Neutrophil Activation by Mineral Microparticles Coated with Methylglyoxal-Glycated Albumin
Abstract
1. Introduction
2. Results
2.1. Effects of Methylglyoxal-Induced Glycation of HSA
2.2. Parameters of Protein Interaction with Vaterite Microparticles
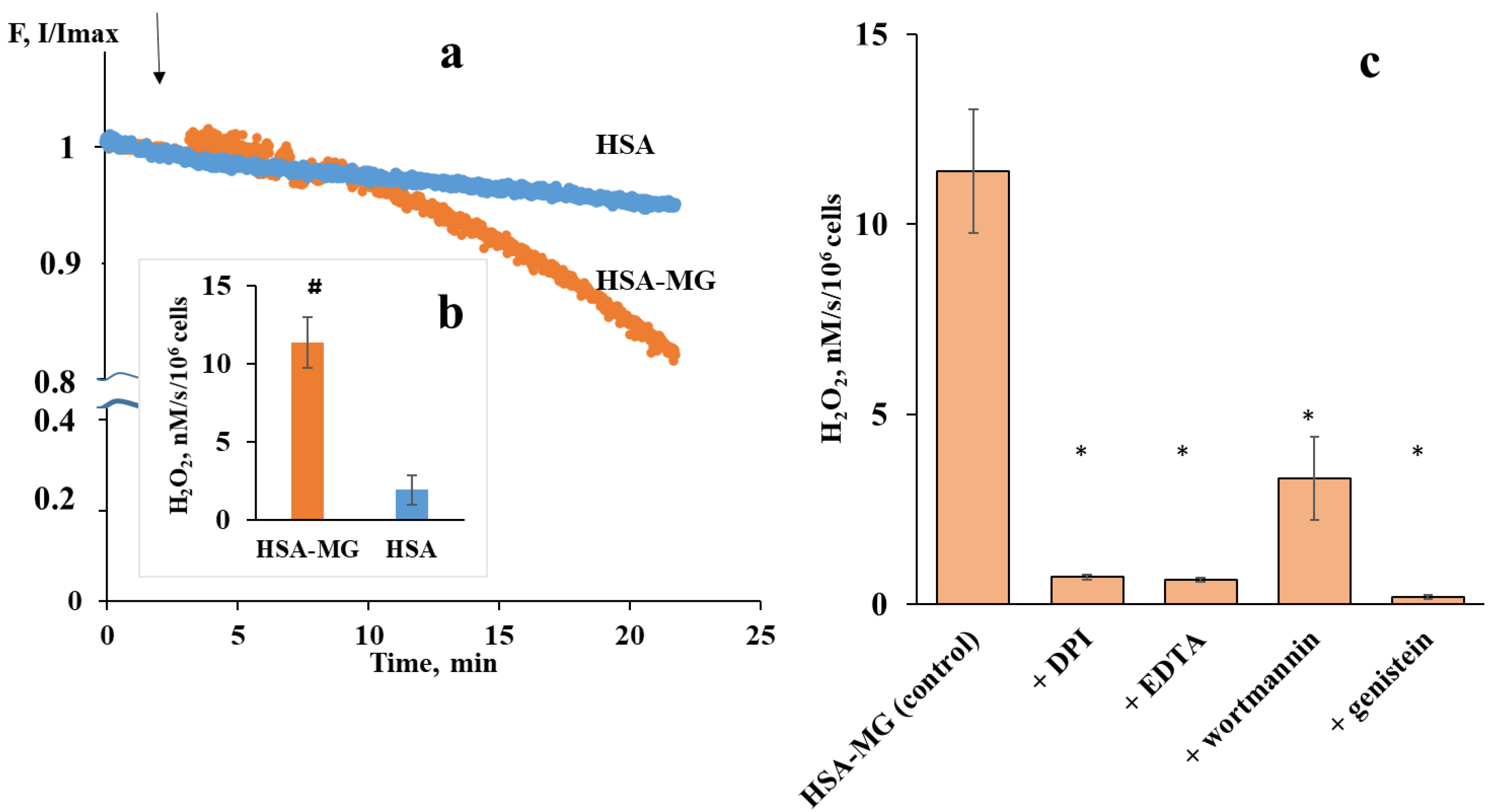
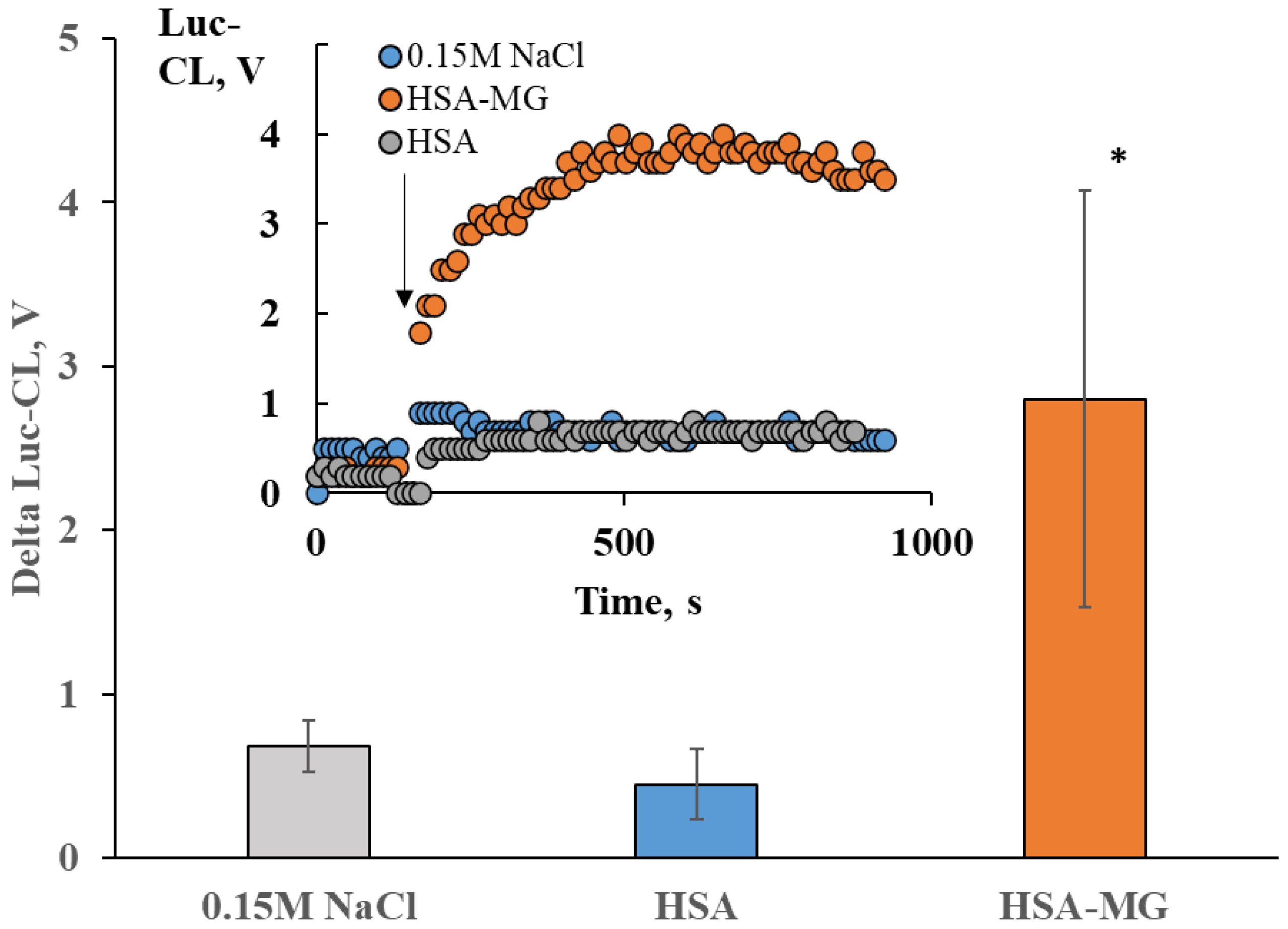
2.3. Neutrophil Activation by Soluble HSA-MG
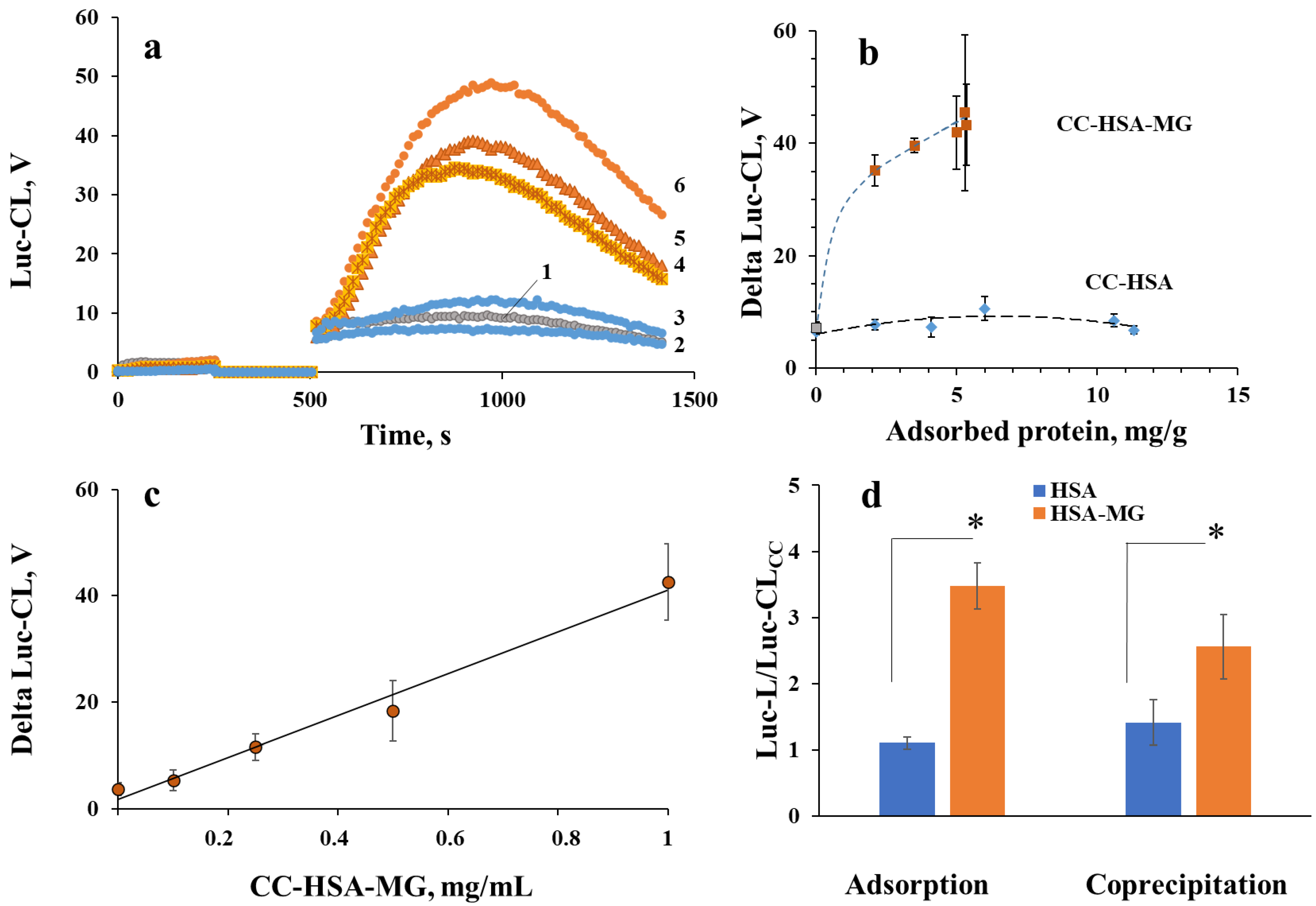
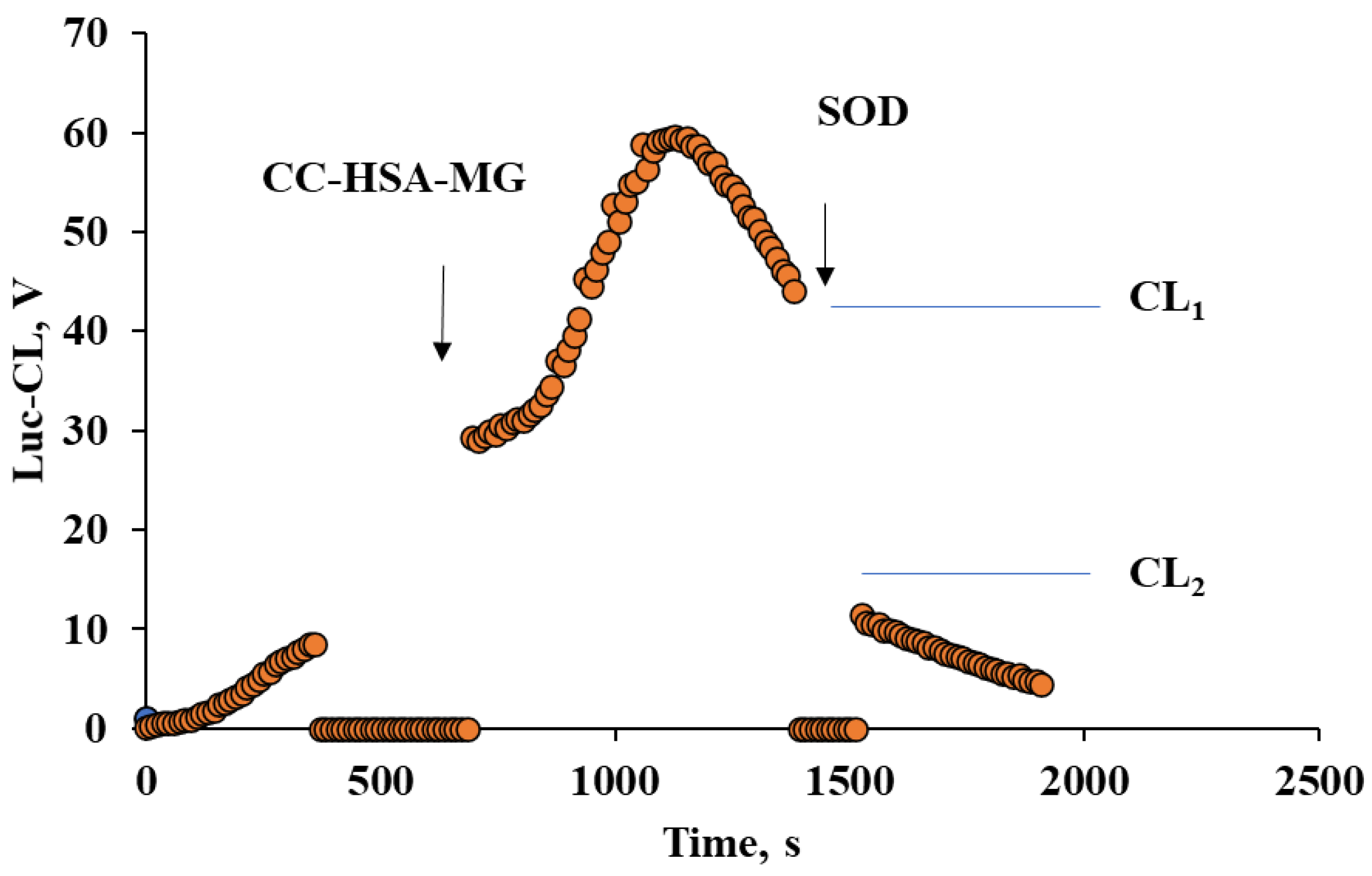
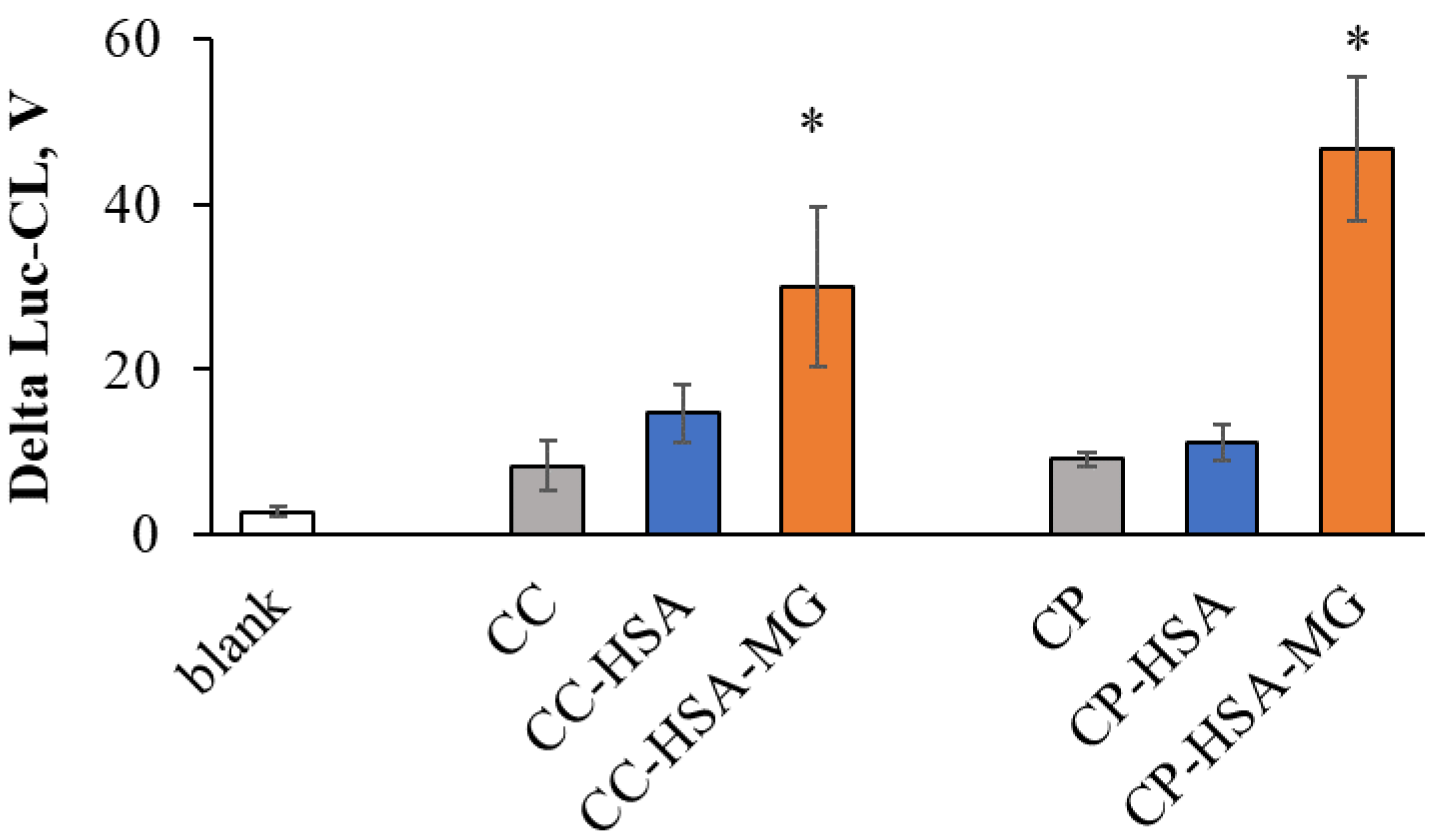
2.4. Neutrophil Activation by Protein–Vaterite Microparticles
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Methylglyoxal-Modified Albumin (HSA-MG)
4.3. The Molecular Mass of HSA and HSA-MG
4.4. Advanced Glycation Product (AGE) Detection
4.5. Two-Dimensional Difference Gel Electrophoresis (DIGE)
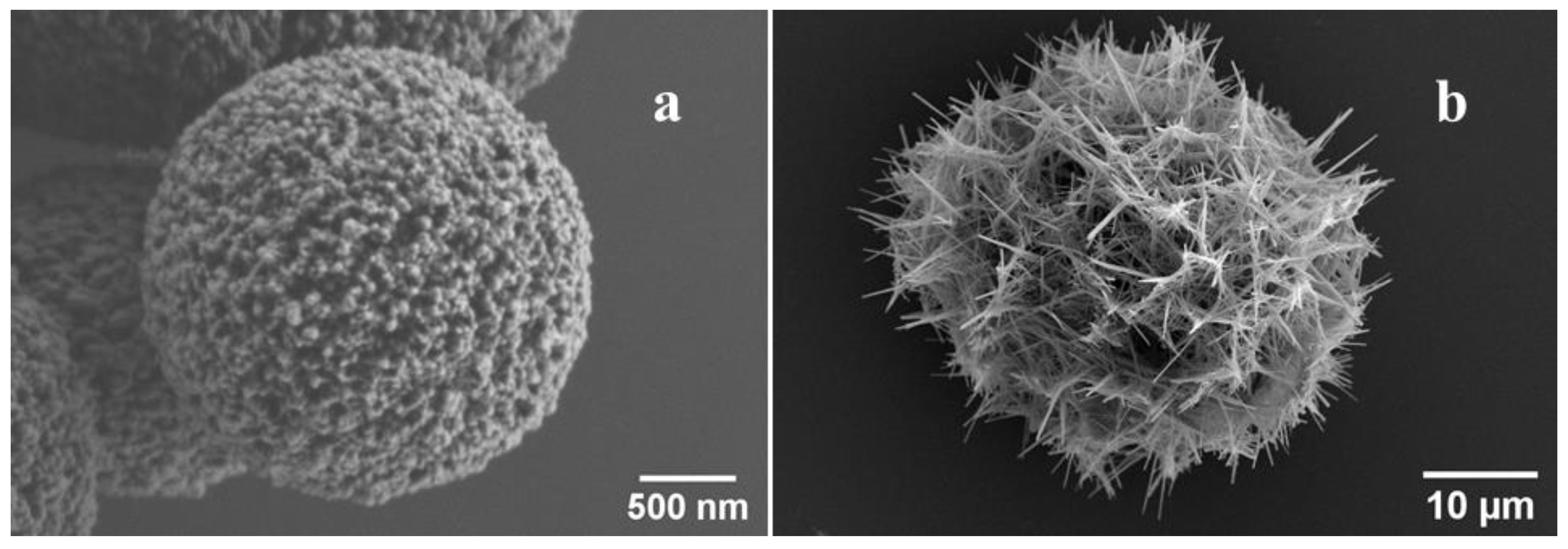
4.6. Scanning Electron Microscopy (SEM)
4.7. Fabrication of Vaterite Microparticles and Vaterite Microparticles with Co-Precipitated Protein
4.8. Light Microscopy of Vaterite Microparticles (CC)
4.9. Calcium Phosphate Nanowire-Composed Microparticles (CP) Preparation
4.10. Protein Adsorption onto CC and CP Particles
4.11. ζ-Potential Measurement
4.12. Blood Collection and Isolation of Neutrophils
4.13. Measurement of H2O2 Production by Human Neutrophils Based on Scopoletin Oxidation
4.14. Chemiluminescence Assay (CL)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Kierdorf, K.; Fritz, G. RAGE regulation and signaling in inflammation and beyond. J. Leuk. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.M.; Simpson, C.L.; Stewart, J.A., Jr. The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef] [PubMed]
- Steitz, S.A.; Speer, M.Y.; Curinga, G.; Yang, H.Y.; Haynes, P.; Aebersold, R.; Schinke, T.; Karsenty, G.; Giachelli, C.M. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef]
- Wei, Q.; Ren, X.; Jiang, Y.; Jin, H.; Liu, N.; Li, J. Advanced glycation end products accelerate rat vascular calcification through RAGE/oxidative stress. BMC Cardiovasc. Disord. 2013, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, M.R.; Bouvet, C.; Bouchard, S.; Moreau, S.; Leblond, J.; Deblois, D.; Moreau, P. Reduction of advanced-glycation end products levels and inhibition of RAGE signaling decreases rat vascular calcification induced by diabetes. PLoS ONE 2014, 9, e85922. [Google Scholar] [CrossRef]
- Prystowsky, J.B.; Huprikar, J.S.; Rademaker, A.W.; Rege, R.V. Human polymorphonuclear leukocyte phagocytosis of crystalline cholesterol, bilirubin, and calcium hydroxyapatite In Vitro. Dig. Dis. Sci. 1995, 40, 412–418. [Google Scholar] [CrossRef]
- Tabei, Y.; Sugino, S.; Eguchi, K.; Tajika, M.; Abe, H.; Nakajima, Y.; Horie, M. Effect of calcium carbonate particle shape on phagocytosis and pro-inflammatory response in differentiated THP-1 macrophages. Biochem. Biophys. Res. Commun. 2017, 490, 499–505. [Google Scholar] [CrossRef]
- Burt, H.M.; Jackson, J.K.; Taylor, D.R.; Crowther, R.S. Activation of human neutrophils by calcium carbonate polymorphs. Dig. Dis. Sci. 1997, 42, 1283–1289. [Google Scholar] [CrossRef]
- Peng, H.H.; Wu, C.Y.; Young, D.; Martel, J.; Young, A.; Ojcius, D.M.; Lee, Y.H.; Young, J.D. Physicochemical and biological properties of biomimetic mineralo-protein nanoparticles formed spontaneously in biological fluids. Small 2013, 9, 2297–2307. [Google Scholar] [CrossRef]
- Miura, Y.; Iwazu, Y.; Shiizaki, K.; Akimoto, T.; Kotani, K.; Kurabayashi, M.; Kurosu, H.; Kuro-O, M. Identification and quantification of plasma calciprotein particles with distinct physical properties in patients with chronic kidney disease. Sci. Rep. 2018, 8, 1256. [Google Scholar] [CrossRef]
- Peng, H.H.; Liu, Y.J.; Ojcius, D.M.; Lee, C.M.; Chen, R.H.; Huang, P.R.; Martel, J.; Young, J.D. Mineral particles stimulate innate immunity through neutrophil extracellular traps containing HMGB1. Sci. Rep. 2017, 7, 16628. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Schmitt, J.; Münch, G.; Gasic-Milencovic, J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal. Biochem. 2005, 338, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G. Kinetics of glycoxidation of bovine serum albumin by methylglyoxal and glyoxal and its prevention by various compounds. Molecules 2014, 19, 4880–4896. [Google Scholar] [CrossRef]
- McLaughlin, J.A.; Pethig, R.; Szent-Györgyi, A. Spectroscopic studies of the protein-methylglyoxal adduct. Proc. Natl. Acad. Sci. USA 1980, 77, 949–951. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; de Guerenu, A.V.L.; Feoktistova, N.A.; Skirtach, A.G.; Volodkin, D. Protein-containing multilayer capsules by templating on mesoporous CaCO3 particles: Post- and pre-loading approaches. Macromol. Biosci. 2016, 16, 95–105. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Le-Deygen, I.M.; Filatova, L.Y.; Dmitry, V.; Vikulina, A.S. Hybrid CaCO3-mucin crystals: Effective approach for loading and controlled release of cationic drugs. Mater. Des. 2019, 182, e108020. [Google Scholar] [CrossRef]
- Ayilavarapu, S.; Kantarci, A.; Fredman, G.; Turkoglu, O.; Omori, K.; Liu, H.; Iwata, T.; Yagi, M.; Hasturk, H.; Van Dyke, T.E. Diabetes-induced oxidative stress is mediated by Ca2+-independent phospholipase A2 in neutrophils. J. Immunol. 2010, 184, 1507–1515. [Google Scholar] [CrossRef]
- Root, R.K.; Metcalf, J.; Oshino, N.; Chance, B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J. Clin. Investig. 1975, 55, 945–955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waddell, T.K.; Fialkow, L.; Chan, C.K.; Kishimoto, T.K.; Downey, G.P. Potentiation of the oxidative burst of human neutrophils. A signaling role for L-selectin. J. Biol. Chem. 1994, 269, 18485–18491. [Google Scholar] [CrossRef]
- Collison, K.S.; Parhar, R.S.; Saleh, S.S.; Meyer, B.F.; Kwaasi, A.A.; Hammami, M.M.; Schmidt, A.M.; Stern, D.M.; Al-Mohanna, F.A. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs). J. Leukoc. Biol. 2002, 71, 433–444. [Google Scholar]
- Lizcano, A.; Secundino, I.; Döhrmann, S.; Corriden, R.; Rohena, C.; Diaz, S.; Ghosh, P.; Deng, L.; Nizet, V.; Varki, A. Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation. Blood 2017, 129, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.; Neufurth, M.; Huang, J.; Wang, K.; Feng, Q.; Schröder, H.C.; Diehl-Seifert, B.; Muñoz-Espí, R.; Wang, X. Nonenzymatic transformation of amorphous CaCO3 into calcium phosphate mineral after exposure to sodium phosphate In Vitro: Implications for In Vivo hydroxyapatite bone formation. Chembiochem 2015, 16, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Sudareva, N.; Popova, H.; Saprykina, N.; Bronnikov, S. Structural optimization of calcium carbonate cores as templates for protein encapsulation. J. Microencapsul. 2014, 31, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Martel, J.; Young, J.D. Ectopic calcification and formation of mineralo-organic particles in arteries of diabetic subjects. Sci. Rep. 2020, 10, 8545. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Wu, C.Y.; Peng, H.H.; Young, J.D. Mineralo-organic nanoparticles in health and disease: An overview of recent fndings. Nanomedicine 2018, 13, 1787–1793. [Google Scholar] [CrossRef]
- Nadra, I.; Mason, J.C.; Philippidis, P.; Florey, O.; Smythe, C.D.; McCarthy, G.M.; Landis, R.C.; Haskard, D.O. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: A vicious cycle of inflammation and arterial calcification? Circ. Res. 2005, 96, 1248–1256. [Google Scholar] [CrossRef]
- Ortiz-Bravo, E.; Sieck, M.; Schumacher, R.H. Changes in the proteins coating monosodium urate crystals during active and subsiding inflammation. Immunogold studies of synovial fluid from patients with gout and of fluid obtained using the rat subcutaneous air pouch model. Arthritis Rheum. 1993, 36, 1274–1285. [Google Scholar] [CrossRef]
- Terkeltaub, R.; Dyer, C.A.; Martin, J.; Curtiss, L.K. Apolipoprotein (apo) E inhibits the capacity of monosodium urate crystals to stimulate neutrophils. Characterization of intraarticular apo E and demonstration of apo E binding to urate crystals In Vivo. J. Clin. Investig. 1991, 87, 20–26. [Google Scholar] [CrossRef]
- Mera, K.; Takeo, K.; Izumi, M.; Maruyama, T.; Nagai, R.; Otagiri, M. Effect of reactive-aldehydes on the modification and dysfunction of human serum albumin. J. Pharm. Sci. 2010, 99, 1614–1625. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Filatova, L.Y.; Kirzhanova, E.A.; Mikhalchik, E.V.; Volodkin, D.; Vikulina, A.S. Hybrid mucin-vaterite microspheres for delivery of proteolytic enzyme chymotrypsin. Macromol. Biosci. 2022, 22, 2200005. [Google Scholar] [CrossRef]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein-calcium carbonate coprecipitation: A tool for protein encapsulation. Biotechnol. Prog. 2005, 21, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Balabushevich, N.G.; Lopez de Guerenu, A.V.; Feoktistova, N.A.; Volodkin, D.V. Protein loading into porous CaCO3 microspheres: Adsorption equilibrium and bioactivity retention. Phys. Chem. Chem. Phys. 2015, 17, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, C.; Karlsson, A.; Bylund, J. Intracellular neutrophil oxidants: From laboratory curiosity to clinical reality. J. Immunol. 2019, 202, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Daffu, G.; Del Pozo, C.H.; O’Shea, K.M.; Ananthakrishnan, R.; Ramasamy, R.; Schmidt, A.M. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int. J. Mol. Sci. 2013, 14, 19891–19910. [Google Scholar] [CrossRef] [PubMed]
- Toure, F.; Zahm, J.M.; Garnotel, R.; Lambert, E.; Bonnet, N.; Schmidt, A.M.; Vitry, F.; Chanard, J.; Gillery, P.; Rieu, P. Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix. Biochem. J. 2008, 416, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Chitayat, S.; Dattilo, B.M.; Schiefner, A.; Diez, J.; Chazin, W.J.; Fritz, G. Structural basis for ligand recognition and activation of RAGE. Structure 2010, 18, 1342–1352. [Google Scholar] [CrossRef]
- Xie, J.; Reverdatto, S.; Frolov, A.; Hoffmann, R.; Burz, D.S.; Shekhtman, A. Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE). J. Biol. Chem. 2008, 283, 27255–27269. [Google Scholar] [CrossRef]
- Jackson, J.K.; Tudan, C.; Sahl, B.; Pelech, S.L.; Burt, H.M. Calcium pyrophosphate dihydrate crystals activate MAP kinase in human neutrophils: Inhibition of MAP kinase, oxidase activation and degranulation responses of neutrophils by taxol. Immunology 2003, 90, 502–510. [Google Scholar] [CrossRef]
- Naftaly, A.; Izgilov, R.; Omari, E.; Benayahu, D. Revealing advanced glycation end products associated structural changes in serum albumin. ACS Biomater. Sci. Eng. 2021, 7, 3179–3189. [Google Scholar] [CrossRef]
- Demina, I.A.; Serebryakova, M.V.; Ladygina, V.G.; Rogova, M.A.; Kondratov, I.G.; Renteeva, A.N.; Govorun, V.M. Proteomic characterization of Mycoplasma gallisepticum nanoforming. Biochemistry 2010, 75, 1252–1257. [Google Scholar] [CrossRef]
- Zhan, J.; Tseng, Y.-H.; Chan, J.C.C.; Mou, C.-Y. Biomimetic formation of hydroxyapatite nanorods by a single-crystal-to-single-crystal transformation. Adv. Funct. Mat. 2005, 15, 2005–2010. [Google Scholar] [CrossRef]
- Grigorieva, D.V.; Gorudko, I.V.; Grudinina, N.A.; Panasenko, O.M.; Semak, I.V.; Sokolov, A.V.; Timoshenko, A.V. Lactoferrin modified by hypohalous acids: Partial loss in activation of human neutrophils. Int. J. Biol. Macromol. 2022, 195, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, A.V.; Gabius, H.J. Efficient induction of superoxide release from human neutrophils by the galactoside-specific lectin from Viscum album. Biol. Chem. Hoppe-Scyler 1993, 374, 237–243. [Google Scholar] [CrossRef]
- Gorudko, I.V.; Mukhortava, A.V.; Caraher, B.; Ren, M.; Cherenkevich, S.N.; Kelly, G.M.; Timoshenko, A.V. Lectin-induced activation of plasma membrane NADPH oxidase in cholesterol-depleted human neutrophils. Arch. Biochem. Biophys. 2011, 516, 173–181. [Google Scholar] [CrossRef] [PubMed]


| Parameter | HSA | HSA-MG | p |
|---|---|---|---|
| Fluorescence, λex = 325 nm/λem = 430 nm | 0.31 ± 0.06 * | 0.41 ± 0.02 * | ˂0.001 |
| Absorbance, λ = 320 nm | 0.016 ± 0.001 * | 1.376 ± 0.002 * | ˂0.001 |
| ζ-potential, mV | −13.8 ± 2.2 | −15.9 ± 2.0 | |
| MW, Da | 67,626 | 75,461 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhalchik, E.V.; Ivanov, V.A.; Borodina, I.V.; Pobeguts, O.V.; Smirnov, I.P.; Gorudko, I.V.; Grigorieva, D.V.; Boychenko, O.P.; Moskalets, A.P.; Klinov, D.V.; et al. Neutrophil Activation by Mineral Microparticles Coated with Methylglyoxal-Glycated Albumin. Int. J. Mol. Sci. 2022, 23, 7840. https://doi.org/10.3390/ijms23147840
Mikhalchik EV, Ivanov VA, Borodina IV, Pobeguts OV, Smirnov IP, Gorudko IV, Grigorieva DV, Boychenko OP, Moskalets AP, Klinov DV, et al. Neutrophil Activation by Mineral Microparticles Coated with Methylglyoxal-Glycated Albumin. International Journal of Molecular Sciences. 2022; 23(14):7840. https://doi.org/10.3390/ijms23147840
Chicago/Turabian StyleMikhalchik, Elena V., Victor A. Ivanov, Irina V. Borodina, Olga V. Pobeguts, Igor P. Smirnov, Irina V. Gorudko, Daria V. Grigorieva, Olga P. Boychenko, Alexander P. Moskalets, Dmitry V. Klinov, and et al. 2022. "Neutrophil Activation by Mineral Microparticles Coated with Methylglyoxal-Glycated Albumin" International Journal of Molecular Sciences 23, no. 14: 7840. https://doi.org/10.3390/ijms23147840
APA StyleMikhalchik, E. V., Ivanov, V. A., Borodina, I. V., Pobeguts, O. V., Smirnov, I. P., Gorudko, I. V., Grigorieva, D. V., Boychenko, O. P., Moskalets, A. P., Klinov, D. V., Panasenko, O. M., Filatova, L. Y., Kirzhanova, E. A., & Balabushevich, N. G. (2022). Neutrophil Activation by Mineral Microparticles Coated with Methylglyoxal-Glycated Albumin. International Journal of Molecular Sciences, 23(14), 7840. https://doi.org/10.3390/ijms23147840






