Impact of Copper-Doped Mesoporous Bioactive Glass Nanospheres on the Polymerisation Kinetics and Shrinkage Stress of Dental Resin Composites
Abstract
1. Introduction
- (1)
- Adding Cu-MBGN has no effect on light transmission, degree of conversion, maximum polymerisation rate, time to reach maximum polymerisation rate, linear shrinkage, shrinkage stress, maximum shrinkage stress rate, time to reach maximum shrinkage stress rate, and depth of cure.
- (2)
- There is no difference between composites containing only Cu-MBGN and those containing combined Cu-MBGN and silica of 12 nm particle size in any of the examined parameters.
- (3)
- There is no difference between Cu-MBGN and the conventional 45S5 BG of 4 μm particle size in any of the examined parameters.
2. Results
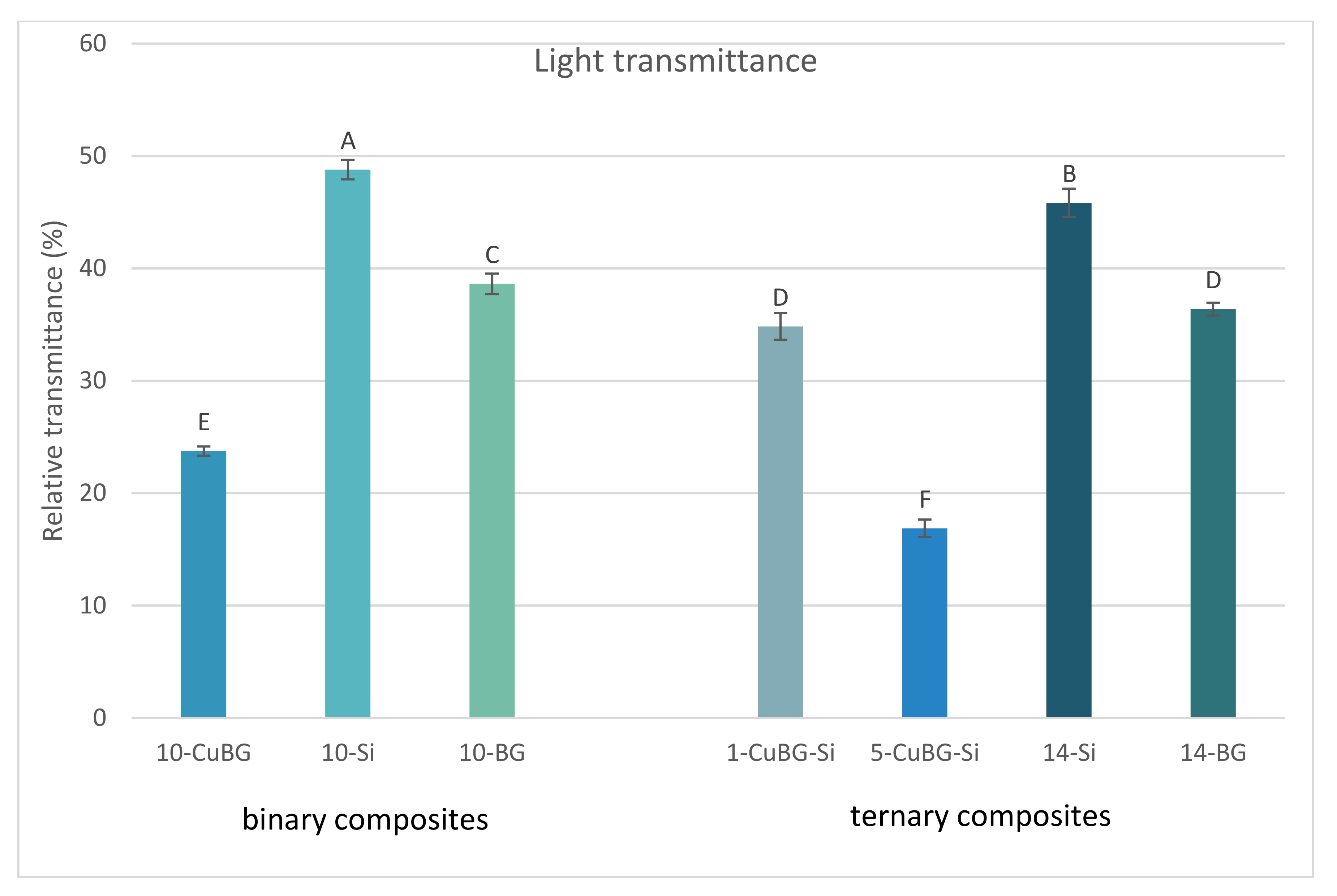
2.1. Light Transmittance
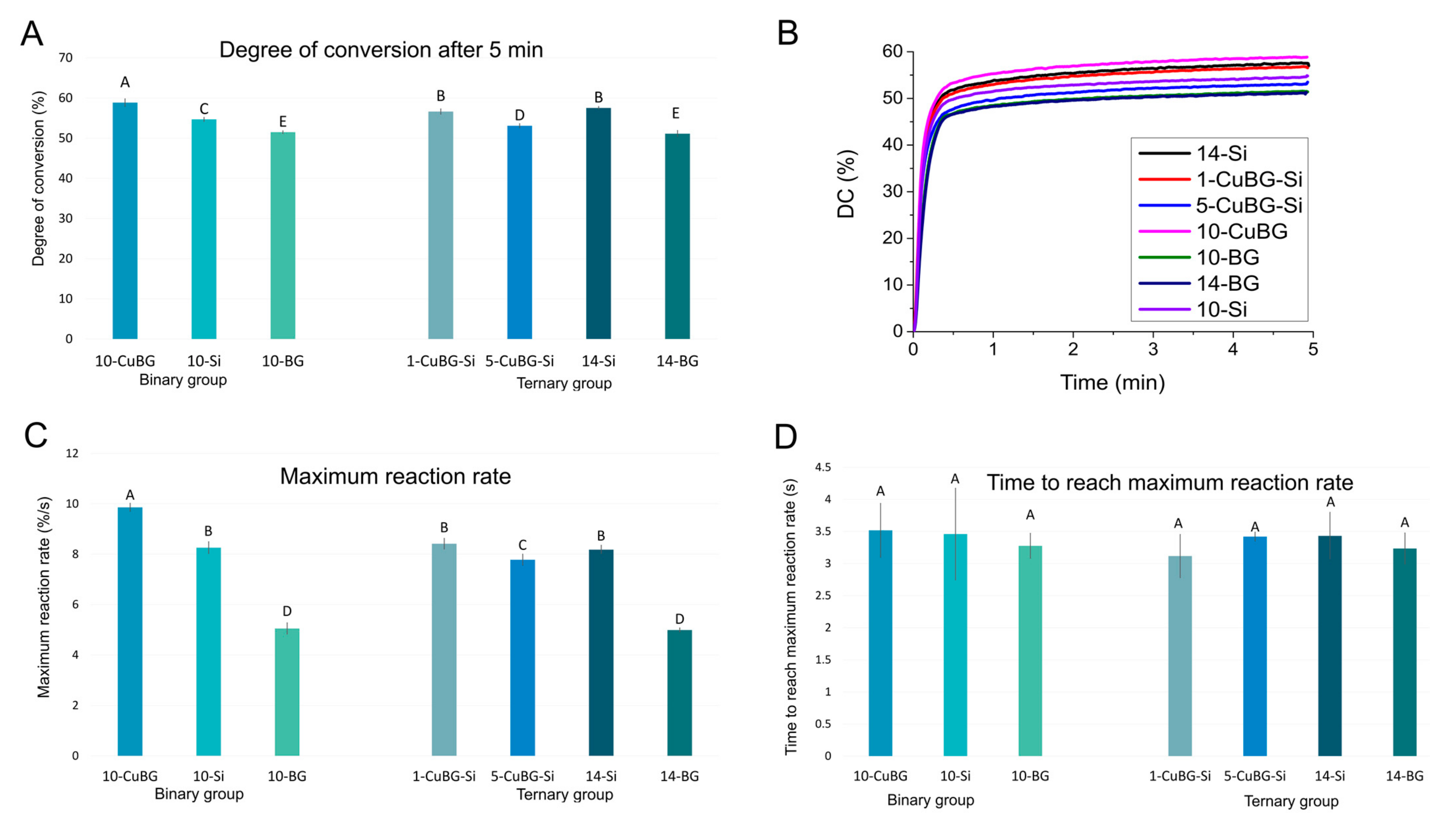
2.2. Polymerisation Kinetics
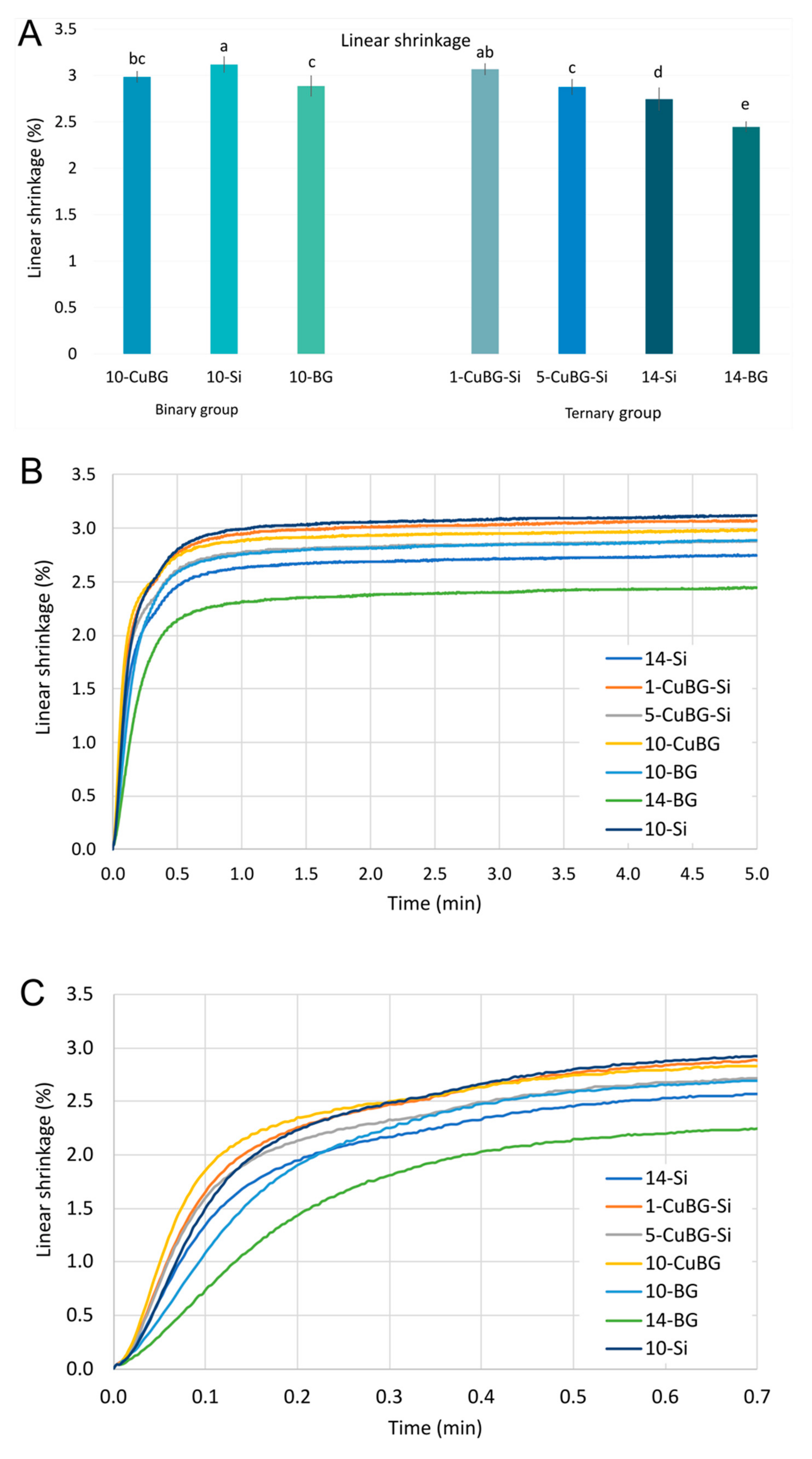
2.3. Linear Shrinkage
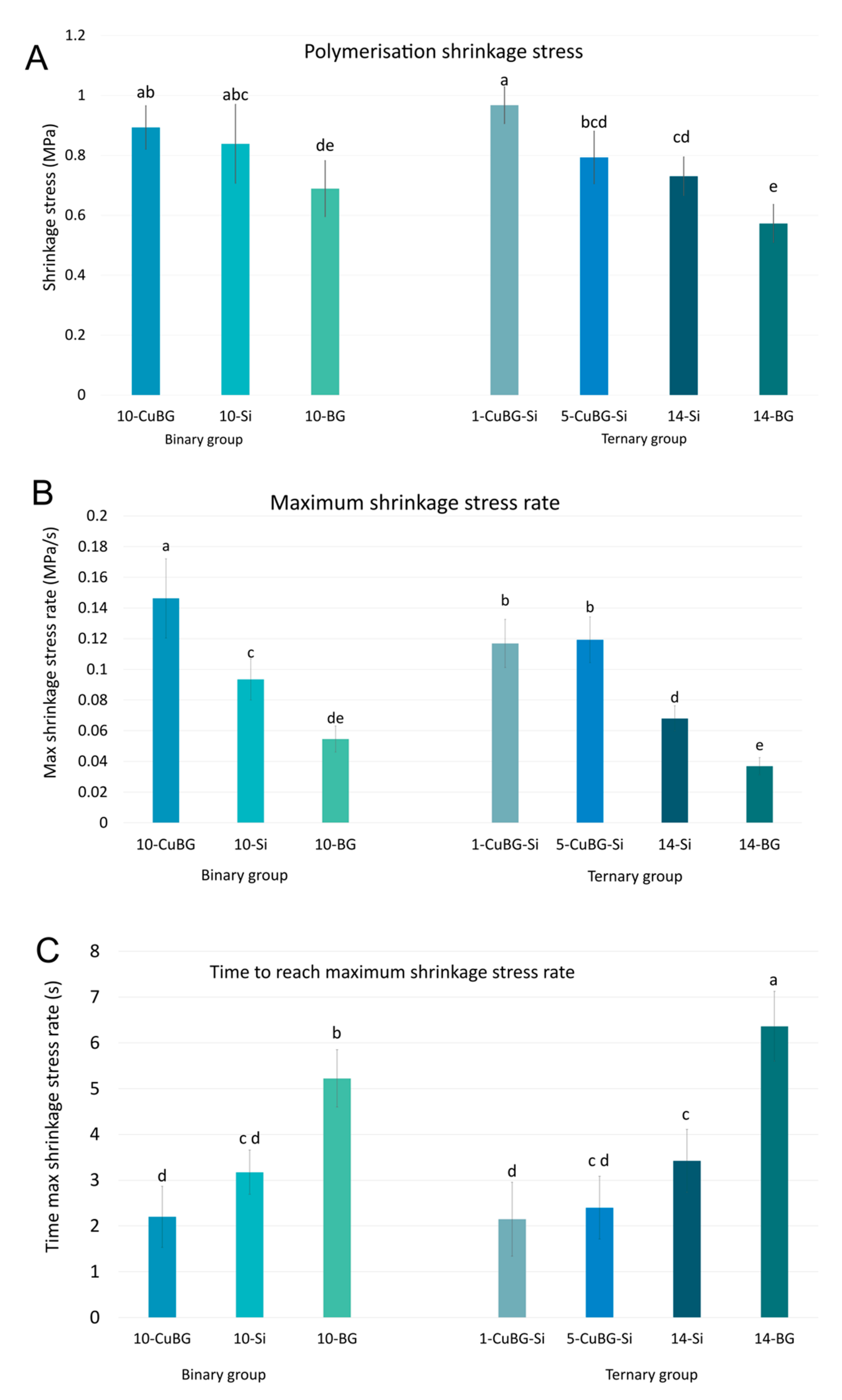
2.4. Polymerisation Shrinkage Stress
2.5. ISO 4049 Depth of Cure
3. Discussion
3.1. Binary Composites
3.2. Ternary Composites
4. Materials and Methods
4.1. Materials
4.2. Light Transmittance
4.3. Polymerisation Kinetics
4.4. Polymerisation Shrinkage
4.5. Polymerisation Shrinkage Stress
4.6. Depth of Cure
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simila, H.O.; Boccaccini, A.R. Sol-gel bioactive glass containing biomaterials for restorative dentistry: A review. Dent. Mater. 2022, 38, 725–747. [Google Scholar] [CrossRef]
- Tiskaya, M.; Shahid, S.; Gillam, D.; Hill, R. The use of bioactive glass (BAG) in dental composites: A critical review. Dent. Mater. 2021, 37, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Marovic, D.; Tarle, Z.; Hiller, K.A.; Muller, R.; Ristic, M.; Rosentritt, M.; Skrtic, D.; Schmalz, G. Effect of silanized nanosilica addition on remineralizing and mechanical properties of experimental composite materials with amorphous calcium phosphate. Clin. Oral Investig. 2014, 18, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Marovic, D.; Tarle, Z.; Hiller, K.A.; Muller, R.; Rosentritt, M.; Skrtic, D.; Schmalz, G. Reinforcement of experimental composite materials based on amorphous calcium phosphate with inert fillers. Dent. Mater. 2014, 30, 1052–1060. [Google Scholar] [CrossRef]
- Marovic, D.; Haugen, H.J.; Negovetic Mandic, V.; Par, M.; Zheng, K.; Tarle, Z.; Boccaccini, A.R. Incorporation of Copper-Doped Mesoporous Bioactive Glass Nanospheres in Experimental Dental Composites: Chemical and Mechanical Characterization. Materials 2021, 14, 2611. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Attin, T.; Tarle, Z.; Taubock, T.T. A New Customized Bioactive Glass Filler to Functionalize Resin Composites: Acid-Neutralizing Capability, Degree of Conversion, and Apatite Precipitation. J. Clin. Med. 2020, 9, 1173. [Google Scholar] [CrossRef]
- Marovic, D.; Sariri, K.; Demoli, N.; Ristic, M.; Hiller, K.A.; Skrtic, D.; Rosentritt, M.; Schmalz, G.; Tarle, Z. Remineralizing amorphous calcium phosphate based composite resins: The influence of inert fillers on monomer conversion, polymerization shrinkage, and microhardness. Croat. Med. J. 2016, 57, 465–473. [Google Scholar] [CrossRef]
- Opdam, N.J.; van de Sande, F.H.; Bronkhorst, E.; Cenci, M.S.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.C.; van Dijken, J.W. Longevity of posterior composite restorations: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef]
- Mjor, I.A.; Moorhead, J.E.; Dahl, J.E. Reasons for replacement of restorations in permanent teeth in general dental practice. Int. Dent. J. 2000, 50, 361–366. [Google Scholar] [CrossRef]
- Eltahlah, D.; Lynch, C.D.; Chadwick, B.L.; Blum, I.R.; Wilson, N.H.F. An update on the reasons for placement and replacement of direct restorations. J. Dent. 2018, 72, 1–7. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; De Munck, J.; Vanloy, A.; Declerck, D.; Lambrechts, P.; Peumans, M.; Teughels, W.; Van Meerbeek, B.; Van Landuyt, K.L. Secondary caries: Prevalence, characteristics, and approach. Clin. Oral Investig. 2020, 24, 683–691. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is secondary caries with composites a material-based problem? Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; De Munck, J.; Slomka, V.; Van Meerbeek, B.; Teughels, W.; Van Landuyt, K.L. Lack of Buffering by Composites Promotes Shift to More Cariogenic Bacteria. J. Dent. Res. 2016, 95, 875–881. [Google Scholar] [CrossRef]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.J.; Faria, E.S.A.L.; Rodrigues, M.P.; Vilela, A.B.F.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization shrinkage stress of composite resins and resin cements—What do we need to know? Braz. Oral Res. 2017, 31, e62. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.A.; Joyston-Bechal, S.; Beighton, D. Marginal ditching and staining as a predictor of secondary caries around amalgam restorations: A clinical and microbiological study. J. Dent. Res. 1995, 74, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Dennison, J.B.; Sarrett, D.C. Prediction and diagnosis of clinical outcomes affecting restoration margins. J. Oral Rehabil. 2012, 39, 301–318. [Google Scholar] [CrossRef]
- Khvostenko, D.; Salehi, S.; Naleway, S.E.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Cyclic mechanical loading promotes bacterial penetration along composite restoration marginal gaps. Dent. Mater. 2015, 31, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Khvostenko, D.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Zheng, K.; Sui, B.; Ilyas, K.; Boccaccini, A.R. Porous bioactive glass micro- and nanospheres with controlled morphology: Developments, properties and emerging biomedical applications. Mater. Horiz. 2021, 8, 300–335. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Kang, J.; Rutkowski, B.; Gaweda, M.; Zhang, J.; Wang, Y.; Founier, N.; Sitarz, M.; Taccardi, N.; Boccaccini, A.R. Toward Highly Dispersed Mesoporous Bioactive Glass Nanoparticles with High Cu Concentration Using Cu/Ascorbic Acid Complex as Precursor. Front. Chem. 2019, 7, 497. [Google Scholar] [CrossRef] [PubMed]
- Paterson, T.E.; Bari, A.; Bullock, A.J.; Turner, R.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C.; MacNeil, S.; Shepherd, J. Multifunctional Copper-Containing Mesoporous Glass Nanoparticles as Antibacterial and Proangiogenic Agents for Chronic Wounds. Front. Bioeng. Biotechnol. 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Yu, O.Y.; Lung, C.Y.K.; Chu, C.H. Application of Copper Nanoparticles in Dentistry. Nanomaterials 2022, 12, 805. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Investigations on mechanical behaviour of dental composites. Clin. Oral Investig. 2009, 13, 427–438. [Google Scholar] [CrossRef]
- Ilie, N.; Bucuta, S.; Draenert, M. Bulk-fill resin-based composites: An in vitro assessment of their mechanical performance. Oper. Dent. 2013, 38, 618–625. [Google Scholar] [CrossRef]
- Balbinot, G.S.; Leitune, V.C.B.; Ogliari, F.A.; Collares, F.M. Niobium silicate particles as bioactive fillers for composite resins. Dent. Mater. 2020, 36, 1578–1585. [Google Scholar] [CrossRef]
- Bai, X.; Lin, C.; Wang, Y.; Ma, J.; Wang, X.; Yao, X.; Tang, B. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 2020, 36, 794–807. [Google Scholar] [CrossRef]
- Damen, J.J.; Ten Cate, J.M. Silica-induced precipitation of calcium phosphate in the presence of inhibitors of hydroxyapatite formation. J. Dent. Res. 1992, 71, 453–457. [Google Scholar] [CrossRef]
- Ilie, N. Impact of light transmittance mode on polymerisation kinetics in bulk-fill resin-based composites. J. Dent. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Musanje, L.; Darvell, B.W. Curing-light attenuation in filled-resin restorative materials. Dent. Mater. 2006, 22, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.; Sjodahl, M.; Soderholm, K.J. How filler properties, filler fraction, sample thickness and light source affect light attenuation in particulate filled resin composites. Dent. Mater. 2005, 21, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Marovic, D.; Skenderovic, H.; Gamulin, O.; Klaric, E.; Tarle, Z. Light transmittance and polymerization kinetics of amorphous calcium phosphate composites. Clin. Oral Investig. 2017, 21, 1173–1182. [Google Scholar] [CrossRef]
- Clewell, D.H. Scattering of Light by Pigment Particles. J. Opt. Soc. Am. 1941, 31, 521–527. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Zhang, D.; Miao, Y.; Li, G. Controllable synthesis of mesoporous multi-shelled ZnO microspheres as efficient photocatalysts for NO oxidation. Appl. Surf. Sci. 2018, 435, 468–475. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Y.; Zhang, H.; Yan, H.; Lv, H.; Jiang, B. Sol–Gel Preparation of Hydrophobic Silica Antireflective Coatings with Low Refractive Index by Base/Acid Two-Step Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 11470–11475. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T. Mesoporous architecture of TiO2 microspheres via controlled template assisted route and their photoelectrochemical properties. J. Mater. Sci. Mater. Electron. 2017, 28, 304–316. [Google Scholar] [CrossRef]
- Zhang, Q.; Myers, D.; Lan, J.; Jenekhe, S.A.; Cao, G. Applications of light scattering in dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2012, 14, 14982–14998. [Google Scholar] [CrossRef]
- Ilie, N.; Keßler, A.; Durner, J. Influence of various irradiation processes on the mechanical properties and polymerisation kinetics of bulk-fill resin based composites. J. Dent. 2013, 41, 695–702. [Google Scholar] [CrossRef]
- Par, M.; Gamulin, O.; Marovic, D.; Klaric, E.; Tarle, Z. Raman spectroscopic assessment of degree of conversion of bulk-fill resin composites—Changes at 24 hours post cure. Oper. Dent. 2015, 40, E92–E101. [Google Scholar] [CrossRef]
- Shortall, A.C.; Palin, W.M.; Burtscher, P. Refractive index mismatch and monomer reactivity influence composite curing depth. J. Dent. Res. 2008, 87, 84–88. [Google Scholar] [CrossRef]
- Lehtinen, J.; Laurila, T.; Lassila, L.V.; Vallittu, P.K.; Raty, J.; Hernberg, R. Optical characterization of bisphenol-A-glycidyldimethacrylate-triethyleneglycoldimethacrylate (BisGMA/TEGDMA) monomers and copolymer. Dent. Mater. 2008, 24, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Mohn, D.; Attin, T.; Tarle, Z.; Taubock, T.T. Polymerization shrinkage behaviour of resin composites functionalized with unsilanized bioactive glass fillers. Sci. Rep. 2020, 10, 15237. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Developing a more complete understanding of stresses produced in dental composites during polymerization. Dent. Mater. 2005, 21, 36–42. [Google Scholar] [CrossRef]
- Condon, J.R.; Ferracane, J.L. Reduction of composite contraction stress through non-bonded microfiller particles. Dent. Mater. 1998, 14, 256–260. [Google Scholar] [CrossRef]
- Condon, J.R.; Ferracane, J.L. Reduced polymerization stress through non-bonded nanofiller particles. Biomaterials 2002, 23, 3807–3815. [Google Scholar] [CrossRef]
- Meereis, C.T.W.; Munchow, E.A.; de Oliveira da Rosa, W.L.; da Silva, A.F.; Piva, E. Polymerization shrinkage stress of resin-based dental materials: A systematic review and meta-analyses of composition strategies. J. Mech. Behav. Biomed. Mater. 2018, 82, 268–281. [Google Scholar] [CrossRef]
- Taubock, T.T.; Jager, F.; Attin, T. Polymerization shrinkage and shrinkage force kinetics of high- and low-viscosity dimethacrylate- and ormocer-based bulk-fill resin composites. Odontology 2019, 107, 103–110. [Google Scholar] [CrossRef]
- Marovic, D.; Taubock, T.T.; Attin, T.; Panduric, V.; Tarle, Z. Monomer conversion and shrinkage force kinetics of low-viscosity bulk-fill resin composites. Acta Odontol. Scand. 2015, 73, 474–480. [Google Scholar] [CrossRef]
- Howard, B.; Wilson, N.D.; Newman, S.M.; Pfeifer, C.S.; Stansbury, J.W. Relationships between conversion, temperature and optical properties during composite photopolymerization. Acta Biomater. 2010, 6, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Björnmalm, M.; Wise, A.K.; Cortez-Jugo, C.; Revalor, E.; Ju, Y.; Feeney, O.M.; Richardson, R.T.; Hanssen, E.; Shepherd, R.K.; et al. Gel-Mediated Electrospray Assembly of Silica Supraparticles for Sustained Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 31019–31031. [Google Scholar] [CrossRef] [PubMed]
- Ruyter, I.E.; Øysæd, H. Conversion in different depths of ultraviolet and visible light activated composite materials. Acta Odontol. Scand. 1982, 40, 179–192. [Google Scholar] [CrossRef]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Polymerization kinetics of experimental bioactive composites containing bioactive glass. J. Dent. 2018, 76, 83–88. [Google Scholar] [CrossRef]
- Taubock, T.T.; Bortolotto, T.; Buchalla, W.; Attin, T.; Krejci, I. Influence of light-curing protocols on polymerization shrinkage and shrinkage force of a dual-cured core build-up resin composite. Eur. J. Oral Sci. 2010, 118, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Lottanti, S.; Taubock, T.T.; Zehnder, M. Shrinkage of backfill gutta-percha upon cooling. J. Endod. 2014, 40, 721–724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Par, M.; Prskalo, K.; Taubock, T.T.; Skenderovic, H.; Attin, T.; Tarle, Z. Polymerization kinetics of experimental resin composites functionalized with conventional (45S5) and a customized low-sodium fluoride-containing bioactive glass. Sci. Rep. 2021, 11, 21225. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Mohn, D.; Attin, T.; Taubock, T.T.; Tarle, Z. Curing potential of experimental resin composites filled with bioactive glass: A comparison between Bis-EMA and UDMA based resin systems. Dent. Mater. 2020, 36, 711–723. [Google Scholar] [CrossRef]

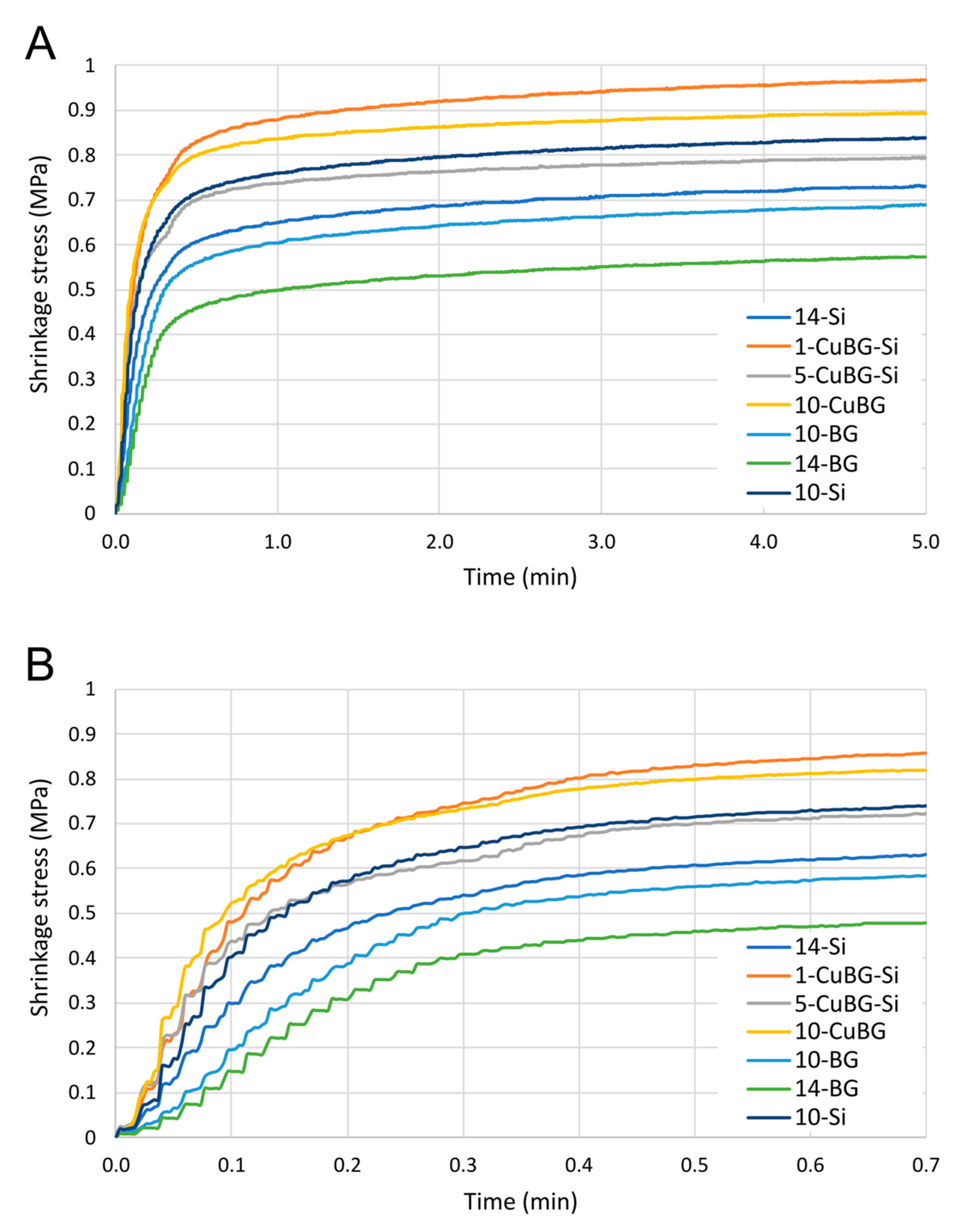
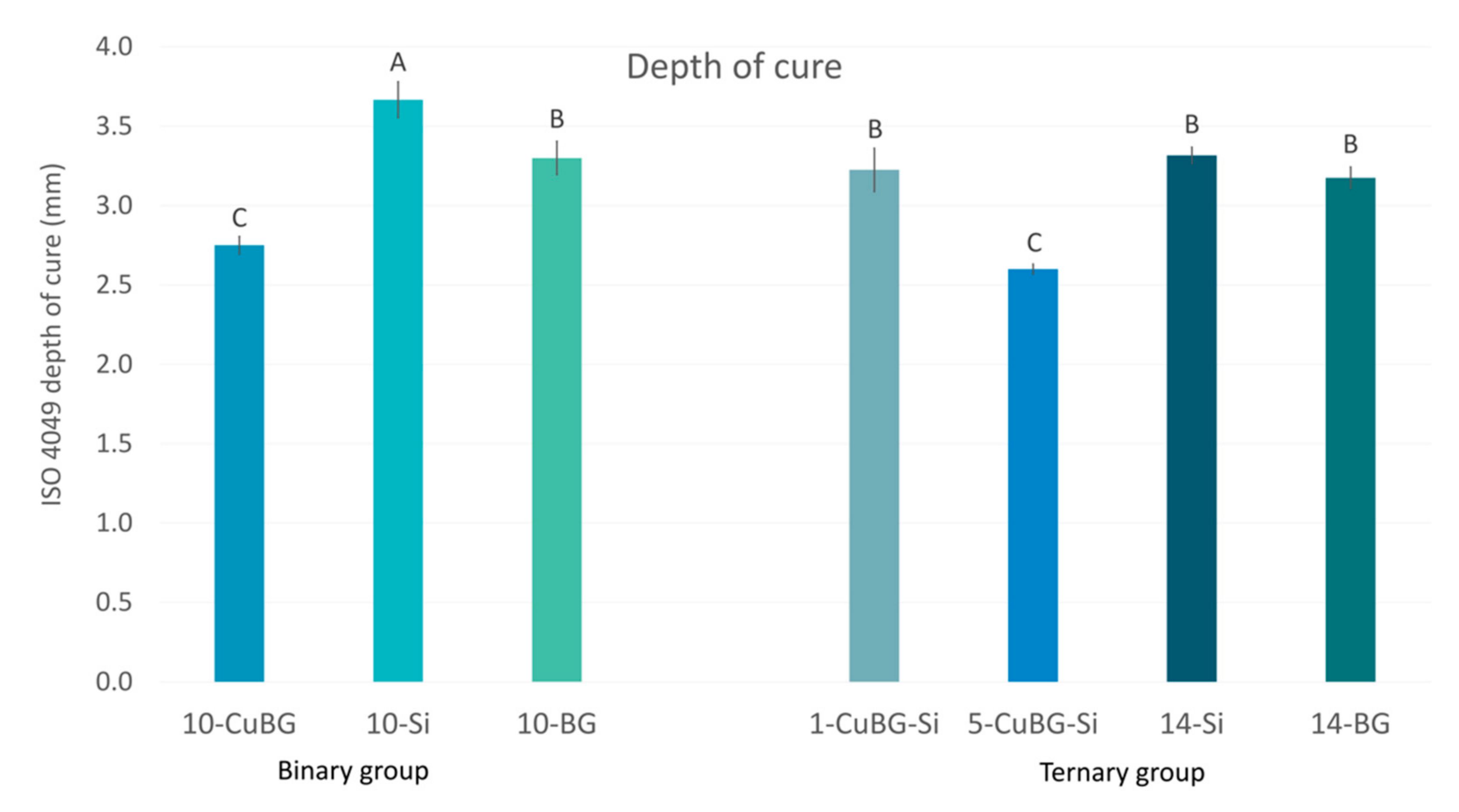
| a | b | c | d | |
|---|---|---|---|---|
| 10-CuBG | 63.34 | 11.63 | 7.55 | 0.65 |
| 10-Si | 59.12 | 10.56 | 5.86 | 0.56 |
| 10-BG | 55.80 | 8.00 | 5.51 | 0.31 |
| 1-CuBG-Si | 58.81 | 11.53 | 7.57 | 0.60 |
| 5-CuBG-Si | 55.92 | 10.96 | 7.01 | 0.66 |
| 14-Si | 61.44 | 10.49 | 7.33 | 0.51 |
| 14-BG | 55.75 | 7.72 | 5.16 | 0.33 |
| Name | Type | Manufacturer/ Product | Composition (wt.%) | Size | Silanisation |
|---|---|---|---|---|---|
| Cu-MBGN | Experimental/bioactive | Produced in-house [22] | SiO2 84.8% CaO 9.4% CuO 5.8% * | ~100 nm | No |
| 45S5 BG | Commercial/bioactive | Schott, Mainz, Germany G018-144 | SiO2 45% Na2O 24.5% CaO 24.5% P2O5 6% | 4.0 μm | No |
| Ba glass | Commercial/inert | Schott, Mainz, Germany GM27884 | SiO2 55.0% BaO 25.0% B2O3 10.0% Al2O3 10.0% | 1.0 μm | Yes 3.2% |
| Silica | Commercial/inert | Evonik Degussa, Hanau, Germany Aerosil DT | SiO2 > 99.8% | 12 nm | Yes 4–6% |
| Group | Material | Resin | Inert Ba Glass Micro Fillers | Silica Nanofillers | Cu-MBGN | 45S5 BG |
|---|---|---|---|---|---|---|
| Binary Composites | 10-CuBG | 35% | 55% | - | 10% | - |
| 10-BG | - | - | 10% | |||
| 10-Si | 10% | - | - | |||
| Ternary Composites | 1-CuBG-Si | 35% | 51% | 13% | 1% | - |
| 5-CuBG-Si | 9% | 5% | - | |||
| 14-BG | - | - | 14% | |||
| 14-Si | 14% | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marovic, D.; Par, M.; Tauböck, T.T.; Haugen, H.J.; Negovetic Mandic, V.; Wüthrich, D.; Burrer, P.; Zheng, K.; Attin, T.; Tarle, Z.; et al. Impact of Copper-Doped Mesoporous Bioactive Glass Nanospheres on the Polymerisation Kinetics and Shrinkage Stress of Dental Resin Composites. Int. J. Mol. Sci. 2022, 23, 8195. https://doi.org/10.3390/ijms23158195
Marovic D, Par M, Tauböck TT, Haugen HJ, Negovetic Mandic V, Wüthrich D, Burrer P, Zheng K, Attin T, Tarle Z, et al. Impact of Copper-Doped Mesoporous Bioactive Glass Nanospheres on the Polymerisation Kinetics and Shrinkage Stress of Dental Resin Composites. International Journal of Molecular Sciences. 2022; 23(15):8195. https://doi.org/10.3390/ijms23158195
Chicago/Turabian StyleMarovic, Danijela, Matej Par, Tobias T. Tauböck, Håvard J. Haugen, Visnja Negovetic Mandic, Damian Wüthrich, Phoebe Burrer, Kai Zheng, Thomas Attin, Zrinka Tarle, and et al. 2022. "Impact of Copper-Doped Mesoporous Bioactive Glass Nanospheres on the Polymerisation Kinetics and Shrinkage Stress of Dental Resin Composites" International Journal of Molecular Sciences 23, no. 15: 8195. https://doi.org/10.3390/ijms23158195
APA StyleMarovic, D., Par, M., Tauböck, T. T., Haugen, H. J., Negovetic Mandic, V., Wüthrich, D., Burrer, P., Zheng, K., Attin, T., Tarle, Z., & Boccaccini, A. R. (2022). Impact of Copper-Doped Mesoporous Bioactive Glass Nanospheres on the Polymerisation Kinetics and Shrinkage Stress of Dental Resin Composites. International Journal of Molecular Sciences, 23(15), 8195. https://doi.org/10.3390/ijms23158195












