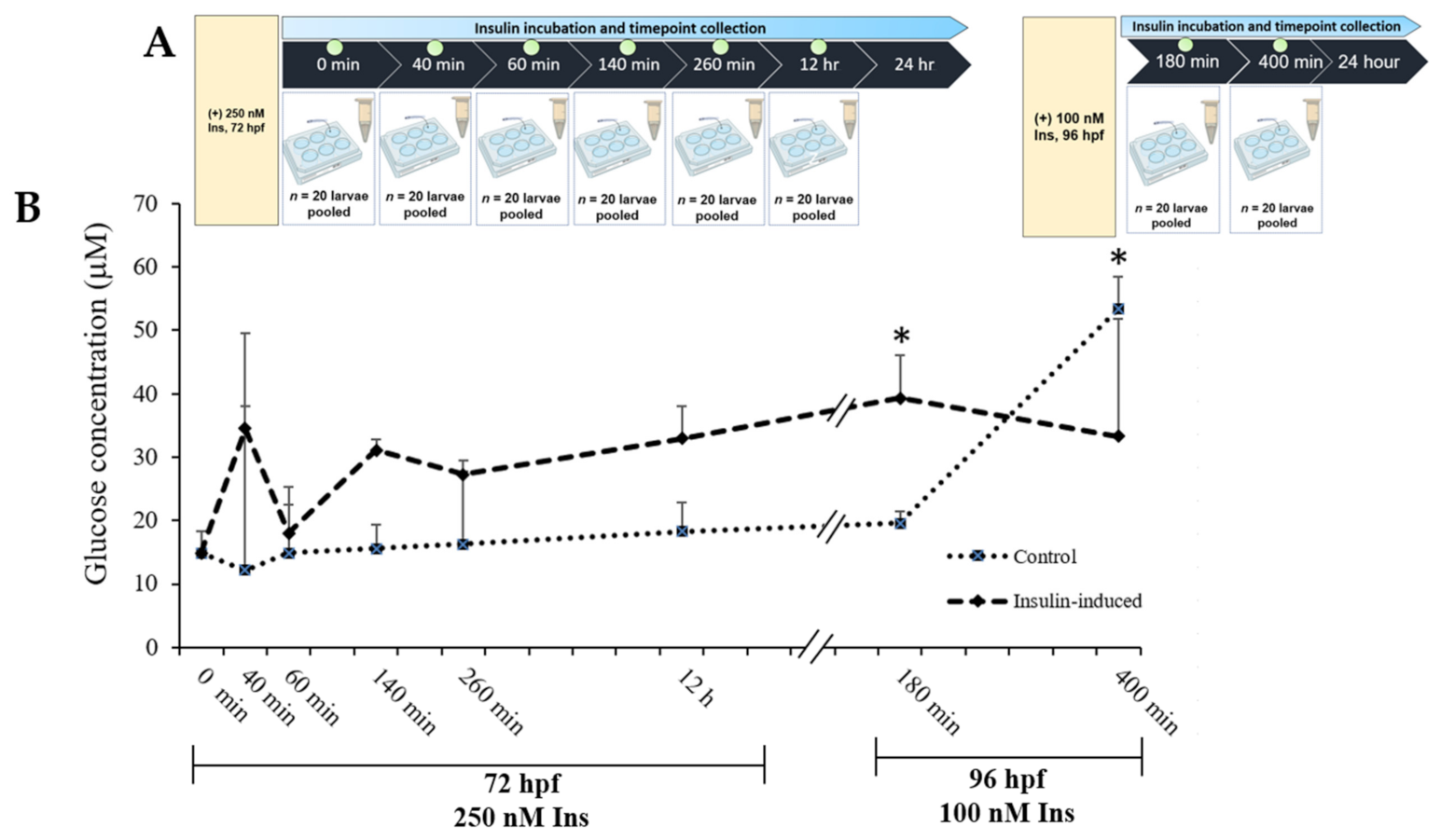
2.1. Glucose Levels’ Measurement
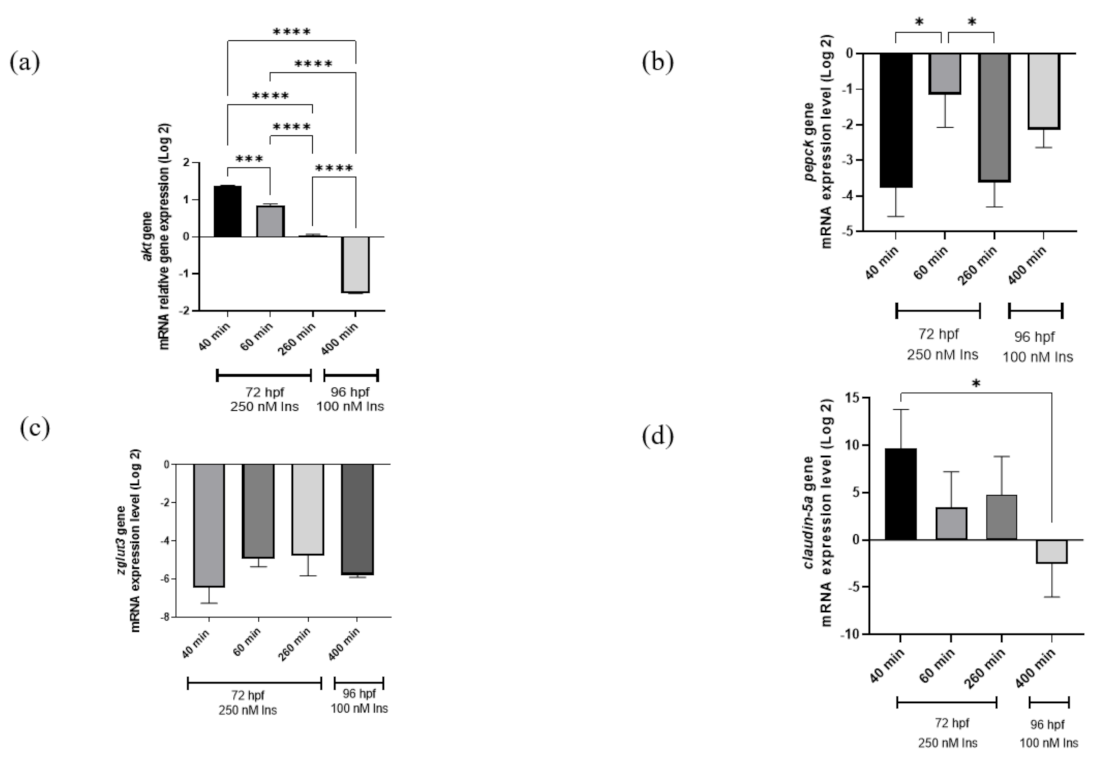
2.2. Gene Expression Analysis
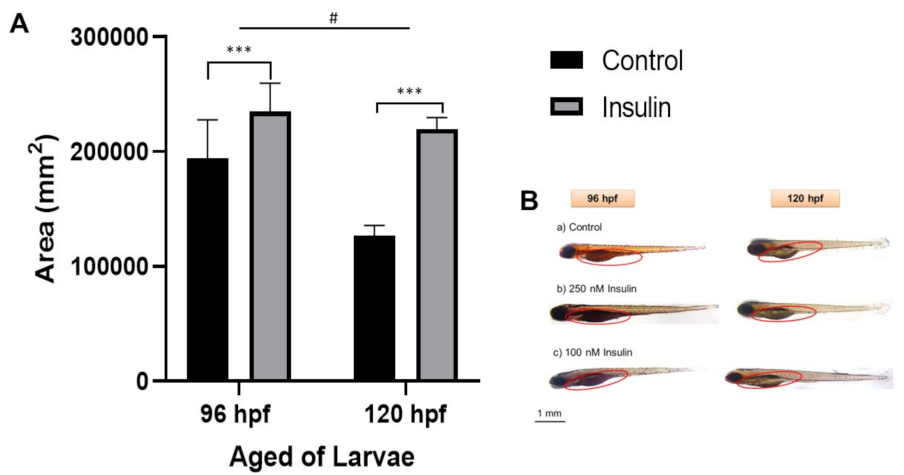
2.3. Lipid Distribution Analysis
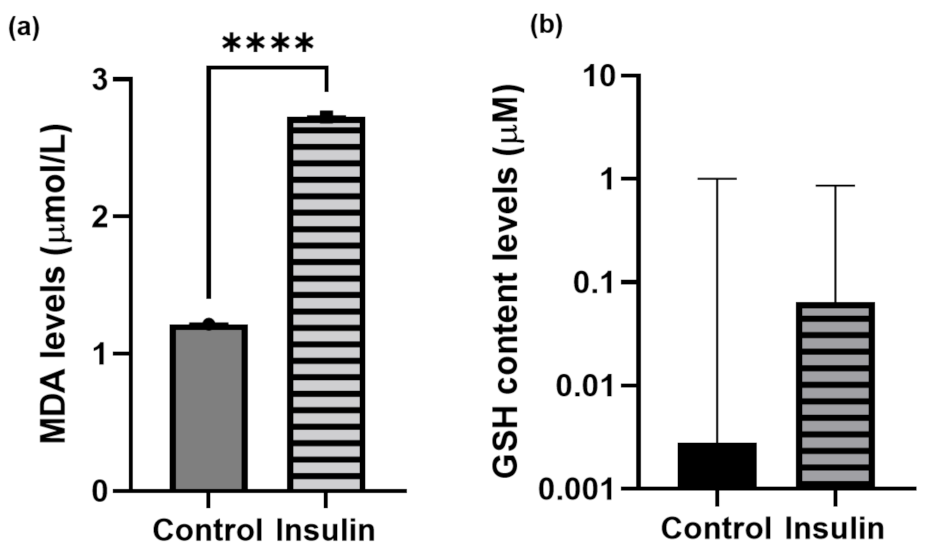
2.4. Oxidative-Stress Measurement
2.5. DAVID Functional-Annotation Analysis from RNA-seq
3. Discussion
4. Materials and Methods
4.1. Reagents and Equipment
4.2. Zebrafish Husbandry
4.3. Insulin Induction
4.4. Glucose Dynamic Study
4.5. Quantitative PCR (qPCR) Analysis for the Relative Genes of Interest Expression Analysis
4.6. Oil Red O (ORO) Staining
4.7. Malondialdehyde (MDA) Assay
4.8. Glutathione (GSH) Assay
4.9. RNA-Seq Transcriptomic Profilings
4.10. Functional and Pathway Enrichment Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene of Interest | Primer Sequences | Accession Number |
|---|---|---|
| beta-actin | 3′-CAACGGAAACGCTCATTGC-5′ 5′-CGAGCAGGAGATGGGAACC-3′ | Keegan et al., (2002) [78] |
| pepck | 3′-ATCACGCATCGCTAAAGAGG-5′ 5′-CCGCTGCGAAATACTTCTTC-3′ | NM_214751.1 |
| claudin-5a | 3′-ATCTTCGTGCTTGTGCCACT-5′ 5′-CAGAGTATGCTTCCCCCGAG-3′ | NM_213274.1 |
| zglut3 | 3′-TCGTCAATGTCTTGGCTCTG-5′ 5′-CAACATACATTGGCGTGAGG-3′ | ENSDART00000016197 |
| akt1 | 3′-TCG GCA GGTG TCTTC TCAAT-5′ 5′-ACCC ATT GCCATACCACGAG-3′ | Sasore and Kennedy (2014) [79] |
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Ganasegeran, K.; Hor, C.P.; Jamil, M.F.A.; Loh, H.C.; Noor, J.M.; Hamid, N.A.; Suppiah, P.D.; Manaf, M.R.A.; Ch’Ng, A.S.H.; Looi, I. A systematic review of the economic burden of type 2 diabetes in Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 5723. [Google Scholar] [CrossRef] [PubMed]
- Haile, B.; Neme, K.; Belachew, T. Evolution of human diet and effect of globalization on regional diet with emphasis to the Mediterranean diet. Nutr. Food Sci. 2017, 47, 869–883. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85035779324&doi=10.1108%2FNFS-02-2017-0017&partnerID=40&md5=6b46d1bc1636b81fa6b3369d27b610f4 (accessed on 18 May 2022). [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018, 17, 122. Available online: /pmc/articles/PMC6119242/ (accessed on 18 May 2022).
- Shpakov, A.O.; Derkach, K.V.; Berstein, L.M. Brain signaling systems in the Type 2 diabetes and metabolic syndrome: Promising target to treat and prevent these diseases. Future Sci. OA 2015, 1, fso.15.23. Available online: http://www.future-science.com/doi/10.4155/fso.15.23 (accessed on 18 May 2021). [CrossRef]
- Matsuzaki, T.; Sasaki, K.; Tanizaki, Y.; Hata, J.; Fujimi, K.; Matsui, Y.; Sekita, A.; Suzuki, S.O.; Kanba, S.; Kiyohara, Y.; et al. Insulin resistance is associated with the pathology of Alzheimer disease: The Hisayama study. Neurology 2010, 75, 764–770. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-77956364857&doi=10.1212%2FWNL.0b013e3181eee25f&partnerID=40&md5=d9c0d3cf66184bb9dab7a83f8d9d1eae (accessed on 25 November 2021). [CrossRef] [PubMed]
- Suzuki, R.; Lee, K.; Jing, E.; Biddinger, S.B.; McDonald, J.G.; Montine, T.J.; Craft, S.; Kahn, C.R. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metab. 2010, 12, 567–579. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Ababei, D.C.; Bild, V.; Bild, W.; Paduraru, L.; Gutu, M.M.; Tamba, B.-I. Renal contributions in the pathophysiology and neuropathological substrates shared by chronic kidney disease and alzheimer’s disease. Brain Sci. 2020, 10, 563. [Google Scholar] [CrossRef]
- Kim, B.; Sullivan, K.A.; Backus, C.; Feldman, E.L. Cortical neurons develop insulin resistance and blunted Akt signaling: A potential mechanism contributing to enhanced ischemic injury in diabetes. Antioxid. Redox Signal. 2011, 14, 1829–1839. Available online: https://pubmed.ncbi.nlm.nih.gov/21194385/ (accessed on 25 November 2021). [CrossRef] [PubMed]
- Zaulkffali, A.S.; Razip, N.N.; Alwi, S.S.S.; Jalil, A.A.; Mutalib, M.S.A.; Gopalsamy, B.; Chang, S.K.; Zainal, Z.; Ibrahim, N.N.; Zakaria, Z.A.; et al. Vitamins D and E stimulate the PI3K-AKT signalling pathway in insulin-resistant SK-N-SH neuronal cells. Nutrients 2019, 11, 2525. [Google Scholar] [CrossRef]
- Gamba, P.; Staurenghi, E.; Testa, G.; Giannelli, S.; Sottero, B.; Leonarduzzi, G. A crosstalk between brain cholesterol oxidation and glucose metabolism in Alzheimer’s disease. Front. Neurosci. 2019, 13, 556. [Google Scholar] [CrossRef] [PubMed]
- Bing, L.; Wu, J.; Zhang, J.; Chen, Y.; Hong, Z.; Zu, H. DHT Inhibits the Aβ25–35-Induced Apoptosis by Regulation of Seladin-1, Survivin, XIAP, bax, and bcl-xl Expression Through a Rapid PI3-K/Akt Signaling in C6 Glial Cell Lines. Neurochem. Res. 2015, 40, 41–48. Available online: https://link.springer.com/article/10.1007/s11064-014-1463-3 (accessed on 21 February 2022). [CrossRef] [PubMed]
- Chen Shang, Y.; Zhong Chong, Z.; Wang, S.; Maiese, K. Tuberous Sclerosis Protein 2 (TSC2) Modulates CCN4 Cytoprotection During Apoptotic Amyloid Toxicity in Microglia. Curr. Neurovasc. Res. 2013, 10, 29–38. [Google Scholar] [CrossRef]
- Shang, Y.C.; Chong, Z.Z.; Wang, S.; Maiese, K. Prevention of β-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012, 4, 187. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3348479/ (accessed on 21 February 2022). [CrossRef]
- Tang, Z.; Bereczki, E.; Zhang, H.; Wang, S.; Li, C.; Ji, X.; Branca, R.M.; Lehtiö, J.; Guan, Z.; Filipcik, P.; et al. Mammalian Target of Rapamycin (mTor) Mediates Tau Protein Dyshomeostasis: Implication for Alzheimer Disease. J. Biol. Chem. 2013, 288, 15556–15570. Available online: https://www.sciencedirect.com/science/article/pii/S0021925820459472 (accessed on 21 February 2022). [CrossRef]
- Sun, N.; Wang, H.; Ma, L.; Lei, P.; Zhang, Q. Ghrelin attenuates brain injury in septic mice via PI3K/Akt signaling activation. Brain Res Bull. 2016, 124, 278–285. Available online: https://pubmed.ncbi.nlm.nih.gov/27288247/ (accessed on 21 February 2022). [CrossRef]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. Available online: /pmc/articles/PMC8082498/ (accessed on 21 February 2022).
- Kim, B.; Feldman, E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Amp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef]
- Vargas, E.; Podder, V.; Sepulveda, M.A.C. Physiology, Glucose Transporter Type 4. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537322/ (accessed on 21 February 2022).
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Metabolic Signalling: Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 31–44. Available online: https://pubmed.ncbi.nlm.nih.gov/28974775/ (accessed on 14 April 2022).
- Chourpiliadis, C.; Mohiuddin, S.S. Biochemistry, Gluconeogenesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544346/ (accessed on 14 April 2022).
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes. 2015, 6, 456. [Google Scholar] [CrossRef]
- Bradford, Y.M.; Toro, S.; Ramachandran, S.; Ruzicka, L.; Howe, D.G.; Eagle, A.; Kalita, P.; Martin, R.; Moxon, S.A.T.; Schaper, K.; et al. Zebrafish Models of Human Disease: Gaining Insight into Human Disease at ZFIN. ILAR J. 2017, 58, 4–16. [Google Scholar] [CrossRef]
- Schlegel, A.; Gut, P. Metabolic insights from zebrafish genetics, physiology, and chemical biology. Cell Mol. Life Sci. 2015, 72, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Marín-Juez, R.; Jong-Raadsen, S.; Yang, S.; Spaink, H.P. Hyperinsulinemia induces insulin resistance and immune suppression via Ptpn6/Shp1 in zebrafish. J. Endocrinol. 2014, 222, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.; Dias, J.; Engrola, S.; Gavaia, P.; Geurden, I.; Dinis, M.T.; Panserat, S. Glucose overload in yolk has little effect on the long-term modulation of carbohydrate metabolic genes in zebrafish (Danio rerio). J. Exp. Biol. 2013, 217, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Rodriguez, I.; Shin, S.; Shim, J.; Kim, N.; Kim, M.; Jeong, S.; Nuankaew, W.; Hong, B.; Kim, H.; et al. Characteristics of the New Insulin-Resistant Zebrafish Model. Pharmaceuticals 2021, 14, 642. Available online: https://www.mdpi.com/1424-8247/14/7/642/htm (accessed on 14 April 2022). [CrossRef]
- Kuwabara, S.; Yamaki, M.; Yu, H.; Itoh, M. Notch signaling regulates the expression of glycolysis-related genes in a context-dependent manner during embryonic development. Biochem. Biophys. Res. Commun. 2018, 503, 803–808. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Chen, R.D.; Lee, J.R.; Liu, S.T.; Lee, S.J.; Hwang, P.P. Specific expression and regulation of glucose transporters in zebrafish ionocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 275–290. [Google Scholar] [CrossRef]
- Zheng, P.P.; Romme, E.; Van Der Spek, P.J.; Dirven, C.M.F.; Willemsen, R.; Kros, J.M. Glut1/SLC2A1 is crucial for the development of the blood–brain barrier in vivo. Ann. Neurol. 2010, 68, 835–844. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/ana.22318 (accessed on 15 May 2022). [CrossRef]
- Ennis, K.; Felt, B.; Georgieff, M.K.; Rao, R. Early Life Iron Deficiency Alters Glucose Transporter-1 Expression in the Adult Rodent Hippocampus. J. Nutr. 2019, 149, 1660–1666. Available online: /pmc/articles/PMC6736205/ (accessed on 14 April 2022).
- Li, Y.; Chen, T.; Miao, X.; Yi, X.; Wang, X.; Zhao, H.; Lee, S.M.-Y.; Zheng, Y. Zebrafish: A promising in vivo model for assessing the delivery of natural products, fluorescence dyes and drugs across the blood–brain barrier. Pharmacol. Res. 2017, 125, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, W. ABC transporters and drug efflux at the blood–brain barrier. Rev. Neurosci. 2010, 21, 29–53. Available online: https://pubmed.ncbi.nlm.nih.gov/20458886/ (accessed on 14 April 2022). [CrossRef]
- Abdelilah-Seyfried, S. Claudin-5a in developing zebrafish brain barriers: Another brick in the wall. BioEssays 2010, 32, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Henson, H.E.; Parupalli, C.; Ju, B.; Taylor, M.R. Functional and genetic analysis of choroid plexus development in zebrafish. Front. Neurosci. 2014, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- O’Brown, N.M.; Pfau, S.J.; Gu, C. Bridging barriers: A comparative look at the blood–brain barrier across organisms. Genes Dev. 2018, 32, 466. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5959231/ (accessed on 14 April 2022). [CrossRef] [PubMed]
- Carnovali, M.; Banfi, G.; Mariotti, M. Zebrafish Models of Human Skeletal Disorders: Embryo and Adult Swimming Together. Biomed. Res. Int. 2019, 2019, 1253710. [Google Scholar] [CrossRef]
- Meng, X.-H.; Chen, B.; Zhang, J.-P. Intracellular insulin and impaired autophagy in a zebrafish model and a cell model of type 2 diabetes. Int. J. Biol. Sci. 2017, 13, 985–995. [Google Scholar] [CrossRef]
- Oyelaja-Akinsipo, O.B.; Dare, E.O.; Katare, D.P. Protective role of diosgenin against hyperglycaemia-mediated cerebral ischemic brain injury in zebrafish model of type II diabetes mellitus. Heliyon 2020, 6, e03296. [Google Scholar] [CrossRef]
- Fraher, D.; Ellis, M.K.; Morrison, S.; McGee, S.L.; Ward, A.; Walder, K.; Gibert, Y. Lipid abundance in zebrafish embryos is regulated by complementary actions of the endocannabinoid system and retinoic acid pathway. Endocrinology 2015, 156, 3596–3609. [Google Scholar] [CrossRef]
- Ka, J.; Pak, B.; Han, O.; Lee, S.; Jin, S.W. Comparison of transcriptomic changes between zebrafish and mice upon high fat diet reveals evolutionary convergence in lipid metabolism. Biochem. Biophys. Res. Commun. 2020, 530, 638–643. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Wang, T.; Liu, H.; Shi, J.; Zhang, X. Evaluation of the oxidative stress status in zebrafish (Danio rerio) liver induced by three typical organic uv filters (BP-4, PABA and PBSA). Int. J. Environ. Res. Public Health 2020, 17, 651. [Google Scholar] [CrossRef]
- Massarsky, A.; Kozal, J.S.; Di Giulio, R.T. Glutathione and zebrafish: Old assays to address a current issue. Chemosphere 2017, 168, 707. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5182135/ (accessed on 20 May 2022). [CrossRef]
- Eames, S.C.; Kinkel, M.D.; Rajan, S.; Prince, V.E.; Philipson, L.H. Transgenic zebrafish model of the C43G human insulin gene mutation. J. Diabetes Investig. 2013, 4, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Lodd, E.; Wiggenhauser, L.M.; Morgenstern, J.; Fleming, T.H.; Poschet, G.; Büttner, M.; Tabler, C.T.; Wohlfart, D.P.; Nawroth, P.P.; Kroll, J. The combination of loss of glyoxalase1 and obesity results in hyperglycemia. JCI Insight 2019, 4, e126154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, C.; Yang, L.; Lu, R.; Zhao, X.; Nie, G. A comparative genomics study of carbohydrate/glucose metabolic genes: From fish to mammals. BMC Genom. 2018, 19, 246. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, R.C.; Krekels, E.H.J.; Kantae, V.; Harms, A.C.; Hankemeier, T.; van der Graaf, P.H.; Spaink, H.P. Impact of post-hatching maturation on the pharmacokinetics of paracetamol in zebrafish larvae. Sci. Rep. 2019, 9, 2149. Available online: https://www.nature.com/articles/s41598-019-38530-w (accessed on 21 May 2022). [CrossRef]
- Holt, G.J. Larval Fish Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2011; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9780470959862 (accessed on 21 May 2022).
- Elo, B.; Villano, C.M.; Govorko, D.; White, L.A. Larval zebrafish as a model for glucose metabolism: Expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J. Mol. Endocrinol. 2007, 38, 433–440. [Google Scholar] [CrossRef]
- Gut, P.; Baeza-Raja, B.; Andersson, O.; Hasenkamp, L.; Hsiao, J.; Hesselson, D.; Akassoglou, K.; Verdin, E.; Hirschey, M.D.; Stainier, D.Y.R. Whole-organism screening for gluconeogenesis identifies activators of fasting metabolism. Nat. Chem. Biol. 2013, 9, 97–104. Available online: https://pubmed.ncbi.nlm.nih.gov/23201900/ (accessed on 21 May 2022). [CrossRef]
- Tanvir, Z.; Nelson, R.F.; DeCicco-Skinner, K.; Connaughton, V.P. One month of hyperglycemia alters spectral responses of the zebrafish photopic electroretinogram. DMM Dis. Model Mech. 2018, 11, dmm035220. [Google Scholar] [CrossRef]
- Tay, S.S.; Kuah, M.K.; Shu-Chien, A.C. Transcriptional activation of zebrafish fads2 promoter and its transient transgene expression in yolk syncytial layer of zebrafish embryos. Sci. Rep. 2018, 8, 2–13. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Conteh, A.M.; Sorrell, C.A.; Mirmira, A.; Tersey, S.A.; Mirmira, R.G.; Linnemann, A.K.; Anderson, R.M. An in vivo zebrafish model for interrogating ROS-mediated pancreatic β-cell injury, response, and prevention. Oxid. Med. Cell. Longev. 2018, 2018, 1324739. [Google Scholar] [CrossRef]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef]
- Landgraf, K.; Schuster, S.; Meusel, A.; Garten, A.; Riemer, T.; Schleinitz, D.; Kiess, W.; Körner, A. Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC Physiol. 2017, 17, 4. Available online: https://pubmed.ncbi.nlm.nih.gov/28327129/ (accessed on 23 May 2021). [CrossRef] [PubMed]
- Xiong, X.; Song, Q.; Han, C.; Gan, W.; He, F.; Wei, S.; Liu, H.; Xu, H. Insulin Promotes the Expression of the Gluconeogenic Rate-Limiting Enzymes Phosphoenolpyruvate Carboxykinase (Pepck) and Glucose 6-Phosphatase (G6pase) through PI3k/Akt/mTOR Signaling Pathway in Goose Hepatocytes. Braz. J. Poult. Sci. 2016, 18, 395–400. Available online: http://www.scielo.br/j/rbca/a/sdN3Gmzxj7CHBZfLWWTtzKr/?lang=en (accessed on 23 May 2021). [CrossRef]
- Ghaddar, B.; Diotel, N. Zebrafish: A New Promise to Study the Impact of Metabolic Disorders on the Brain. Int. J. Mol. Sci. 2022, 23, 5372. [Google Scholar] [CrossRef] [PubMed]
- Talchai, S.C.; Accili, D. Legacy effect of foxo1 in pancreatic endocrine progenitors on adult β-cell mass and function. Diabetes 2015, 64, 2868–2879. Available online: https://pmc/articles/PMC4512230/ (accessed on 13 April 2022).
- Al-Masri, M.; Krishnamurthy, M.; Li, J.; Fellows, G.F.; Dong, H.H.; Goodyer, C.G.; Wang, R. Effect of forkhead box O1 (FOXO1) on beta cell development in the human fetal pancreas. Diabetologia. 2010, 53, 699–711. Available online: https://link.springer.com/article/10.1007/s00125-009-1632-0 (accessed on 13 April 2022). [CrossRef]
- Qi, Y.; Zhu, Q.; Zhang, K.; Thomas, C.; Wu, Y.; Kumar, R.; Baker, K.M.; Xu, Z.; Chen, S.; Guo, S. Activation of foxo1 by insulin resistance promotes cardiac dysfunction and βmyosin heavy chain gene expression. Circ. Hear Fail. 2015, 8, 198–208. Available online: https://www.ahajournals.org/doi/abs/10.1161/circheartfailure.114.001457 (accessed on 13 April 2022). [CrossRef]
- Liu, G.M.; Zhang YMTargeting, F.B. Pase is an emerging novel approach for cancer therapy. Cancer Cell Int. 2018, 18, 36. Available online: https://pmc/articles/PMC5845355/ (accessed on 12 April 2022). [CrossRef]
- Kratzer, I.; Vasiljevic, A.; Rey, C.; Fevre-Montange, M.; Saunders, N.; Strazielle, N.; Ghersi-Egea, J.-F. Complexity and developmental changes in the expression pattern of claudins at the blood-CSF barrier. Histochem. Cell Biol. 2012, 138, 861–879. Available online: https://pubmed.ncbi.nlm.nih.gov/22886143/ (accessed on 12 April 2022). [CrossRef]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525. Available online: https://pmc/articles/PMC3768107/ (accessed on 12 April 2022). [CrossRef]
- Carvalho, L.; Heisenberg, C.P. The yolk syncytial layer in early zebrafish development. Trends Cell Biol. 2010, 20, 586–592. Available online: https://pubmed.ncbi.nlm.nih.gov/20674361/ (accessed on 12 April 2022). [CrossRef]
- Flannery, C.; Dufour, S.; Rabøl, R.; Shulman, G.I.; Petersen, K.F. Skeletal Muscle Insulin Resistance Promotes Increased Hepatic De Novo Lipogenesis, Hyperlipidemia, and Hepatic Steatosis in the Elderly. Diabetes 2012, 61, 2711. Available online: https://pmc/articles/PMC3478531/ (accessed on 20 November 2021). [CrossRef]
- Takeuchi, M.; Yamagishi, S.-I. Insulin resistance is an independent correlate of high serum levels of advanced glycation end products (AGEs) and low testosterone in non-diabetic men. Oxid. Med. Cell. Longev. 2010, 3, 262–265. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-77957366321&doi=10.4161%2Foxim.3.4.12734&partnerID=40&md5=c18a2918aaa2fdb0ed7aece89b244c30 (accessed on 20 November 2021).
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Thompson, B.J.; Hietakangas, V.; Cohen, S.M. MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in Drosophila. PLoS Genet. 2011, 7, 1002429. Available online: https://pmc/articles/PMC3248469/ (accessed on 20 November 2021). [CrossRef]
- Alvira-Botero, X.; Carro, M.E. Clearance of Amyloid-β Peptide Across the Choroid Plexus in Alzheimers Disease. Curr. Aging Sci. 2010, 3, 219–229. Available online: https://pubmed.ncbi.nlm.nih.gov/20735345/ (accessed on 20 November 2021). [CrossRef] [PubMed]
- Camara, A.Y.; Wan, Y.; Yu, Y.; Wang, Q.; Wang, K.; Li, H. Effect of Endogenous Selenium on Arsenic Uptake and Antioxidative Enzymes in As-Exposed Rice Seedlings. Int. J. Environ. Res. Public Health 2019, 16, 3350. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Arenas, E. Emerging roles of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2010, 11, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Abdul, H.M.; Furman, J.L.; Sama, M.A.; Mathis, D.M.; Norris, C.M. NFATs and Alzheimer’s Disease. Mol. Cell Pharmacol. 2010, 2, 7. Available online: https://pmc/articles/PMC2855852/ (accessed on 20 November 2021).
- Killick, R.; Ribe, E.M.; Al-Shawi, R.; Malik, B.; Hooper, C.; Fernandes, C.; Dobson, R.; Nolan, P.M.; Lourdusamy, A.; Furney, S.; et al. Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt–PCP–JNK pathway. Mol. Psychiatry. 2014, 19, 88–98. Available online: https://www.nature.com/articles/mp2012163 (accessed on 1 December 2021). [CrossRef]
- Caricasole, A.; Copani, A.; Caraci, F.; Aronica, E.; Rozemuller, A.J.; Caruso, A.; Storto, M.; Gaviraghi, G.; Terstappen, G.C.; Nicoletti, F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J. Neurosci. 2004, 24, 6021–6027. Available online: https://pubmed.ncbi.nlm.nih.gov/15229249/ (accessed on 1 December 2021). [CrossRef]
- Mahboudi, H.; Heidari, N.M.; Rashidabadi, Z.I.; Anbarestani, A.H.; Karimi, S.; Darestani, K.D. Prospect and Competence of Quantitative Methods via Real-time PCR in a Comparative Manner: An Experimental Review of Current Methods. Open Bioinform. J. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R. Stat. Soc. Ser. B Stat. Method. 1995, 57, 289. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. Available online: https://pubmed.ncbi.nlm.nih.gov/20132535/ (accessed on 20 June 2022). [CrossRef] [PubMed]
- Keegan, B.R.; Feldman, J.L.; Lee, D.H.; Koos, D.S.; Ho, R.K.; Stainier, D.Y.R.; Yelon, D. The elongation factors Pandora/Spt6 and Foggy/Spt5 promote transcription in the zebrafish embryo. Development 2002, 129, 1623–1632. Available online: https://journals.biologists.com/dev/article/129/7/1623/51996/The-elongation-factors-Pandora-Spt6-and-Foggy-Spt5 (accessed on 20 June 2022). [CrossRef] [PubMed]
- Sasore, T.; Kennedy, B. Deciphering combinations of PI3K/AKT/mTOR pathway drugs augmenting anti-angiogenic efficacy in vivo. PLoS ONE 2014, 9, e105280. [Google Scholar] [CrossRef][Green Version]
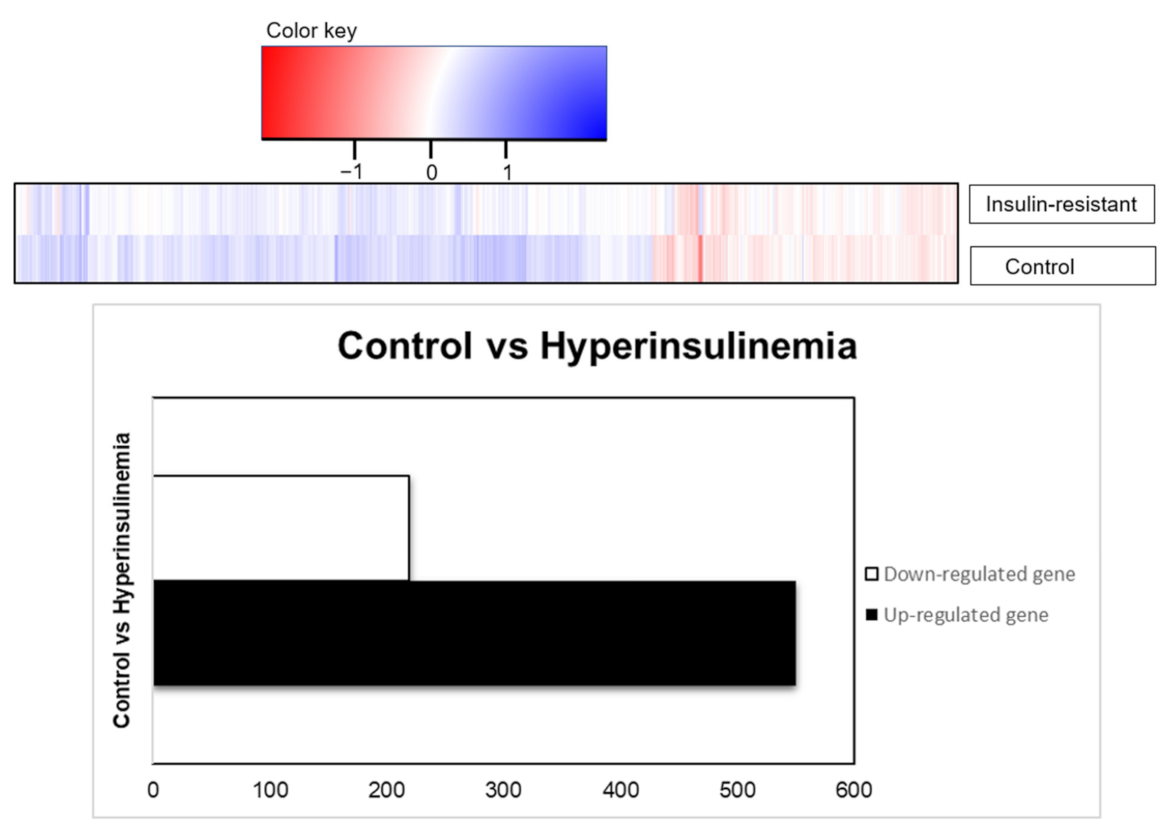
| Upregulated | |||
|---|---|---|---|
| TopGO | Term | Total Genes | Adjusted p-Value EASE (<0.05) |
| GO_BP | Intracellular signal transduction | 48 | 2.50 × 10−10 |
| Calcium ion import | 6 | 9.50 × 10−5 | |
| Regulation of ion transmembrane transport | 12 | 1.10 × 10−4 | |
| Homophilic cell adhesion via plasma membrane adhesion molecules | 14 | 2.40 × 10−4 | |
| Calcium ion transmembrane transport | 9 | 4.40 × 10−4 | |
| Calcium ion transport | 8 | 4.40 × 10−4 | |
| Transmembrane transport | 21 | 3.90 × 10−3 | |
| Axonogenesis involved in innervation | 3 | 6.10 × 10−3 | |
| Regulation of endocytosis | 4 | 6.50 × 10−3 | |
| Positive regulation of kinase activity | 6 | 1.20 × 10−2 | |
| GO_CC | Plasma membrane | 74 | 2.00 × 10−6 |
| Voltage-gated calcium channel complex | 8 | 7.90 × 10−6 | |
| Integral component of plasma membrane | 39 | 6.30 × 10−5 | |
| Membrane | 139 | 3.80 × 10−4 | |
| Basal plasma membrane | 4 | 9.10 × 10−4 | |
| Axon | 10 | 1.00 × 10−3 | |
| Axonal growth cone | 3 | 7.80 × 10−3 | |
| Integral component of plasma membrane | 128 | 8.50 × 10−3 | |
| Receptor complex | 8 | 1.20 × 10−2 | |
| Synaptic vesicle | 6 | 1.20 × 10−2 | |
| Synapse | 15 | 1.60 × 10−2 | |
| GO_MF | Voltage-gated ion channel activity | 12 | 2.90 × 10−5 |
| Voltage-gated calcium channel activity | 8 | 4.10 × 10−5 | |
| Tau protein binding | 4 | 4.30 × 10−5 | |
| Metal ion binding | 81 | 6.80 × 10−5 | |
| High-voltage-gated calcium channel activity | 5 | 1.10 × 10−4 | |
| Ion channel activity | 12 | 1.30 × 10−3 | |
| Calcium channel activity | 7 | 3.60 × 10−3 | |
| Transmembrane-receptor-protein tyrosine kinase activity | 7 | 1.50 × 10−2 | |
| Protein tyrosine kinase activity | 7 | 1.60 × 10−2 | |
| Protein kinase activity | 18 | 9.80 × 10−2 | |
| Downregulated | |||
|---|---|---|---|
| TopGO | Term | Total Genes | Adjusted p-Value EASE (<0.05) |
| GO_BP | piRNA metabolic process | 6 | 3.70 × 10−8 |
| Proteolysis | 16 | 2.70 × 10−6 | |
| Gene silencing by RNA | 4 | 2.00 × 10−3 | |
| Transmembrane transport | 11 | 4.00 × 10−3 | |
| Lipid metabolic process | 7 | 1.70 × 10−2 | |
| Neutrophil chemotaxis | 4 | 1.70 × 10−2 | |
| High-density-lipoprotein-particle assembly | 2 | 2.50 × 10−2 | |
| Reverse cholesterol transport | 2 | 2.50 × 10−2 | |
| Very-low-density-lipoprotein-particle modelling | 2 | 2.50 × 10−2 | |
| Cholesterol efflux | 2 | 8.40 × 10−2 | |
| GO_CC | Extracellular region | 24 | 6.00 × 10−8 |
| Extracellular space | 23 | 1.50 × 10−6 | |
| P granule | 5 | 1.30 × 10−5 | |
| pi-body | 3 | 2.00 × 10−4 | |
| High-density-lipoprotein particle | 3 | 1.50 × 10−3 | |
| Chylomicron | 2 | 2.90 × 10−2 | |
| Integral component of postsynaptic specialization membrane | 2 | 5.70 × 10−2 | |
| GO_MF | Hydrolase activity | 30 | 4.70 × 10−9 |
| Peptidase activity | 16 | 7.3–8 | |
| Serine-type peptidase activity | 9 | 1.30 × 10−6 | |
| piRNA binding | 3 | 1.10 × 10−4 | |
| Carboxypeptidase | 4 | 3.60 × 10−4 | |
| Endoribonuclease activity, producing 5′-Phosphomonoesters | 3 | 3.70 × 10−4 | |
| Metallocarboxypeptidase activity | 4 | 4.10 × 10−4 | |
| Transmembrane transporter activity | 7 | 7.10 × 10−3 | |
| Cholinesterase activity | 2 | 3.00 × 10−3 | |
| Sterol esterase | 2 | 4.20 × 10−2 | |
| KEGG Pathways from the DAVID Platform | |
|---|---|
| Upregulated | Downregulated |
|
|
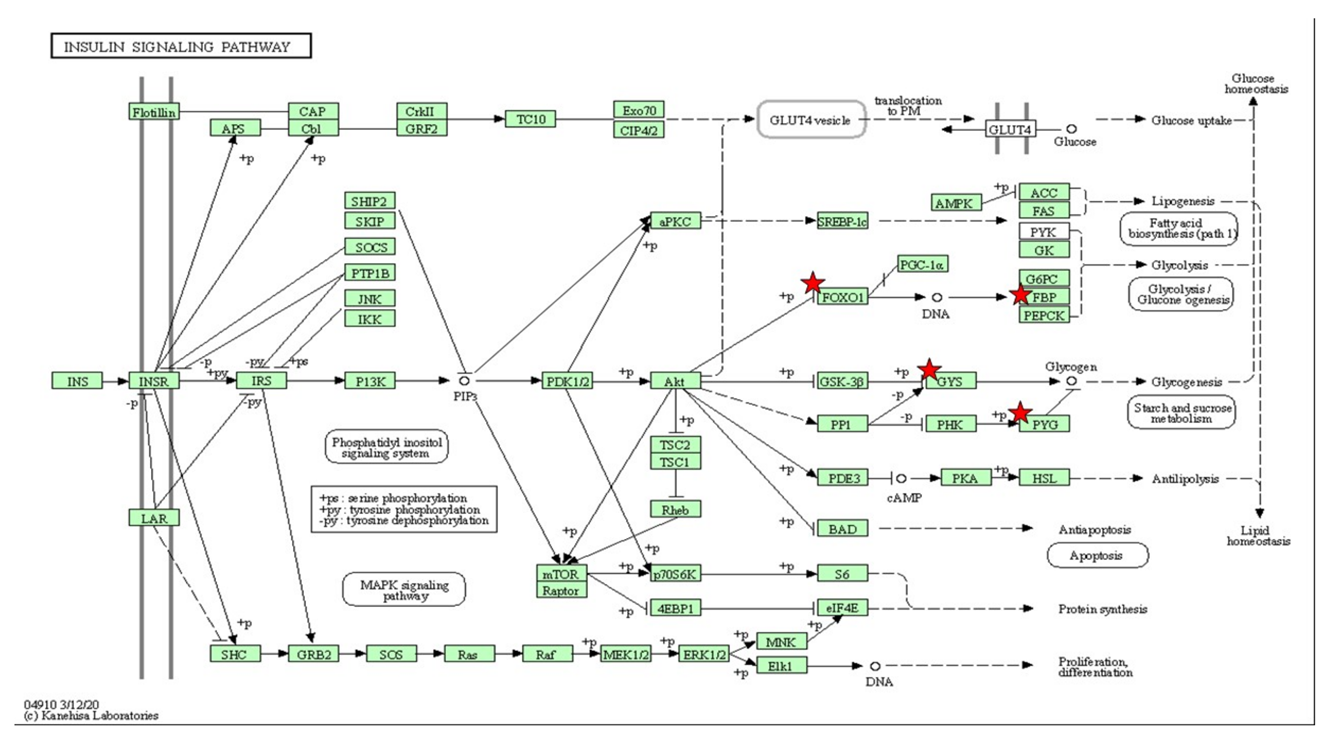
| Insulin-Signaling Pathway | ||
|---|---|---|
| Gene List | LogFC | (p < 0.05) |
| foxo1b | 2.068 | 0.05 |
| bachla | 1.459 | 0.05 |
| ehf | −2.393 | 0.01 |
| Magfb | 2.505 | <0.01 |
| pou6f2 | 1.812 | 0.03 |
| mafk | 1.812 | 0.03 |
| fosl1a | 1.404 | 0.04 |
| dmrt1 | −5.803 | 0.03 |
| sp8b | 2.384 | 0.04 |
| hsf4 | 1.911 | 0.01 |
| crebrf | 3.031 | <0.01 |
| gatad2b | 1.643 | 0.02 |
| lrrfip1b | 1.459 | 0.04 |
| her4.2 | 3.324 | <0.01 |
| rxarb | 7.163 | <0.01 |
| Metabolic pathways | ||
| cel.1 | −2.303 | <0.01 |
| cel.2 | −2.464 | <0.01 |
| amy2a | −1.901 | <0.01 |
| fbp2 | −2.507 | <0.01 |
| chia.6 | −1.372 | 0.05 |
| glsl | −2.033 | 0.02 |
| gsta.2 | −1.928 | 0.01 |
| gys2 | −1.459 | 0.03 |
| hmox1a | −1.459 | 0.03 |
| hao1 | −1.310 | 0.05 |
| mthfd1l | −2.463 | <0.01 |
| mthfd1a | −1.804 | 0.01 |
| pla2g1b | −1.610 | 0.02 |
| paics | −2.239 | <0.01 |
| pygl | −1.412 | 0.03 |
| sprb | −2.422 | 0.02 |
| si:ch211-264e16.2 | −6.904 | <0.01 |
| si:dkey-266f7.9 | −2.070 | <0.01 |

| MAPK-Signaling Pathway | ||
|---|---|---|
| Gene List | LogFC | p < 0.05 |
| rasgrp3 | 1.75 | 0.03 |
| rasgrf2b | 1.71 | 0.03 |
| angpt2b | 2.28 | 0.05 |
| erbb2 | 1.53 | 0.04 |
| erbb4a | 1.63 | 0.04 |
| erbb4b | 2.28 | 0.01 |
| ntrk2b | 2.72 | 0.00 |
| nfkb1 | 2.76 | 0.01 |
| prkcg | 7.92 | 0.00 |
| rps6ka5 | 2.38 | 0.01 |
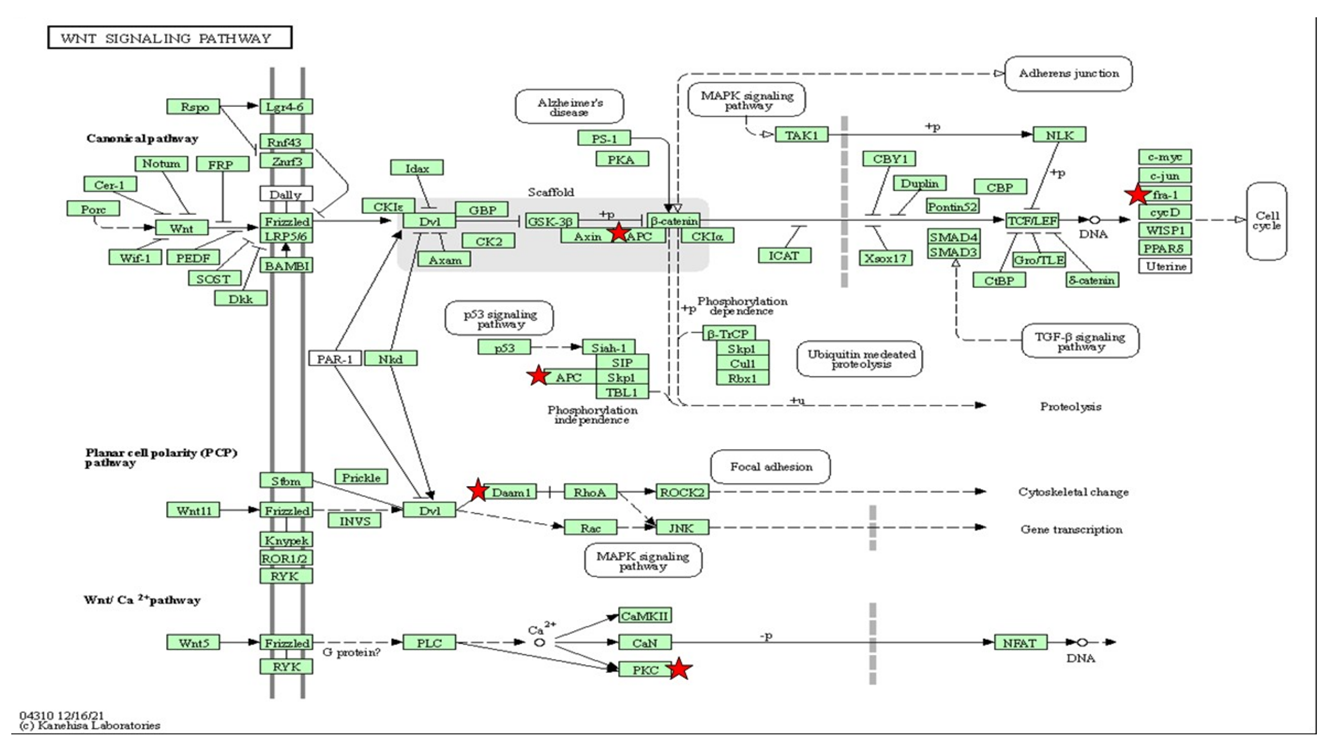
| Wnt/Ca2+ Pathway | ||
|---|---|---|
| Gene List | LogFC | (p < 0.05) |
| Prkcg | 7.92 | <0.05 |
| rps6ka5 | 2.38 | 0.01 |
| cdc42bpaa | 1.57 | 0.05 |
| Camkla | 1.78 | 0.02 |
| cdk6 | 1.58 | 0.05 |
| erbb4a | 1.63 | 0.04 |
| Itk | 2.97 | 0.04 |
| acvr2ab | 1.96 | 0.01 |
| erbb4b | 2.28 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md Razip, N.N.; Mohd Noor, S.; Norazit, A.; Nordin, N.; Sakeh, N.M.; Khaza’ai, H. An Association between Insulin Resistance and Neurodegeneration in Zebrafish Larval Model (Danio rerio). Int. J. Mol. Sci. 2022, 23, 8290. https://doi.org/10.3390/ijms23158290
Md Razip NN, Mohd Noor S, Norazit A, Nordin N, Sakeh NM, Khaza’ai H. An Association between Insulin Resistance and Neurodegeneration in Zebrafish Larval Model (Danio rerio). International Journal of Molecular Sciences. 2022; 23(15):8290. https://doi.org/10.3390/ijms23158290
Chicago/Turabian StyleMd Razip, Nurliyana Najwa, Suzita Mohd Noor, Anwar Norazit, Norshariza Nordin, Nurshafika Mohd Sakeh, and Huzwah Khaza’ai. 2022. "An Association between Insulin Resistance and Neurodegeneration in Zebrafish Larval Model (Danio rerio)" International Journal of Molecular Sciences 23, no. 15: 8290. https://doi.org/10.3390/ijms23158290
APA StyleMd Razip, N. N., Mohd Noor, S., Norazit, A., Nordin, N., Sakeh, N. M., & Khaza’ai, H. (2022). An Association between Insulin Resistance and Neurodegeneration in Zebrafish Larval Model (Danio rerio). International Journal of Molecular Sciences, 23(15), 8290. https://doi.org/10.3390/ijms23158290






