Discovery of the Anticancer Activity for Lung and Gastric Cancer of a Brominated Coelenteramine Analog
Abstract
:1. Introduction
2. Results
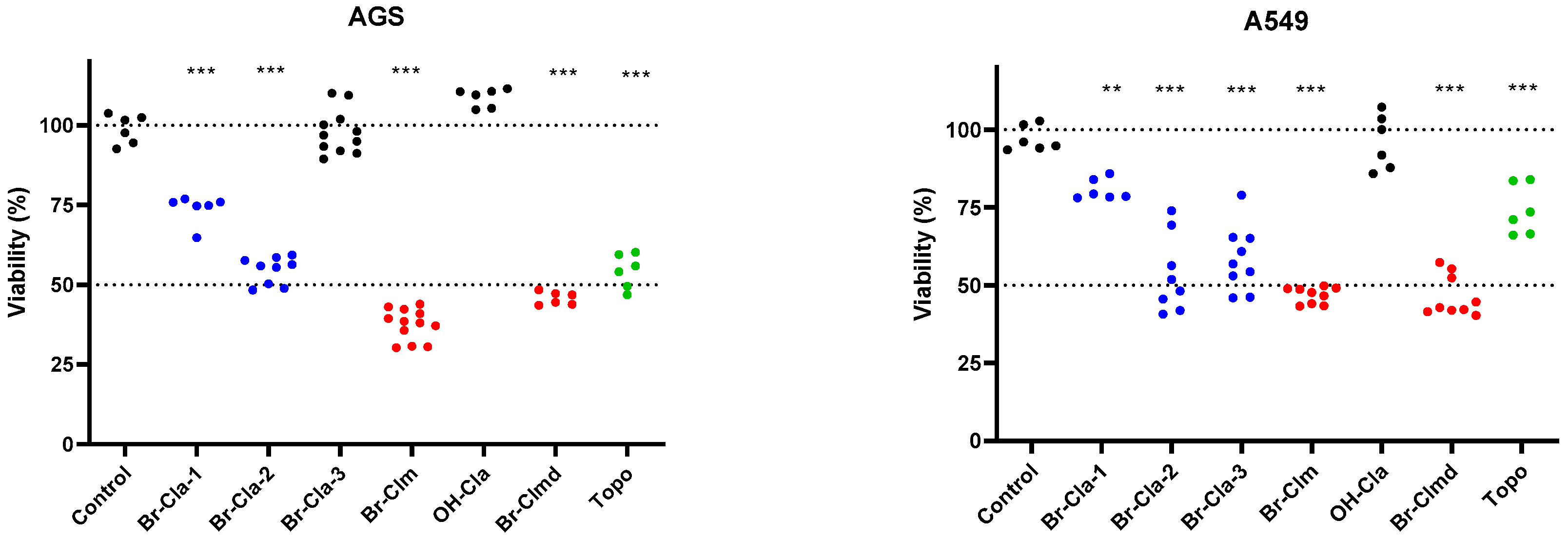
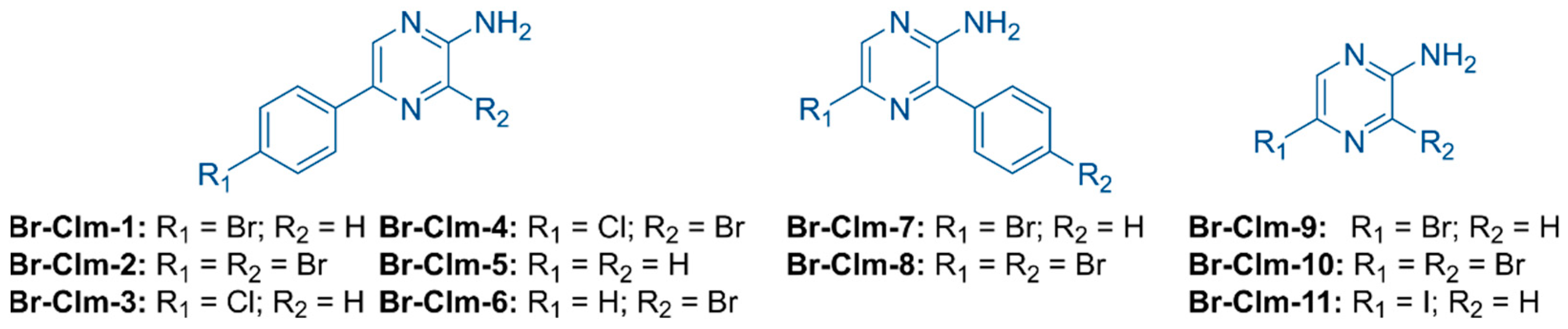
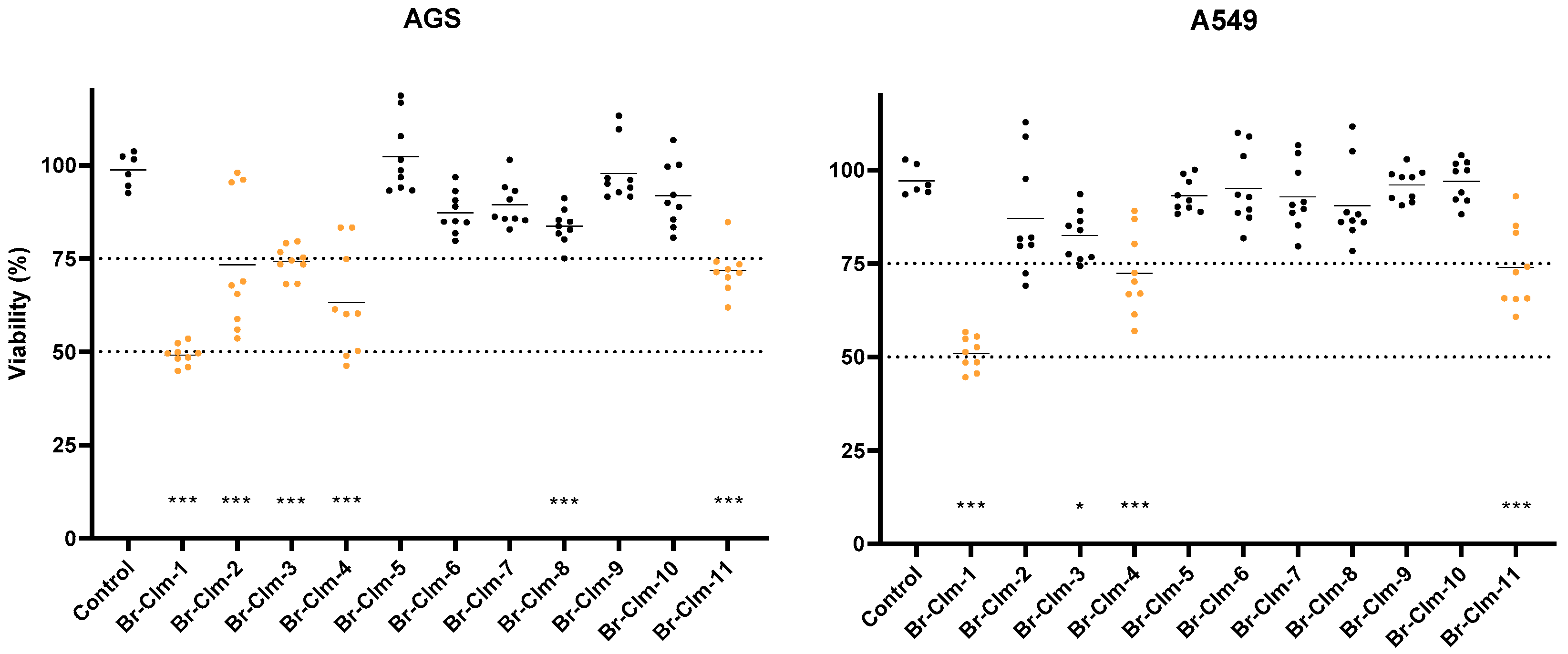
2.1. Br-Cla-1/2/3, Br-Clm, and Br-Clmd Display Toxicity towards Human Cancer Cells
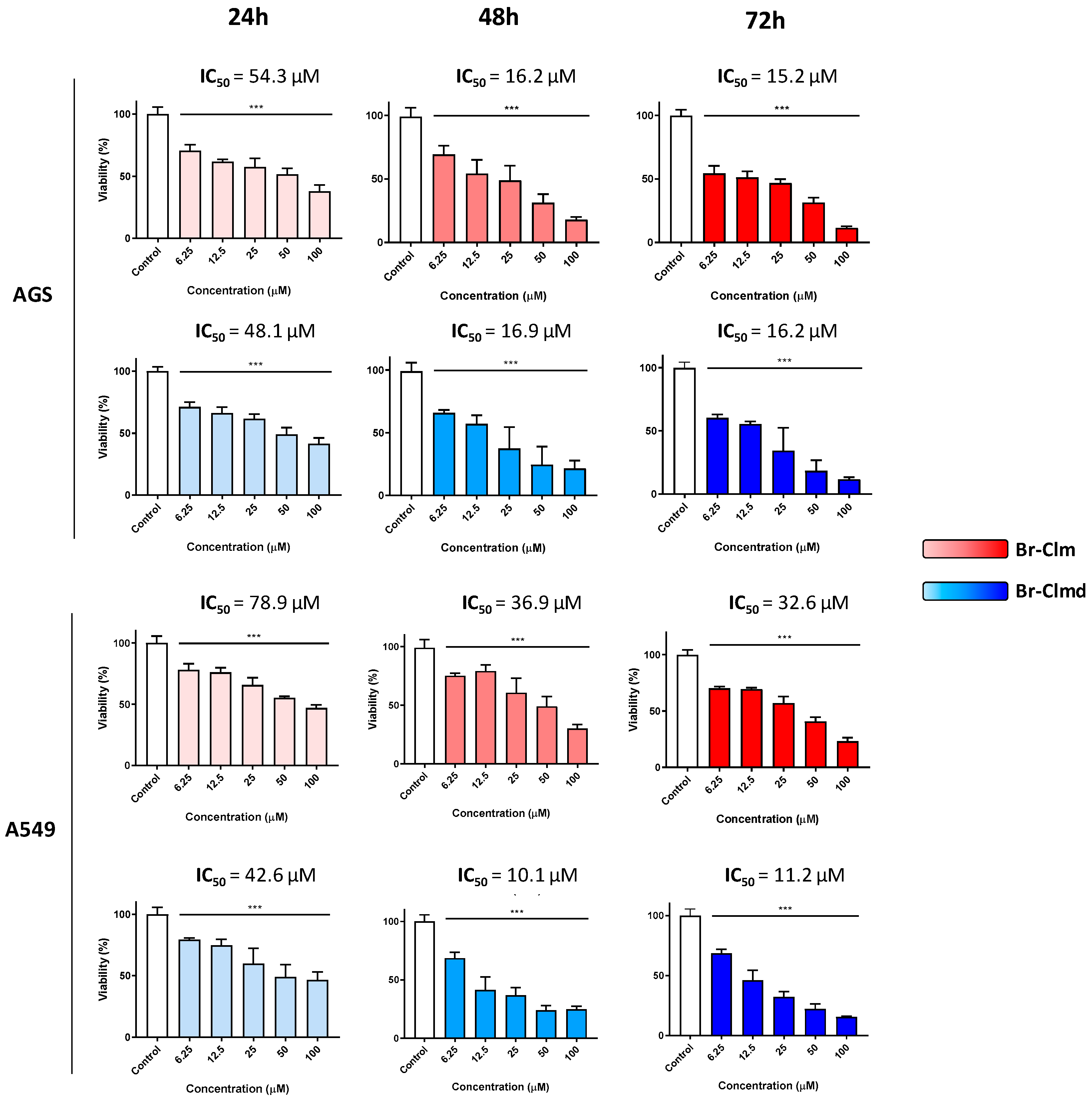
2.2. Cytotoxicity of Br-Clm and Br-Clmd Is Time- and Concentration-Dependent
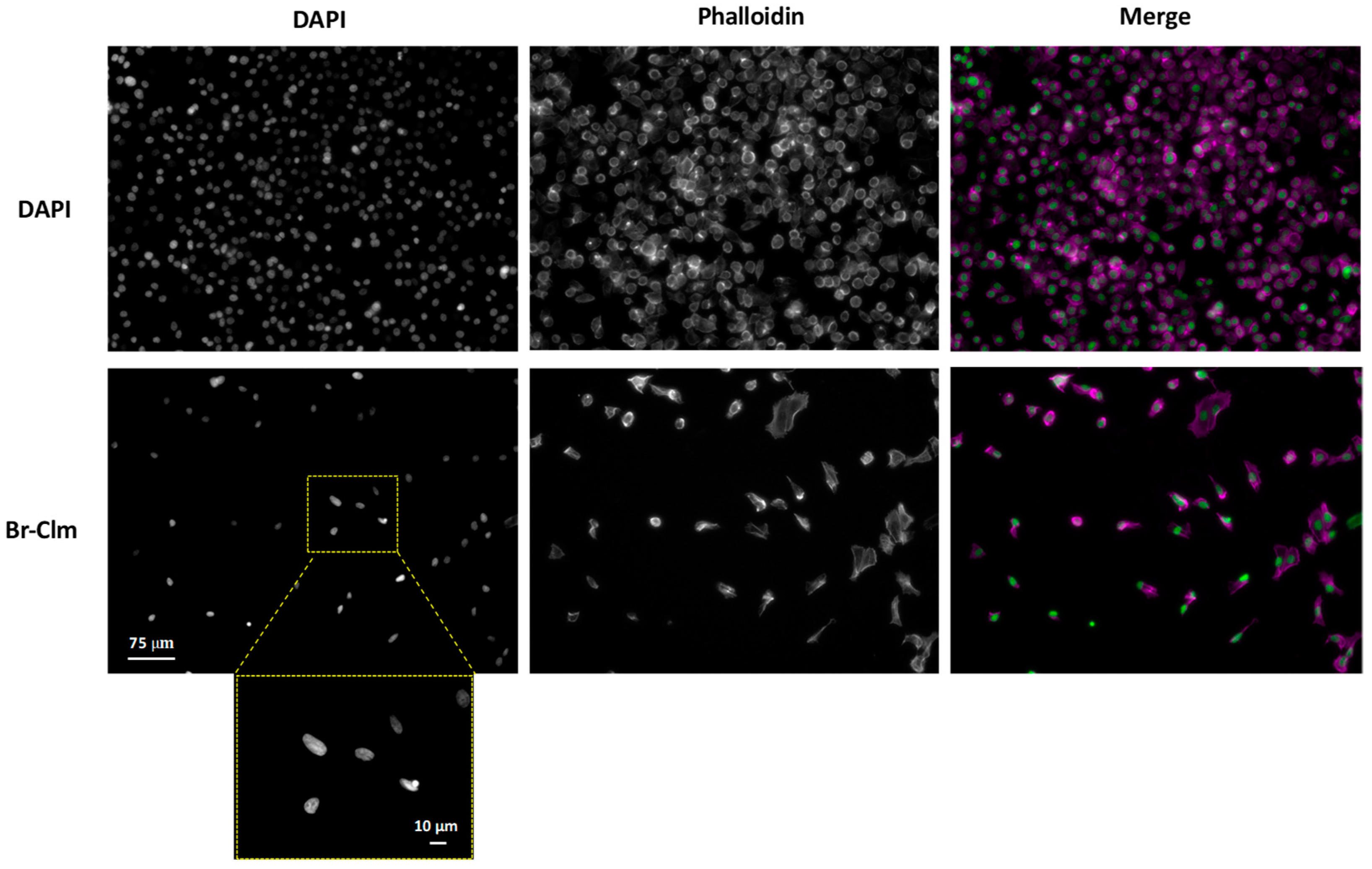
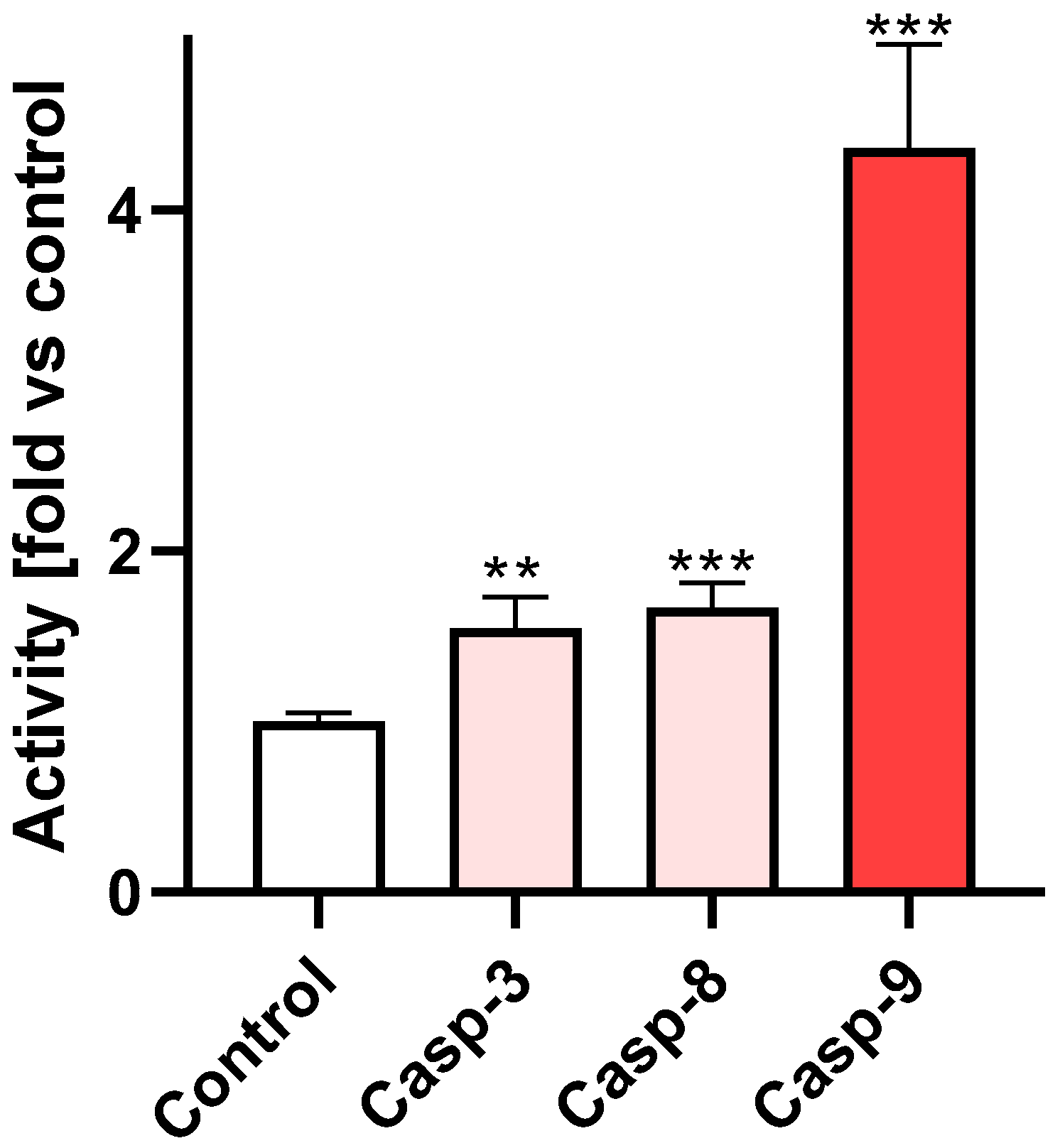
2.3. Br-Clm Triggers Cancer Cell Death via Apoptosis
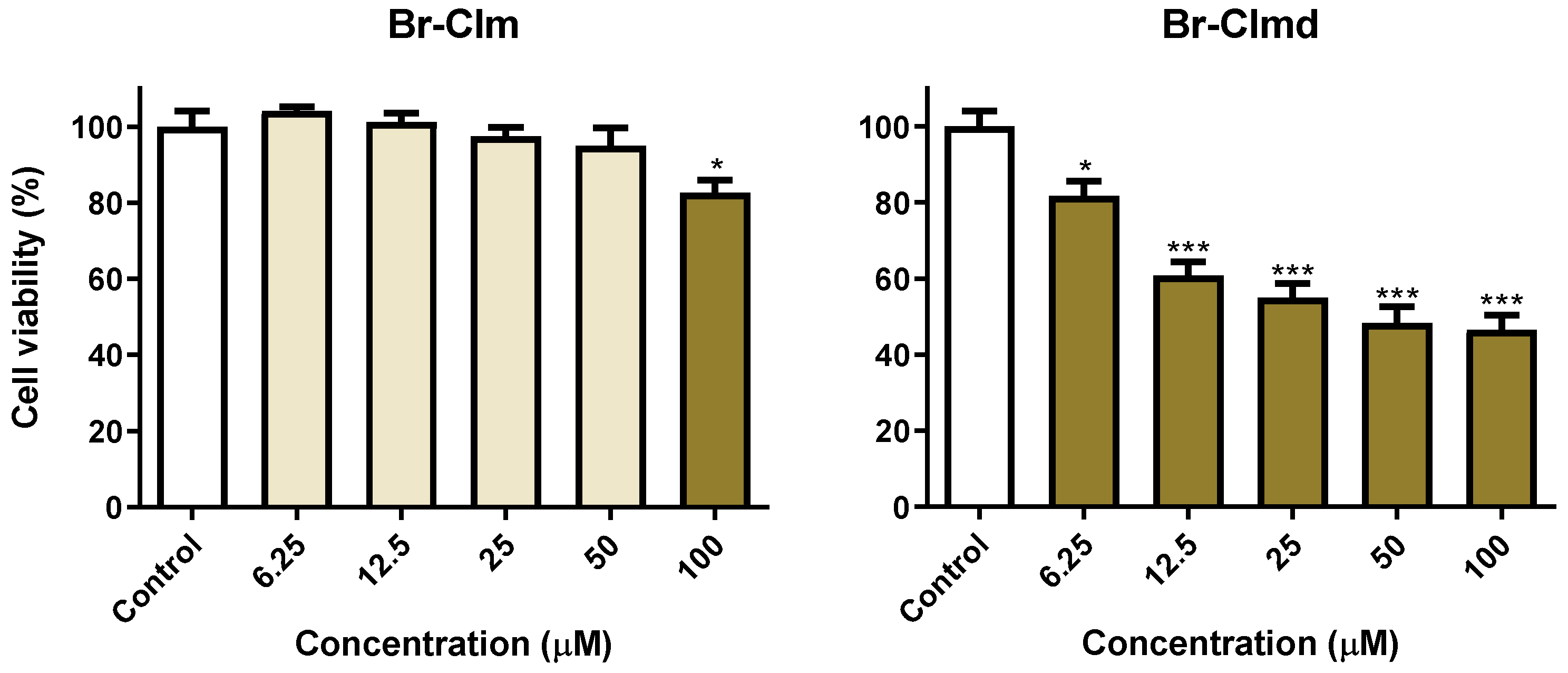
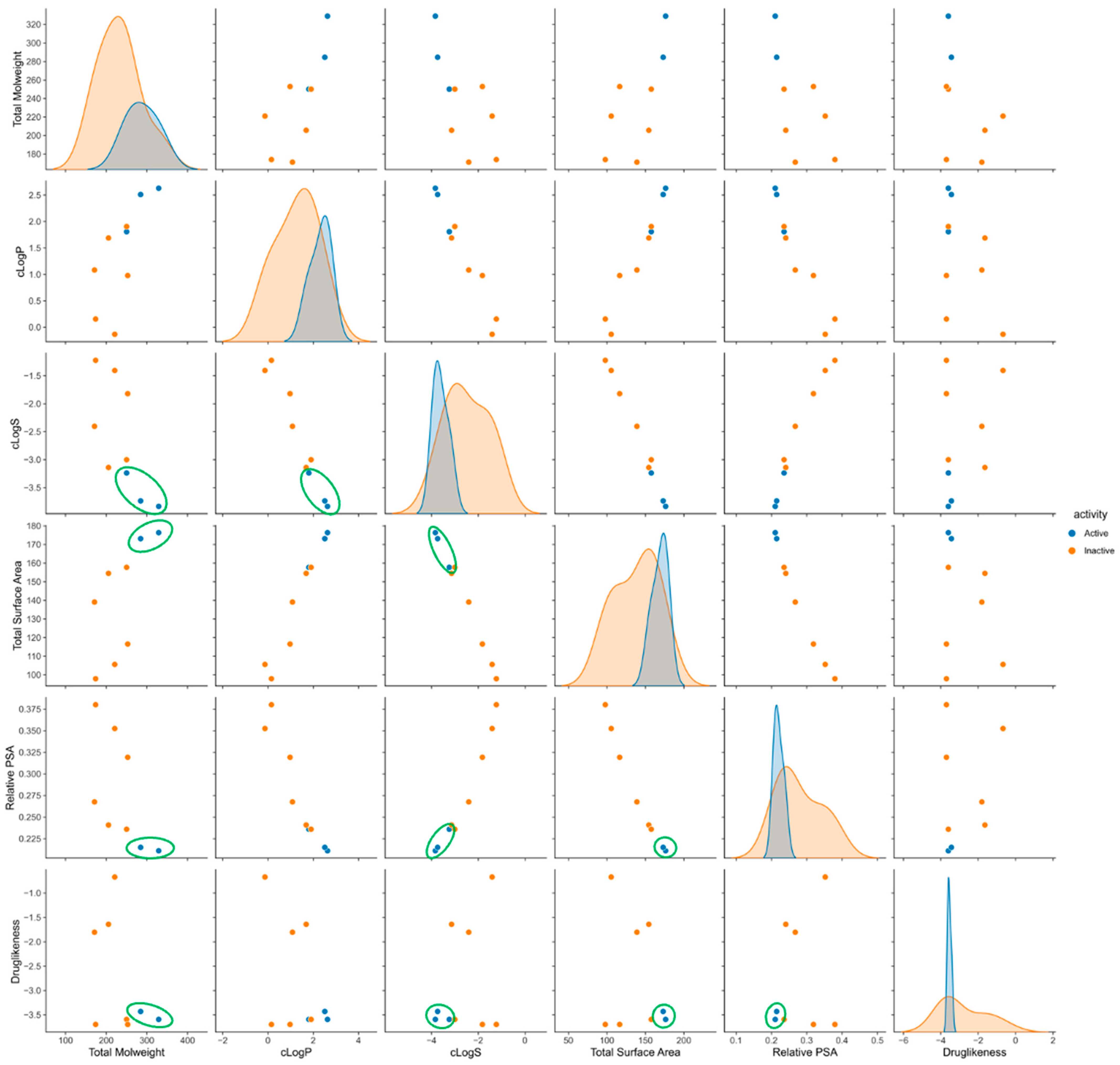
2.4. Exploring the Br-Clm Chemical Space
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Photophysical Characterization and Stability Studies
4.3. Cell Culture
4.4. Assessment of Viability—MTT Assay
4.5. Morphological Analysis
4.6. Caspase Activity Assay
4.7. Chemometric Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer Research UK. Worldwide Cancer Statistics. Available online: Cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer#heading-One (accessed on 28 March 2022).
- Kreso, A.; Dick, J.E. Evolution of the Cancer Stem Cell Model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishayee, A.; Block, K. A broad-spectrum integrative design for cancer prevention and therapy: The challenge ahead. Semin. Cancer Biol. 2015, 35, S1–S4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dienstmann, R.; Serpico, D.; Rodón, J.; Saura, C.; Macarulla, T.; Elez, E.; Alsina, M.; Capdevila, J.; Pérez-García, J.; Sánchez-Ollé, G.; et al. Molecular Profiling of Patients with Colorectal Cancer and Matched Targeted Therapy in Phase I Clinical Trials. Mol. Cancer Ther. 2012, 11, 2062–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Zhou, L.; Wei, S. A 2-pyridone modified zinc phthalocyanine with three-in-one multiple functions for photodynamic therapy. Chem. Commun. 2021, 57, 3127–3130. [Google Scholar] [CrossRef]
- Li, X.; Shi, Z.; Wu, J.; Wu, J.; He, C.; Hao, X.; Duan, C. Lighting up metallohelices: From DNA binders to chemotherapy and photodynamic therapy. Chem. Commun. 2020, 56, 7537–7548. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-F.; Chen, J.-X.; Chen, W.-C.; Zheng, X.; Cao, C.; Tan, J.; Cui, X.; Yuan, Z.; Ji, S.; Lu, G.; et al. Achieving high singlet-oxygen generation by applying the heavy-atom effect to thermally activated delayed fluorescent materials. Chem. Commun. 2021, 57, 4902–4905. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.-I.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Magalhães, C.M.; da Silva, J.C.G.E.; da Silva, L.P. Chemiluminescence and Bioluminescence as an Excitation Source in the Photodynamic Therapy of Cancer: A Critical Review. ChemPhysChem 2016, 17, 2286–2294. [Google Scholar] [CrossRef]
- da Silva, L.P.; Núñez-Montenegro, A.; Magalhães, C.M.; Ferreira, P.J.O.; Duarte, D.; González-Berdullas, P.; Rodríguez-Borges, J.E.; Vale, N.; da Silva, J.C.E. Single-molecule chemiluminescent photosensitizer for a self-activating and tumor-selective photodynamic therapy of cancer. Eur. J. Med. Chem. 2019, 183, 111683. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Magalhães, C.M.; Núñez-Montenegro, A.; Ferreira, P.J.O.; Duarte, D.; Rodríguez-Borges, J.E.; Vale, N.; da Silva, J.C.E. Study of the Combination of Self-Activating Photodynamic Therapy and Chemotherapy for Cancer Treatment. Biomolecules 2019, 9, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, C.M.; González-Berdullas, P.; Duarte, D.; Correia, A.S.; Rodríguez-Borges, J.E.; Vale, N.; da Silva, J.C.E.; da Silva, L.P. Target-Oriented Synthesis of Marine Coelenterazine Derivatives with Anticancer Activity by Applying the Heavy-Atom Effect. Biomedicines 2021, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Bronsart, L.L.; Stokes, C.; Contag, C.H. Multimodality Imaging of Cancer Superoxide Anion Using the Small Molecule Coelenterazine. Mol. Imaging Biol. 2016, 18, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Kaskova, Z.M.; Tsarkova, A.S.; Yampolsky, I.V. 1001 Lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Magalhães, C.M.; da Silva, J.C.E. Interstate Crossing-Induced Chemiexcitation Mechanism as the Basis for Imidazopyrazinone Bioluminescence. ChemistrySelect 2016, 1, 3343–3356. [Google Scholar] [CrossRef]
- Magalhães, C.M.; da Silva, J.C.E.; da Silva, L.P. Study of coelenterazine luminescence: Electrostatic interactions as the controlling factor for efficient chemiexcitation. J. Lumin. 2018, 199, 339–347. [Google Scholar] [CrossRef]
- Magalhães, C.M.; da Silva, J.C.E.; da Silva, L.P. Comparative study of the chemiluminescence of coelenterazine, coelenterazine-e and Cypridina luciferin with an experimental and theoretical approach. J. Photochem. Photobiol. B Biol. 2019, 190, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Johnson, F.H. Chemical nature of bioluminescence systems in coelenterates. Proc. Natl. Acad. Sci. USA 1975, 72, 1546–1549. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Ribeiro, V.; Andrade, P.B.; Valentão, P.; Pereira, D.M. Benzoquinones from Cyperus spp. trigger IRE1α-independent and PERK-dependent ER stress in human stomach cancer cells and are novel proteasome inhibitors. Phytomedicine 2019, 63, 153017. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.C.; Andrade, P.B.; Valentão, P.; Pereira, D.M. Neurotoxicity of the steroidal alkaloids tomatine and tomatidine is RIP1 kinase- and caspase-independent and involves the eIF2α branch of the endoplasmic reticulum. J. Steroid Biochem. Mol. Biol. 2017, 171, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.R.; Pereira, D.M.; Aroso, I.M.; Santos, T.; Batista, M.T.; Cerqueira, M.T.; Marques, A.P.; Reis, R.L.; Pires, R.A. Cork extracts reduce UV-mediated DNA fragmentation and cell death. RSC Adv. 2015, 5, 96151–96157. [Google Scholar] [CrossRef] [Green Version]
- Andrés, A.; Rosés, M.; Ràfols, C.; Bosch, E.; Espinosa, S.; Segarra, V.; Huerta, J.M. Setup and validation of shake-flask procedures for the determination of partition coefficients (logD) from low drug amounts. Eur. J. Pharm. Sci. 2015, 76, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lipinski, C.A. Rule of five in 2015 and beyond: Target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug Deliv. Rev. 2016, 101, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Pereira, R.B.; Pinto, N.F.S.; Coelho, C.M.M.; Fernandes, M.J.G.; Fortes, A.G.; Gonçalves, M.S.T.; Pereira, D.M. Eugenol β-Amino/β-Alkoxy Alcohols with Selective Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 3759. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program For Chemistry Aware Data Visualization And Analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Reback, J.; Jbrockmendel; McKinney, W.; Bossche, J.V.D.; Augspurger, T.; Roeschke, M.; Hawkins, S.; Cloud, P.; Gfyoung; Sinhrks; et al. Pandas, v1.4.2. 2020. Available online: https://zenodo.org/record/6408044#.Yt6mvIRBzIU (accessed on 28 March 2022).
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Berdullas, P.; Pereira, R.B.; Teixeira, C.; Silva, J.P.; Magalhães, C.M.; Rodríguez-Borges, J.E.; Pereira, D.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Discovery of the Anticancer Activity for Lung and Gastric Cancer of a Brominated Coelenteramine Analog. Int. J. Mol. Sci. 2022, 23, 8271. https://doi.org/10.3390/ijms23158271
González-Berdullas P, Pereira RB, Teixeira C, Silva JP, Magalhães CM, Rodríguez-Borges JE, Pereira DM, Esteves da Silva JCG, Pinto da Silva L. Discovery of the Anticancer Activity for Lung and Gastric Cancer of a Brominated Coelenteramine Analog. International Journal of Molecular Sciences. 2022; 23(15):8271. https://doi.org/10.3390/ijms23158271
Chicago/Turabian StyleGonzález-Berdullas, Patricia, Renato B. Pereira, Cláudia Teixeira, José Pedro Silva, Carla M. Magalhães, José E. Rodríguez-Borges, David M. Pereira, Joaquim C. G. Esteves da Silva, and Luís Pinto da Silva. 2022. "Discovery of the Anticancer Activity for Lung and Gastric Cancer of a Brominated Coelenteramine Analog" International Journal of Molecular Sciences 23, no. 15: 8271. https://doi.org/10.3390/ijms23158271
APA StyleGonzález-Berdullas, P., Pereira, R. B., Teixeira, C., Silva, J. P., Magalhães, C. M., Rodríguez-Borges, J. E., Pereira, D. M., Esteves da Silva, J. C. G., & Pinto da Silva, L. (2022). Discovery of the Anticancer Activity for Lung and Gastric Cancer of a Brominated Coelenteramine Analog. International Journal of Molecular Sciences, 23(15), 8271. https://doi.org/10.3390/ijms23158271











