Prediction of Response to Cisplatin-Based Neoadjuvant Chemotherapy of Muscle-Invasive Bladder Cancer Patients by Molecular Subtyping including KRT and FGFR Target Gene Assessment
Abstract
:1. Introduction
2. Results
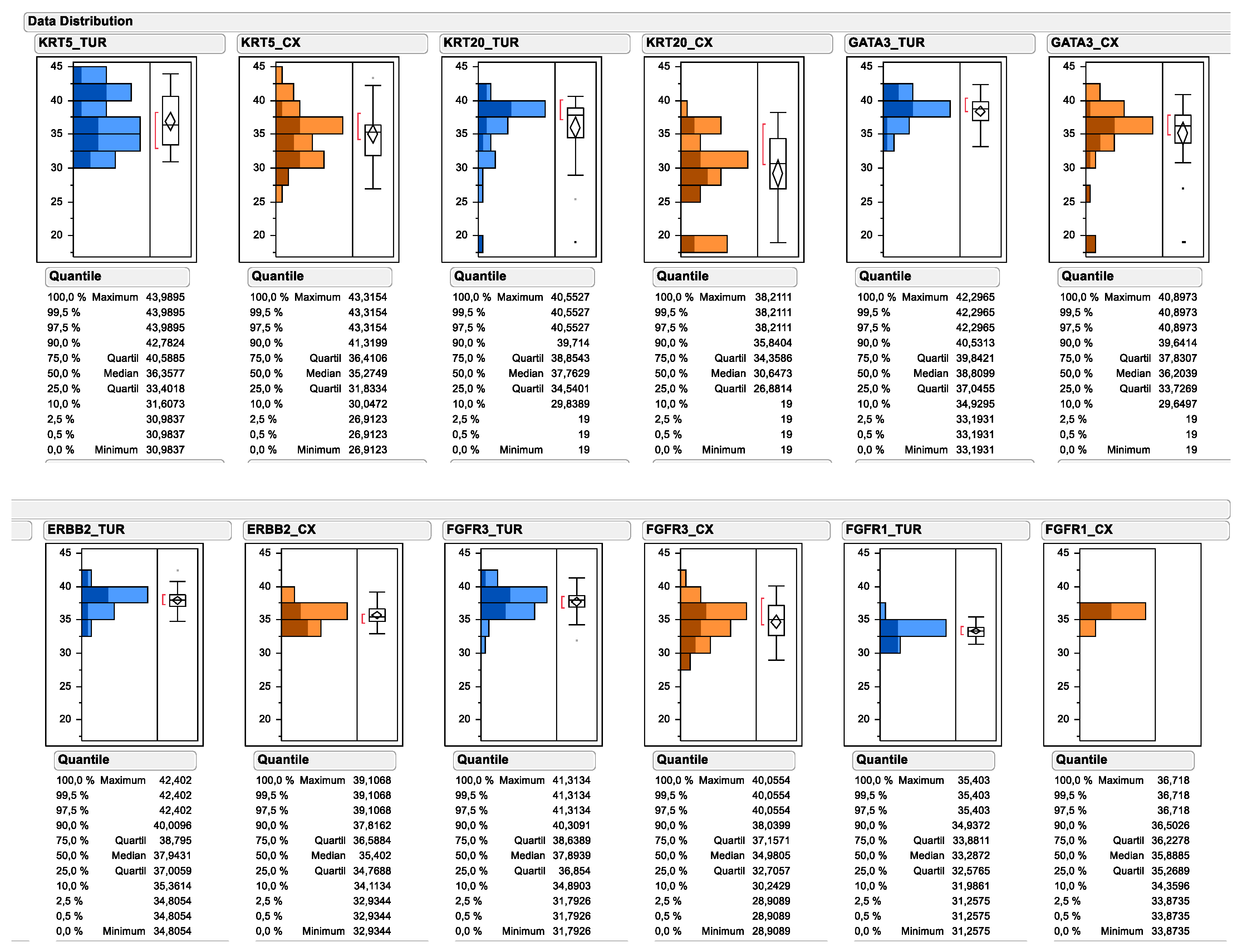
2.1. Distribution of Assessed Protein and mRNA Markers across the Study Cohort
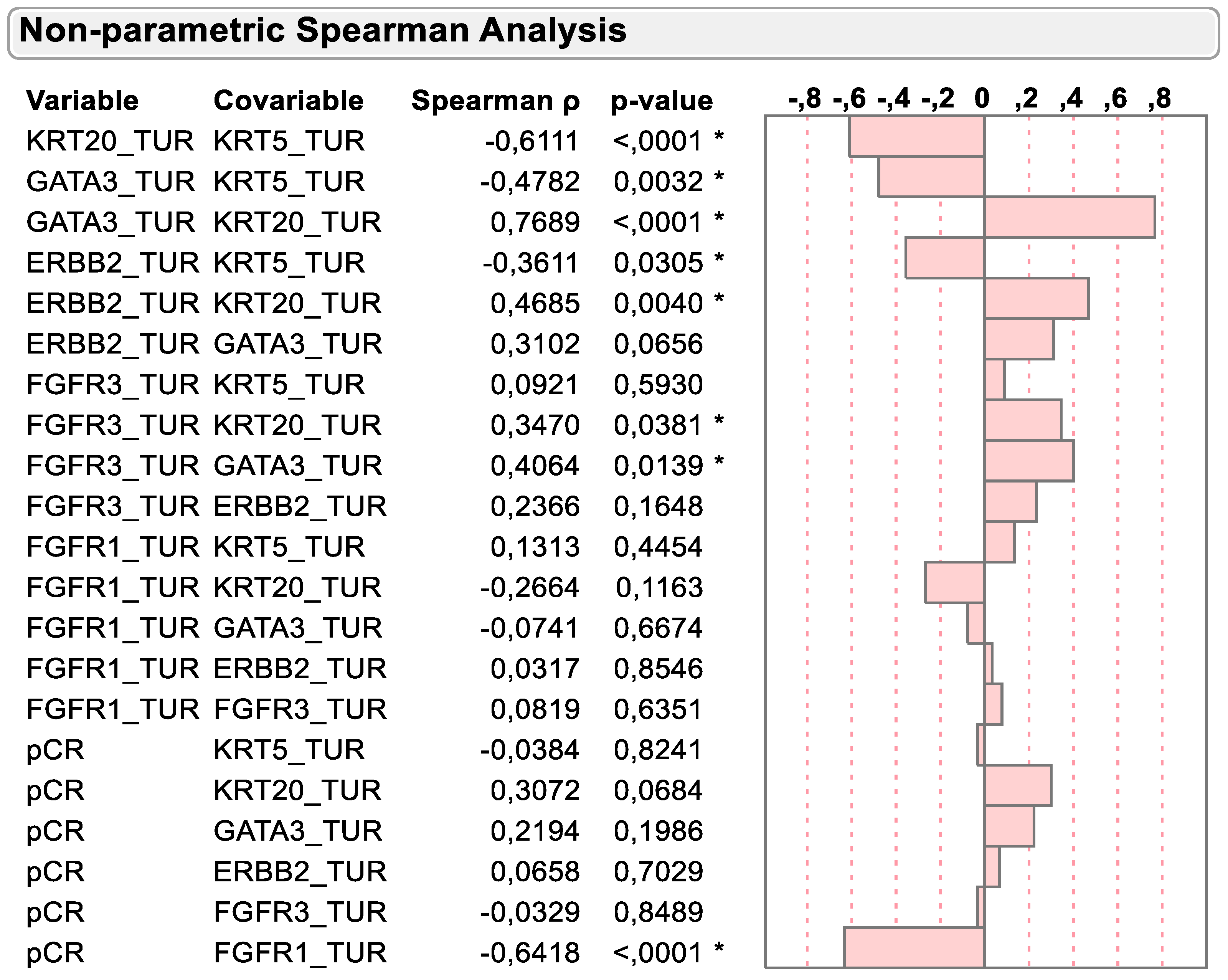
2.2. Correlation of mRNA Markers on Basis of Molecular Subtyping and Clinical Variables
2.3. Hierarchical Clustering Defines Subgroup of Chemotherapy Resistant Tumors
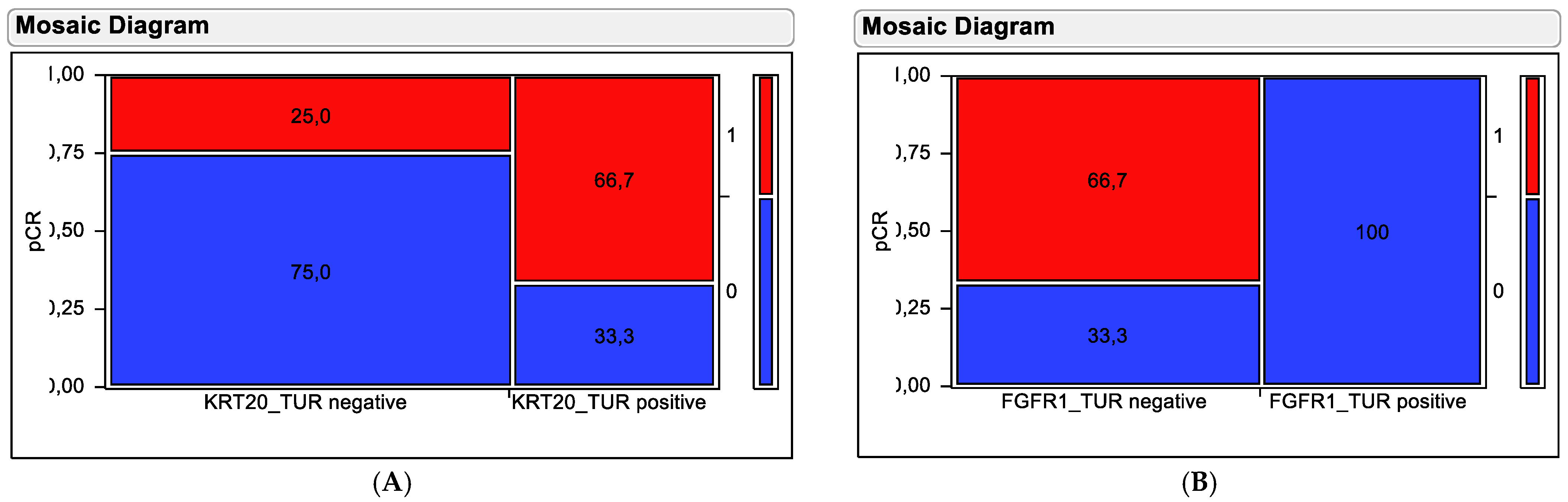
2.4. Contingency Testing to Evaluate Predictive Value of Marker Genes
3. Discussion
4. Materials and Methods
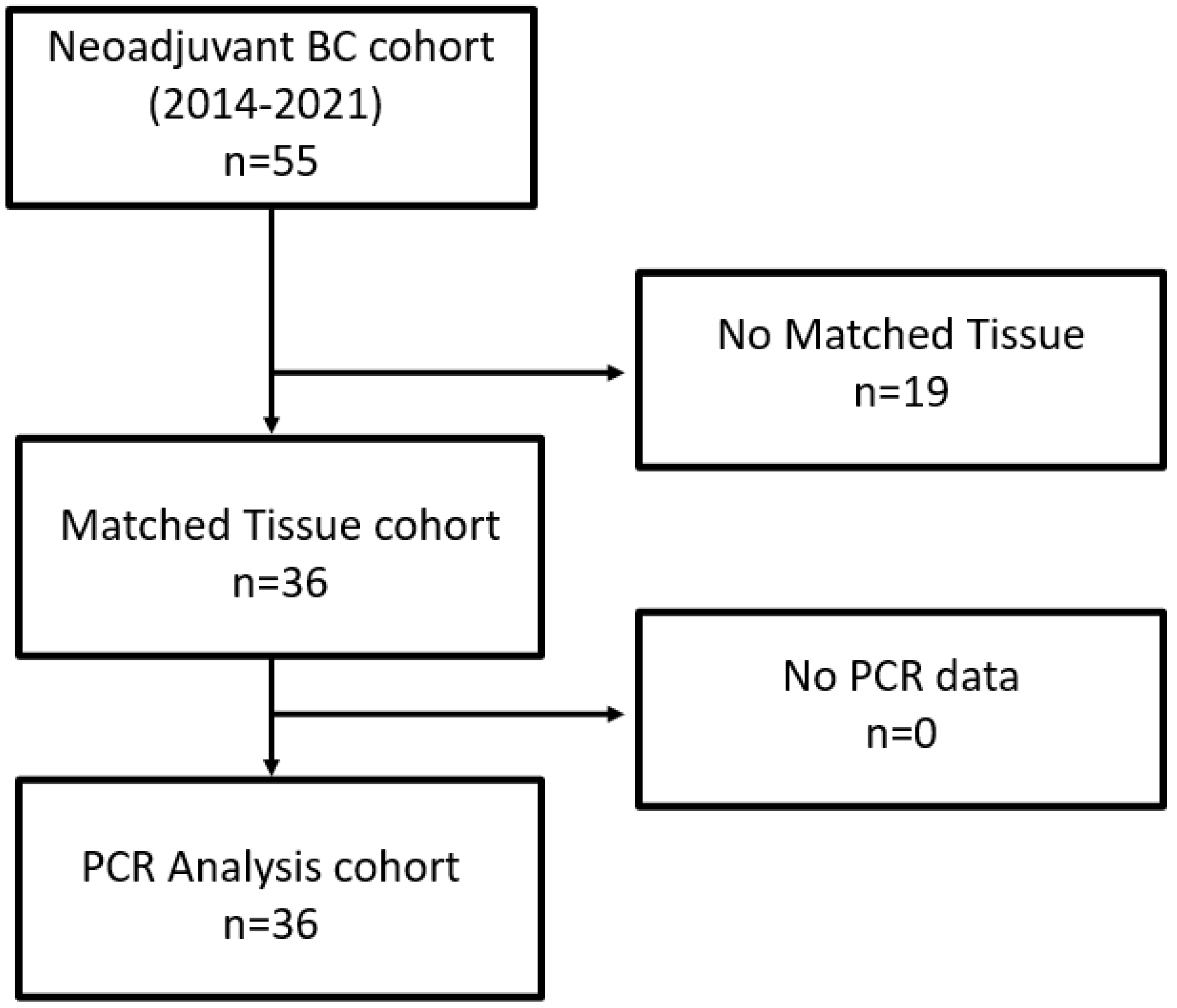
4.1. Patients
Patient Population
4.2. Eligibility
4.3. Pretreatment Evaluation
4.4. Assessment of Treatment Efficacy
4.5. Dose Modifications
4.6. Criteria for Follow-Up
4.7. Surgical Intervention
4.8. Isolation of Tumor RNA
4.9. Gene Expression by RT-qPCR
4.10. Statistical Analysis
4.11. Ethics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | bladder cancer |
| Cis | carcinoma in situ |
| CR | complete response |
| DCT | delta cycle threshold (gene expression based on difference of threshold |
| passing of individual genes when using qPCR) | |
| DSS | disease-specific survival |
| FFPE | formalin fixed paraffin embedded |
| GC | gemcitabine and cisplatin chemotherapy |
| IVD | in vitro diagnostic |
| NAC | neoadjuvant chemotherapy |
| MIBC | muscle-invasive bladder cancer |
| NC | no change |
| NMIBC | non-muscle invasive bladder cancer |
| OS | overall survival |
| PCR | polymerase chain reaction |
| pCR | pathological complete response |
| PD | progressive disease |
| PFS | Progression-free survival |
| PLND | pelvic lymph node dissection |
| PR | partial response |
| RC | radical cystectomy |
| RECIST | response evaluation criteria in solid tumors |
| TURB | transurethral resection of bladder |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witjes, J.A.; Comperat, E.; Cowan, N.C.; De Santis, M.; Gakis, G.; Lebret, T.; Ribal, M.J.; Van der Heijden, A.G.; Sherif, A. EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur. Urol. 2014, 65, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Alfred Witjes, J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Kluth, L.A.; Black, P.C.; Bochner, B.H.; Catto, J.; Lerner, S.P.; Stenzl, A.; Sylvester, R.; Vickers, A.J.; Xylinas, E.; Shariat, S.F. Prognostic and Prediction Tools in Bladder Cancer: A Comprehensive Review of the Literature. Eur. Urol. 2015, 68, 238–253. [Google Scholar] [CrossRef]
- Lavery, H.J.; Stensland, K.D.; Niegisch, G.; Albers, P.; Droller, M.J. Pathological T0 following radical cystectomy with or without neoadjuvant chemotherapy: A useful surrogate. J. Urol. 2014, 191, 898–906. [Google Scholar] [CrossRef]
- Hoffmann, A.C.; Wild, P.; Leicht, C.; Bertz, S.; Danenberg, K.D.; Danenberg, P.V.; Stöhr, R.; Stöckle, M.; Lehmann, J.; Schuler, M.; et al. MDR1 and ERCC1 expression predict outcome of patients with locally advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia 2010, 12, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.-L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Damrauer, J.S.; Hoadley, K.A.; Chism, D.D.; Fan, C.; Tiganelli, C.J.; Wobker, S.E.; Yeh, J.J.; Milowsky, M.I.; Iyer, G.; Parker, J.S.; et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl. Acad. Sci. USA 2014, 111, 3110–3115. [Google Scholar] [CrossRef] [Green Version]
- Breyer, J.; on behalf of the BRIDGE Consortium; Wirtz, R.M.; Otto, W.; Erben, P.; Kriegmair, M.C.; Stoehr, R.; Eckstein, M.; Eidt, S.; Denzinger, S.; et al. In stage pT1 non-muscle-invasive bladder cancer (NMIBC), high KRT20 and low KRT5 mRNA expression identify the luminal subtype and predict recurrence and survival. Virchows Arch. 2017, 470, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Wirtz, R.M.; Gross-Weege, M.; Breyer, J.; Otto, W.; Stoehr, R.; Sikic, D.; Keck, B.; Eidt, S.; Burger, M.; et al. mRNA-Expression of KRT5 and KRT20 Defines Distinct Prognostic Subgroups of Muscle-Invasive Urothelial Bladder Cancer Correlating with Histological Variants. Int. J. Mol. Sci. 2018, 19, 3396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjödahl, G.; Abrahamsson, J.; Bernardo, C.; Eriksson, P.; Höglund, M.; Liedberg, F. Molecular Subtypes as a Basis for Stratified Use of Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer-A Narrative Review. Cancers 2022, 14, 1692. [Google Scholar] [CrossRef] [PubMed]
- Jütte, H.; Reike, M.; Wirtz, R.M.; Kriegmair, M.; Erben, P.; Tully, K.; Weyerer, V.; Eckstein, M.; Hartmann, A.; Eidt, S.; et al. KRT20, KRT5, ESR1 and ERBB2 Expression Can Predict Pathologic Outcome in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. J. Pers. Med. 2021, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- McConkey, D.J.; Choi, W.; Shen, Y.; Lee, I.L.; Porten, S.; Matin, S.F.; Kamat, A.M.; Corn, P.; Millikan, R.E.; Dinney, C.; et al. A Prognostic Gene Expression Signature in the Molecular Classification of Chemotherapy-naïve Urothelial Cancer is Predictive of Clinical Outcomes from Neoadjuvant Chemotherapy: A Phase 2 Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin with Bevacizumab in Urothelial Cancer. Eur. Urol. 2016, 69, 855–862. [Google Scholar]
- Seiler, R.; Ashab, H.A.D.; Erho, N.; van Rhijn, B.W.; Winters, B.; Douglas, J.; Van Kessel, K.E.; van de Putte, E.E.F.; Sommerlad, M.; Wang, N.Q.; et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur. Urol. 2017, 72, 544–554. [Google Scholar] [CrossRef]
- Filipits, M.; Dafni, U.; Gnant, M.; Polydoropoulou, V.; Hills, M.; Kiermaier, A.; de Azambuja, E.; Larsimont, D.; Rojo, F.; Viale, G.; et al. Association of p27 and Cyclin D1 Expression and Benefit from Adjuvant Trastuzumab Treatment in HER2-Positive Early Breast Cancer: A TransHERA Study. Clin. Cancer Res. 2018, 24, 3079–3086. [Google Scholar] [CrossRef] [Green Version]
- Dubsky, P.; Filipits, M.; Jakesz, R.; Rudas, M.; Singer, C.F.; Greil, R.; Dietze, O.; Luisser, I.; Klug, E.; Sedivy, R.; et al. EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann. Oncol. 2013, 24, 640–647. [Google Scholar] [CrossRef]
- Wirtz, R.M.; Sihto, H.; Isola, J.; Heikkilä, P.; Kellokumpu-Lehtinen, P.L.; Auvinen, P.; Turpeenniemi-Hujanen, T.; Jyrkkiö, S.; Lakis, S.; Schlombs, K.; et al. Biological subtyping of early breast cancer: A study comparing RT-qPCR with immunohistochemistry. Breast Cancer Res. Treat. 2016, 157, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laible, M.; Schlombs, K.; Kaiser, K.; Veltrup, E.; Herlein, S.; Lakis, S.; Stöhr, R.; Eidt, S.; Hartmann, A.; Wirtz, R.M.; et al. Technical validation of an RT-qPCR in vitro diagnostic test system for the determination of breast cancer molecular subtypes by quantification of ERBB2, ESR1, PGR and MKI67 mRNA levels from formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer 2016, 16, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyton, C.C.; Tang, D.; Reich, R.R.; Azizi, M.; Chipollini, J.; Pow-Sang, J.M.; Manley, B.; Spiess, P.E.; Poch, M.A.; Sexton, W.J.; et al. Downstaging and Survival Outcomes Associated with Neoadjuvant Chemotherapy Regimens Among Patients Treated with Cystectomy for Muscle-Invasive Bladder Cancer. JAMA Oncol. 2018, 4, 1535–1542. [Google Scholar] [CrossRef]
- Milowsky, M.I.; Rumble, R.B.; Booth, C.M.; Gilligan, T.; Eapen, L.J.; Hauke, R.J.; Boumansour, P.; Lee, C.T. Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J. Clin. Oncol. 2016, 34, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Strissel, P.; Strick, R.; Weyerer, V.; Wirtz, R.; Pfannstiel, C.; Wullweber, A.; Lange, F.; Erben, P.; Stoehr, R.; et al. Cytotoxic T-cell-related gene expression signature predicts improved survival in muscle-invasive urothelial bladder cancer patients after radical cystectomy and adjuvant chemotherapy. J. Immunother. Cancer 2020, 8, e000162. [Google Scholar] [CrossRef]
- Eckstein, M.; Wirtz, R.M.; Pfannstil, C.; Wach, S.; Stoehr, R.; Breyer, J.; Erlmeier, F.; Günes, C.; Nitschcke, K.; Weichert, W.; et al. A multicenter round robin test of PD-L1 expression assessment in urothelial bladder cancer by immunohistochemistry and RT-qPCR with emphasis on prognosis prediction after radical cystectomy. Oncotarget 2018, 9, 15001–15014. [Google Scholar] [CrossRef] [Green Version]
- Sinn, H.P.; Schneeweiss, A.; Keller, M.; Schlombs, K.; Laible, M.; Seitz, J.; Lakis, S.; Veltrup, E.; Altevogt, P.; Eidt, S.; et al. Comparison of immunohistochemistry with PCR for assessment of ER, PR, and Ki-67 and prediction of pathological complete response in breast cancer. BMC Cancer 2017, 17, 124. [Google Scholar] [CrossRef] [Green Version]

| Cohort | Total Cohort |
|---|---|
| Size (n) | 36 |
| Age (years) | |
| Average | 69 |
| Range | 53–85 |
| Gender | |
| Male | 30 (83%) |
| Female | 6 (17%) |
| ECOG performance status | |
| 0 | 28 (78%) |
| 1 | 8 (22%) |
| 2 | 0 (0%) |
| Lymph node metastases before chemotherapy | |
| cN0 | 29 (81%) |
| cN1 | 5 (14%) |
| cN2 | 2 (5%) |
| Response to chemotherapy | |
| Complete response (ypT0) | 14 (39%) |
| lymph status (ypN0) | 32 (89%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ecke, T.H.; Voß, P.C.; Schlomm, T.; Rabien, A.; Friedersdorff, F.; Barski, D.; Otto, T.; Waldner, M.; Veltrup, E.; Linden, F.; et al. Prediction of Response to Cisplatin-Based Neoadjuvant Chemotherapy of Muscle-Invasive Bladder Cancer Patients by Molecular Subtyping including KRT and FGFR Target Gene Assessment. Int. J. Mol. Sci. 2022, 23, 7898. https://doi.org/10.3390/ijms23147898
Ecke TH, Voß PC, Schlomm T, Rabien A, Friedersdorff F, Barski D, Otto T, Waldner M, Veltrup E, Linden F, et al. Prediction of Response to Cisplatin-Based Neoadjuvant Chemotherapy of Muscle-Invasive Bladder Cancer Patients by Molecular Subtyping including KRT and FGFR Target Gene Assessment. International Journal of Molecular Sciences. 2022; 23(14):7898. https://doi.org/10.3390/ijms23147898
Chicago/Turabian StyleEcke, Thorsten H., Paula Carolin Voß, Thorsten Schlomm, Anja Rabien, Frank Friedersdorff, Dimitri Barski, Thomas Otto, Michael Waldner, Elke Veltrup, Friederike Linden, and et al. 2022. "Prediction of Response to Cisplatin-Based Neoadjuvant Chemotherapy of Muscle-Invasive Bladder Cancer Patients by Molecular Subtyping including KRT and FGFR Target Gene Assessment" International Journal of Molecular Sciences 23, no. 14: 7898. https://doi.org/10.3390/ijms23147898
APA StyleEcke, T. H., Voß, P. C., Schlomm, T., Rabien, A., Friedersdorff, F., Barski, D., Otto, T., Waldner, M., Veltrup, E., Linden, F., Hake, R., Eidt, S., Roggisch, J., Heidenreich, A., Rieger, C., Kastner, L., Hallmann, S., Koch, S., & Wirtz, R. M. (2022). Prediction of Response to Cisplatin-Based Neoadjuvant Chemotherapy of Muscle-Invasive Bladder Cancer Patients by Molecular Subtyping including KRT and FGFR Target Gene Assessment. International Journal of Molecular Sciences, 23(14), 7898. https://doi.org/10.3390/ijms23147898







