Protocadherin Gamma C3 (PCDHGC3) Is Strongly Expressed in Glioblastoma and Its High Expression Is Associated with Longer Progression-Free Survival of Patients
Abstract
:1. Introduction
2. Results
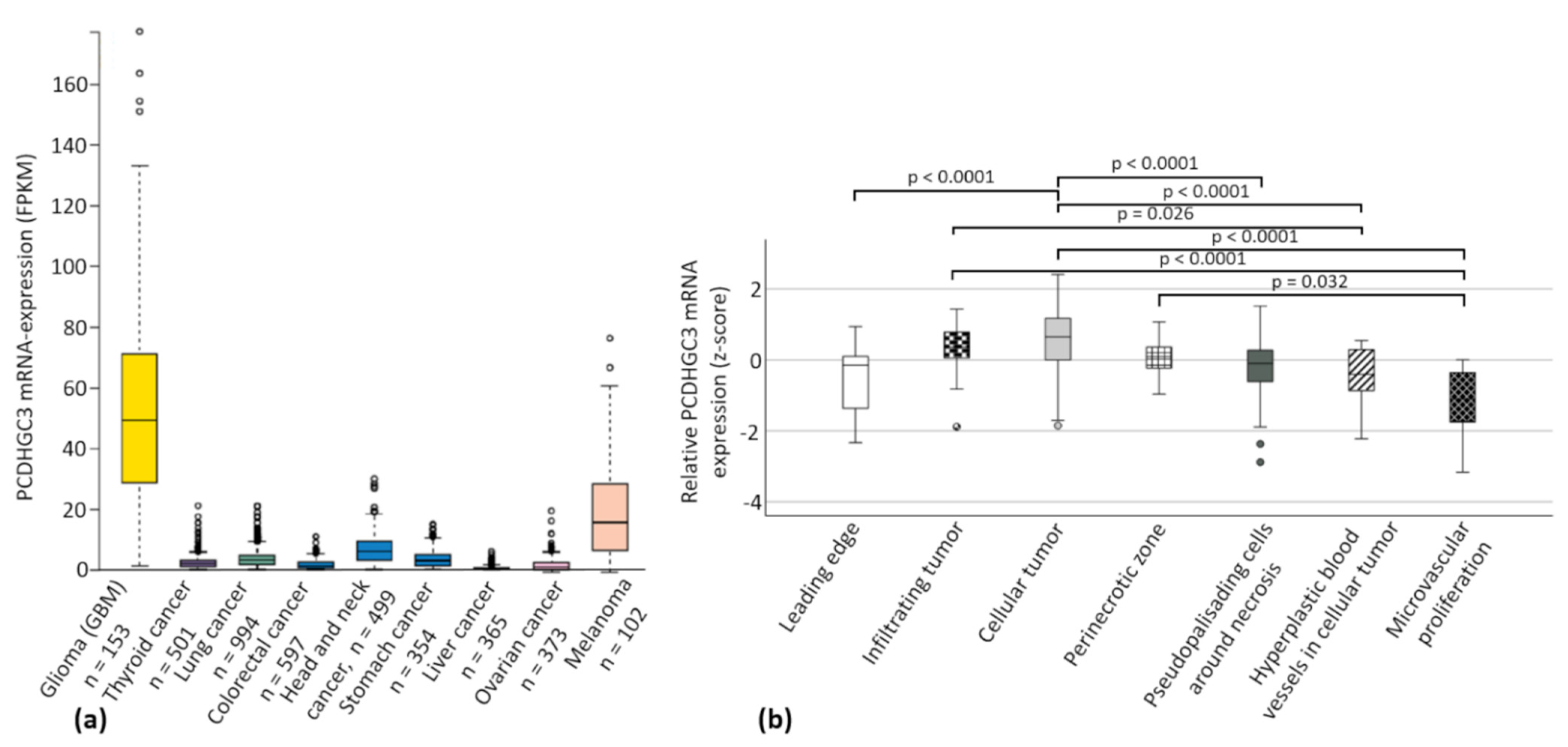
2.1. The Cancer Genome ATLAS (TCGA) Data Show Increased PCDHGC3 mRNA Expression in Gliomas and Melanomas
2.2. Patient Cohorts Characteristics
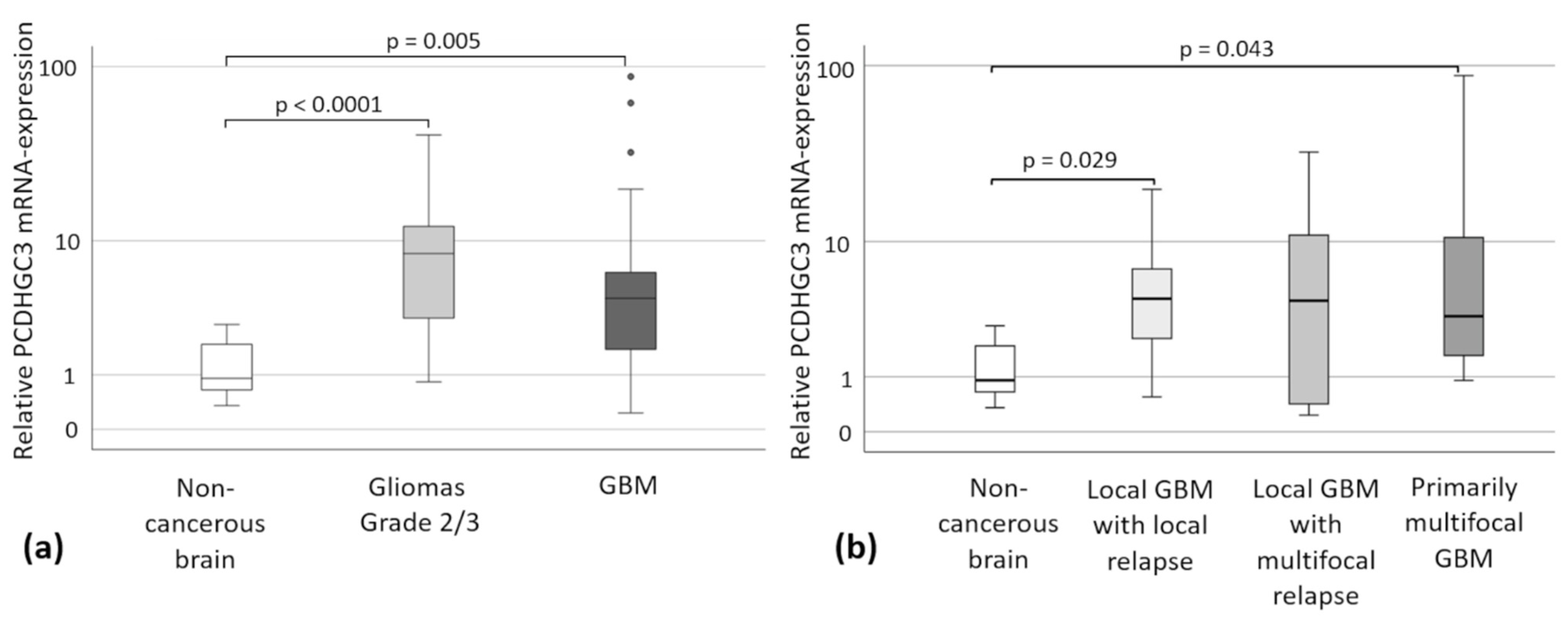
2.3. PCDHGC3 mRNA Expression in Gliomas Grade 2/3 and GBM-Subtypes
2.4. Kaplan-Meier Analyses
2.5. Correlation of Clinical Data with PCDHGC3 Expression
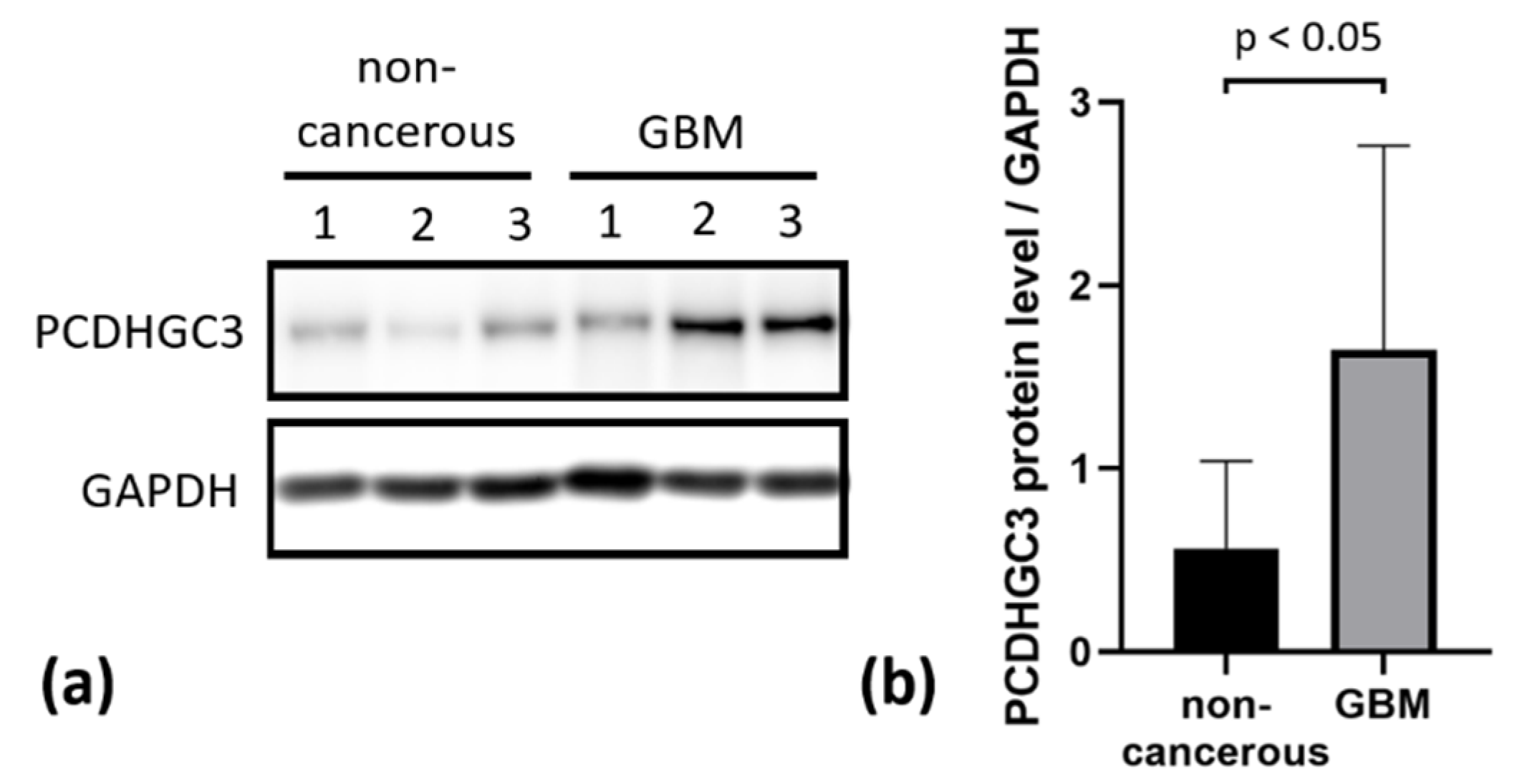
2.6. PCDHGC3 Protein Level Is Increased in GBM
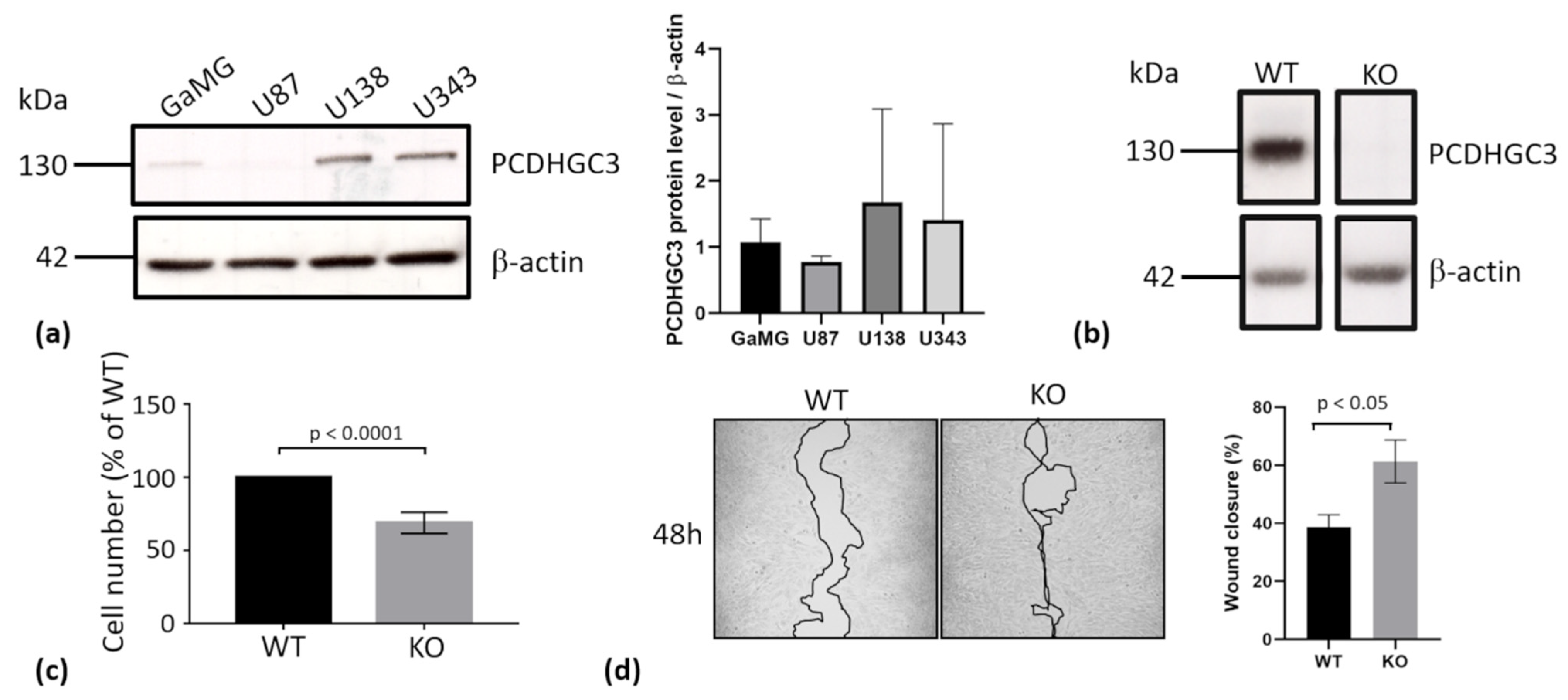
2.7. Deletion of PCDHGC3 in a GBM Cell Line Results in Faster Migration of Knockout Cells
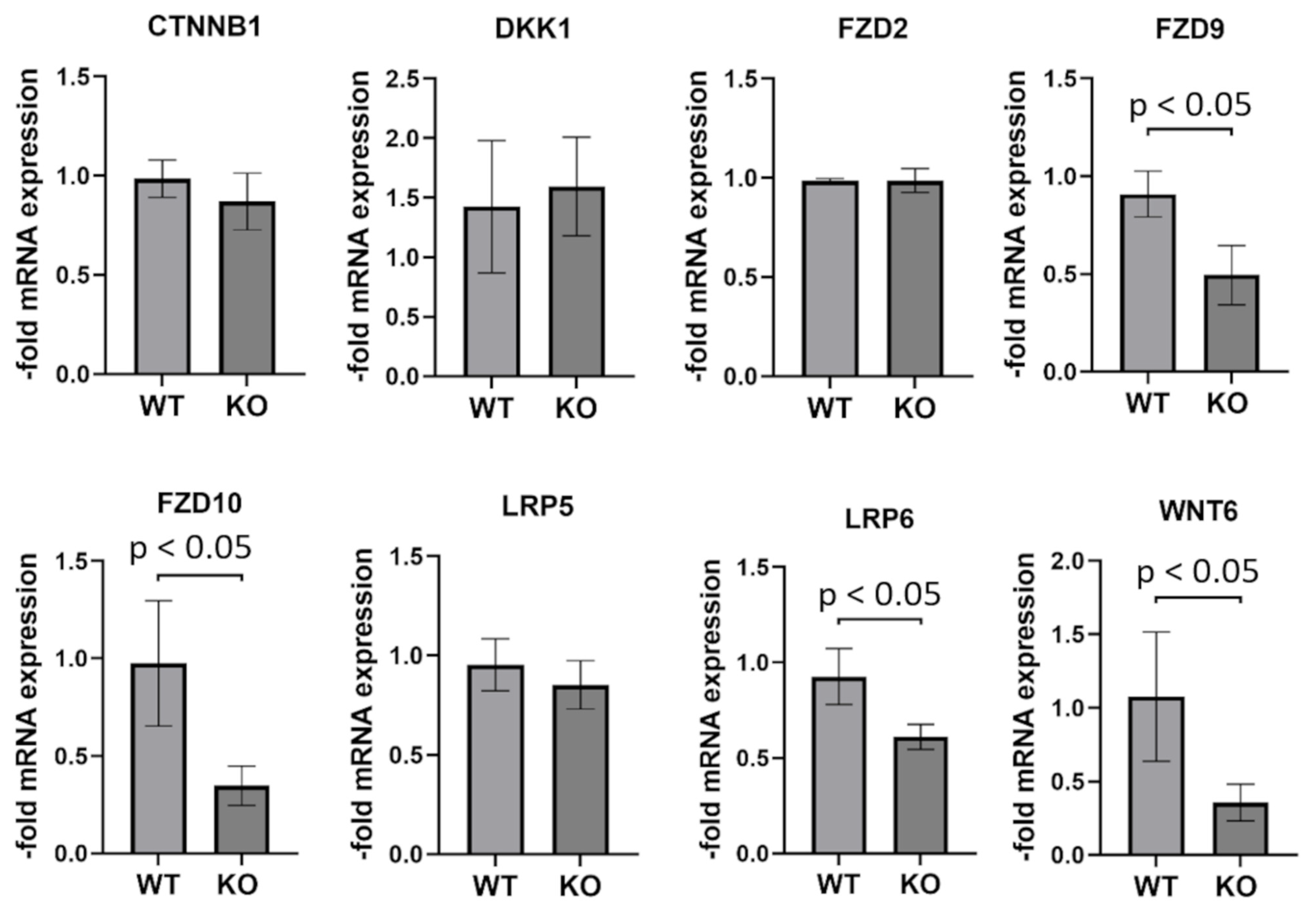
2.8. Genes Involved in WNT (Wingless/Integrated) Signaling Are Partially Downregulated in PCDHGC3 KO U343 Cells
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
4.2. Quantitative Real-Time PCR
4.3. Western Blot
4.4. Generation of the PCDHGC3 Knockout Cell Line
4.5. Wound Healing Assay
4.6. Cell Viability Measurement
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, G.; Collins, V.P. Pathology and molecular genetics of astrocytic gliomas. J. Mol. Med. 2004, 82, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Ius, T.; Mazzucchi, E.; Tomasino, B.; Pauletto, G.; Sabatino, G.; Della Pepa, G.M.; La Rocca, G.; Battistella, C.; Olivi, A.; Skrap, M. Multimodal integrated approaches in low grade glioma surgery. Sci. Rep. 2021, 11, 9964. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Figrarella-Branger, D.; Fuller, G.N.; Giannini, C.; Holland, E.C.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.; Komori, T.; et al. cIMPACT-NOW update 5: Recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020, 139, 603–608. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.K.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. cIMPACT-NOW update 3: Recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018, 136, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Marenco-Hillembrand, L.; Wijesekera, O.; Suarez-Meade, P.; Mampre, D.; Jackson, C.; Peterson, J.; Trifiletti, D.; Hammack, J.; Ortiz, K.; Lesser, E.; et al. Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence based analysis. J. Neuro Oncol. 2020, 147, 297–307. [Google Scholar] [CrossRef]
- Carlsson, S.K.; Brothers, S.P.; Wahlestedt, C. Emerging treatment strategies for glioblastoma multiforme. Embo Mol. Med. 2014, 6, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.; Kemler, R. Protocadherins. Curr. Opin. Cell Biol. 2002, 14, 557–562. [Google Scholar] [CrossRef]
- Gul, I.S.; Hulpiau, P.; Saeys, Y.; van Roy, F. Evolution and diversity of cadherins and catenins. Exp. Cell Res. 2017, 358, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Keeler, A.B.; Molumby, M.J.; Weiner, J.A. Protocadherins branch out: Multiple roles in dendrite development. Cell Adhes. Migr. 2015, 9, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Sano, K.; Tanihara, H.; Heimark, R.L.; Obata, S.; Davidson, M.; St John, T.; Taketani, S.; Suzuki, S. Protocadherins: A large family of cadherin-related molecules in central nervous system. EMBO J. 1993, 12, 2249–2256. [Google Scholar] [CrossRef]
- Wang, X.; Weiner, J.A.; Levi, S.; Craig, A.M.; Bradley, A.; Sanes, J.R. Gamma protocadherins are required for survival of spinal interneurons. Neuron 2002, 36, 843–854. [Google Scholar] [CrossRef] [Green Version]
- Morishita, H.; Yagi, T. Protocadherin family: Diversity, structure, and function. Curr. Opin. Cell Biol. 2007, 19, 584–592. [Google Scholar] [CrossRef]
- Vega-Benedetti, A.F.; Loi, E.; Moi, L.; Blois, S.; Fadda, A.; Antonelli, M.; Arcella, A.; Badiali, M.; Giangaspero, F.; Morra, I.; et al. Clustered protocadherins methylation alterations in cancer. Clin. Epigenetics 2019, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Maroofian, R.; Cavdarli, B.; Riccardi, F.; Field, M.; Banka, S.; Bubshait, D.K.; Li, Y.; Hertecant, J.; Baig, S.M.; et al. Biallelic variants in PCDHGC4 cause a novel neurodevelopmental syndrome with progressive microcephaly, seizures, and joint anomalies. Genet. Med. 2021, 23, 2138–2149. [Google Scholar] [CrossRef]
- Dallosso, A.R.; Oster, B.; Greenhough, A.; Thorsen, K.; Curry, T.J.; Owen, C.; Hancock, A.L.; Szemes, M.; Paraskeva, C.; Frank, M.; et al. Long-range epigenetic silencing of chromosome 5q31 protocadherins is involved in early and late stages of colorectal tumorigenesis through modulation of oncogenic pathways. Oncogene 2012, 31, 4409–4419. [Google Scholar] [CrossRef] [Green Version]
- Dallosso, A.R.; Hancock, A.L.; Szemes, M.; Moorwood, K.; Chilukamarri, L.; Tsai, H.H.; Sarkar, A.; Barasch, J.; Vuononvirta, R.; Jones, C.; et al. Frequent long-range epigenetic silencing of protocadherin gene clusters on chromosome 5q31 in Wilms’ tumor. PLoS Genet. 2009, 5, e1000745. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Absher, D.M.; Gulzar, Z.G.; Young, S.R.; McKenney, J.K.; Peehl, D.M.; Brooks, J.D.; Myers, R.M.; Sherlock, G. DNA methylation profiling reveals novel biomarkers and important roles for DNA methyltransferases in prostate cancer. Genome Res. 2011, 21, 1017–1027. [Google Scholar] [CrossRef] [Green Version]
- Novak, P.; Jensen, T.; Oshiro, M.M.; Watts, G.S.; Kim, C.J.; Futscher, B.W. Agglomerative epigenetic aberrations are a common event in human breast cancer. Cancer Res. 2008, 68, 8616–8625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, P.; Jensen, T.J.; Garbe, J.C.; Stampfer, M.R.; Futscher, B.W. Stepwise DNA methylation changes are linked to escape from defined proliferation barriers and mammary epithelial cell immortalization. Cancer Res. 2009, 69, 5251–5258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puchalski, R.B.; Shah, N.; Miller, J.; Dalley, R.; Nomura, S.R.; Yoon, J.G.; Smith, K.A.; Lankerovich, M.; Bertagnolli, D.; Bickley, K.; et al. An anatomic transcriptional atlas of human glioblastoma. Science 2018, 360, 660–663. [Google Scholar] [CrossRef] [Green Version]
- Dilling, C.; Roewer, N.; Forster, C.Y.; Burek, M. Multiple protocadherins are expressed in brain microvascular endothelial cells and might play a role in tight junction protein regulation. J. Cereb. Blood Flow Metab. 2017, 37, 3391–3400. [Google Scholar] [CrossRef] [Green Version]
- Gabbert, L.; Dilling, C.; Meybohm, P.; Burek, M. Deletion of Protocadherin Gamma C3 Induces Phenotypic and Functional Changes in Brain Microvascular Endothelial Cells In Vitro. Front. Pharmacol. 2020, 11, 590144. [Google Scholar] [CrossRef]
- Wang, C.; Yu, G.; Liu, J.; Wang, J.; Zhang, Y.; Zhang, X.; Zhou, Z.; Huang, Z. Downregulation of PCDH9 predicts prognosis for patients with glioma. J. Clin. Neurosci. 2012, 19, 541–545. [Google Scholar] [CrossRef]
- Zong, Z.; Pang, H.; Yu, R.; Jiao, Y. PCDH8 inhibits glioma cell proliferation by negatively regulating the AKT/GSK3beta/beta-catenin signaling pathway. Oncol. Lett. 2017, 14, 3357–3362. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Tao, B.; Li, S.; Li, B.; Wang, X.; Hu, G.; Li, W.; Yu, Y.; Lu, Y.; Liu, J. Characterizing the role of PCDH9 in the regulation of glioma cell apoptosis and invasion. J. Mol. Neurosci. 2014, 52, 250–260. [Google Scholar] [CrossRef]
- Mah, K.M.; Houston, D.W.; Weiner, J.A. The gamma-Protocadherin-C3 isoform inhibits canonical Wnt signalling by binding to and stabilizing Axin1 at the membrane. Sci. Rep. 2016, 6, 31665. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Feldheim, J.; Kessler, A.F.; Schmitt, D.; Wilczek, L.; Linsenmann, T.; Dahlmann, M.; Monoranu, C.M.; Ernestus, R.I.; Hagemann, C.; Lohr, M. Expression of activating transcription factor 5 (ATF5) is increased in astrocytomas of different WHO grades and correlates with survival of glioblastoma patients. OncoTargets Ther. 2018, 11, 8673–8684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, M.; Ebert, M.; Shan, W.; Phillips, G.R.; Arndt, K.; Colman, D.R.; Kemler, R. Differential expression of individual gamma-protocadherins during mouse brain development. Mol. Cell. Neurosci. 2005, 29, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Burmester, S.; Salvador, E.; Moller-Ehrlich, K.; Schneider, R.; Roewer, N.; Nagai, M.; Forster, C.Y. Kidney Ischemia/Reperfusion Injury Induces Changes in the Drug Transporter Expression at the Blood-Brain Barrier in vivo and in vitro. Front. Physiol. 2020, 11, 569881. [Google Scholar] [CrossRef] [PubMed]
- Curtaz, C.J.; Schmitt, C.; Herbert, S.L.; Feldheim, J.; Schlegel, N.; Gosselet, F.; Hagemann, C.; Roewer, N.; Meybohm, P.; Wockel, A.; et al. Serum-derived factors of breast cancer patients with brain metastases alter permeability of a human blood-brain barrier model. Fluids Barriers CNS 2020, 17, 31. [Google Scholar] [CrossRef]
- Blecharz-Lang, K.G.; Prinz, V.; Burek, M.; Frey, D.; Schenkel, T.; Krug, S.M.; Fromm, M.; Vajkoczy, P. Gelatinolytic activity of autocrine matrix metalloproteinase-9 leads to endothelial de-arrangement in Moyamoya disease. J. Cereb. Blood Flow Metab. 2018, 38, 1940–1953. [Google Scholar] [CrossRef]
- Burek, M.; Konig, A.; Lang, M.; Fiedler, J.; Oerter, S.; Roewer, N.; Bohnert, M.; Thal, S.C.; Blecharz-Lang, K.G.; Woitzik, J.; et al. Hypoxia-Induced MicroRNA-212/132 Alter Blood-Brain Barrier Integrity Through Inhibition of Tight Junction-Associated Proteins in Human and Mouse Brain Microvascular Endothelial Cells. Transl. Stroke Res. 2019, 10, 672–683. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Feldheim, J.; Kessler, A.F.; Schmitt, D.; Salvador, E.; Monoranu, C.M.; Feldheim, J.J.; Ernestus, R.I.; Lohr, M.; Hagemann, C. Ribosomal Protein S27/Metallopanstimulin-1 (RPS27) in Glioma-A New Disease Biomarker? Cancers 2020, 12, 1085. [Google Scholar] [CrossRef]

| Patients’ Characteristics | |
|---|---|
| Sex | Female: 19/41.3% Male: 27/58.7% |
| Age (median, quartiles) | 57.5 years (49.0–69.25 years) |
| ECOG (median, quartiles) | 0 (0–1) |
| Tumor Characteristics | |
| Volume (median, quartile) | 25.5 cm3 (15.3–54.2 cm3) |
| Tumor localization | Left hemisphere: 26/56.5% Right hemisphere: 17/37% Both hemispheres: 3/6.5% |
| Localization in the lobe of the brain | Frontal: 15/32.6% Occipital: 5/10.9% Temporal: 7/15.2% Parietal: 5/10.9% Multiple: 13/28.3% Cerebellar: 1/2.2% |
| MGMT promoter methylation | Unmethylated: 10/29.4% Methylated: 24/70.6% |
| Ki67 staining (median, quartile) | 25% (20–30%) |
| Therapy | |
| Period between diagnosis and therapy (median, quartile) | 6 days (4–13 days) |
| Surgical intervention | Biopsy: 6/13.3% Complete resection: 10/22.2% Incomplete resection: 29/64.4% |
| Radiotherapy | Yes: 43/93.0% No: 8/17.4% |
| Chemotherapy with temozolomide | Yes: 43/93.5% No: 8/17.7% |
| PFS (median, quartiles) | 9 months (6–13 months) |
| Relapse | Local GBM (multifocal relapse): 13/28.3% Local GBM (local relapse): 26/56.5% Multifocal GBM: 7/15.2% |
| OS (median, quartiles) | 18 months (12–25 months) |
| Patient’s Characteristics | |
|---|---|
| Sex | Female: 7/35.0% Male: 13/65.0% |
| Age (median, quartile) | 36.0 years (30.8–45.8 years) |
| OS (median, quartiles) | 34.0 months (9.8–46.0 months) |
| Hazard Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Age | 1.115 | 1.046–1.189 | 0.001 |
| MGMT promoter methylation | 0.130 | 0.032–0.532 | 0.005 |
| PCDHGC3 mRNA expression | 1.038 | 1.008–1.068 | 0.012 |
| Extent of resection | 0.073 | 0.010–0.517 | 0.009 |
| Patient and Tumor Characteristics | Correlation Coefficient r | p-Value |
|---|---|---|
| Sex (female/male) * | 0.703 | |
| Age | 0.056 | 0.813 |
| Tumor growth pattern * | >0.99 | |
| OS | 0.509 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldheim, J.; Wend, D.; Lauer, M.J.; Monoranu, C.M.; Glas, M.; Kleinschnitz, C.; Ernestus, R.-I.; Braunger, B.M.; Meybohm, P.; Hagemann, C.; et al. Protocadherin Gamma C3 (PCDHGC3) Is Strongly Expressed in Glioblastoma and Its High Expression Is Associated with Longer Progression-Free Survival of Patients. Int. J. Mol. Sci. 2022, 23, 8101. https://doi.org/10.3390/ijms23158101
Feldheim J, Wend D, Lauer MJ, Monoranu CM, Glas M, Kleinschnitz C, Ernestus R-I, Braunger BM, Meybohm P, Hagemann C, et al. Protocadherin Gamma C3 (PCDHGC3) Is Strongly Expressed in Glioblastoma and Its High Expression Is Associated with Longer Progression-Free Survival of Patients. International Journal of Molecular Sciences. 2022; 23(15):8101. https://doi.org/10.3390/ijms23158101
Chicago/Turabian StyleFeldheim, Jonas, David Wend, Mara J. Lauer, Camelia M. Monoranu, Martin Glas, Christoph Kleinschnitz, Ralf-Ingo Ernestus, Barbara M. Braunger, Patrick Meybohm, Carsten Hagemann, and et al. 2022. "Protocadherin Gamma C3 (PCDHGC3) Is Strongly Expressed in Glioblastoma and Its High Expression Is Associated with Longer Progression-Free Survival of Patients" International Journal of Molecular Sciences 23, no. 15: 8101. https://doi.org/10.3390/ijms23158101
APA StyleFeldheim, J., Wend, D., Lauer, M. J., Monoranu, C. M., Glas, M., Kleinschnitz, C., Ernestus, R.-I., Braunger, B. M., Meybohm, P., Hagemann, C., & Burek, M. (2022). Protocadherin Gamma C3 (PCDHGC3) Is Strongly Expressed in Glioblastoma and Its High Expression Is Associated with Longer Progression-Free Survival of Patients. International Journal of Molecular Sciences, 23(15), 8101. https://doi.org/10.3390/ijms23158101







