1. Introduction
Osteoporosis is a clinically intractable disease that often causes fractures. In 2000, 9.0 million people were affected with osteoporotic fractures, with 5.8 million total disability-adjusted life years (DALYs) lost, of which 51% were fractures that occurred in Europe and the Americas [
1]. Most cases occur in postmenopausal women owing to homeostasis imbalance from the dramatic decrease in estrogen levels, which accelerates the activation of osteoclasts for bone resorption [
2]. As osteoclasts play a major role in bone resorption, they can destroy the bone matrix, which is the direct cause of osteoporosis [
3]. Thus, understanding the effects of the available therapeutic agents on osteoclasts is important for the effective treatment of osteoporosis.
The mouse monocyte RAW 264.7 cell line has been widely used to study the mechanism of osteoclastogenesis. RAW 264.7 cells differentiate into osteoclasts by the stimulation of receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL) [
4]. It has been demonstrated that RANKL-induced osteoclast differentiation in these cells is regulated by multiple pathways. Binding of RANKL to its receptor, RANK, promotes osteoclast differentiation through the recruitment of tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), subsequently activating downstream signaling pathways, namely the NF-κB signal [
5,
6,
7] and mitogen-activated protein kinase (MAPK) pathway [
8,
9,
10]. RANKL-mediated up-regulation of the NF-κB signal and MAPK pathway activates the essential transcription factor of nuclear factor of activated T cells 1 (NFATc1), and activated NFATc1 induces expression of osteoclastic molecules essential for osteoclast differentiation and function, such as tartrate-resistant acid phosphatase (TRAP), which is a cytochemical marker for both mononuclear and mature osteoclasts, dendritic cell-specific transmembrane protein (DC-STAMP) to promote cell-cell fusion, and MMP-9 and cathepsin K (CTSK) to degrade bone matrix and collagen proteins [
11,
12,
13]. On the other hand, recently, novel transcriptional repressors, B lymphocyte-induced maturation protein-1 (Blimp1) and B cell lymphoma 6 (Bcl6), controlling osteoclastogenesis were identified. Bcl-6 negatively regulates expression of osteoclast-specific genes, which are NFATc1 targets. It also has been shown that Blimp1 binds to the Bcl6 promotor and suppresses its expression [
14]. Therefore, inhibition of NFATc1 is an initial approach to prevent osteoclastogenesis. In addition to inhibiting osteoclast differentiation, the induction of osteoclast apoptosis is another approach to prevent pathological bone resorption. It has been reported that calcitonin induces osteoclast apoptosis and increases its efficacy in an in vivo model of osteoporosis [
15].
Kampo medicines are compound granules extracted from herbal drugs originating from classical or traditional Chinese medicine, which are frequently used in Japan due to their therapeutic efficacy and minimal side effects despite long-term use. In this study, we screened four Kampo medicines, namely, Unkeito (UKT), Kamishoyosan (KSS), Kamikihito (KKT), and Ninjinyoeito (NYT), which are clinically used in Japan to treat menopausal women with various symptoms.
Herein, we investigated their effectiveness and whether they have any effect on osteoclast differentiation. We also sought to understand the molecular mechanism underlying the action of the most effective Kampo medicine by studying key signaling pathways.
3. Discussion
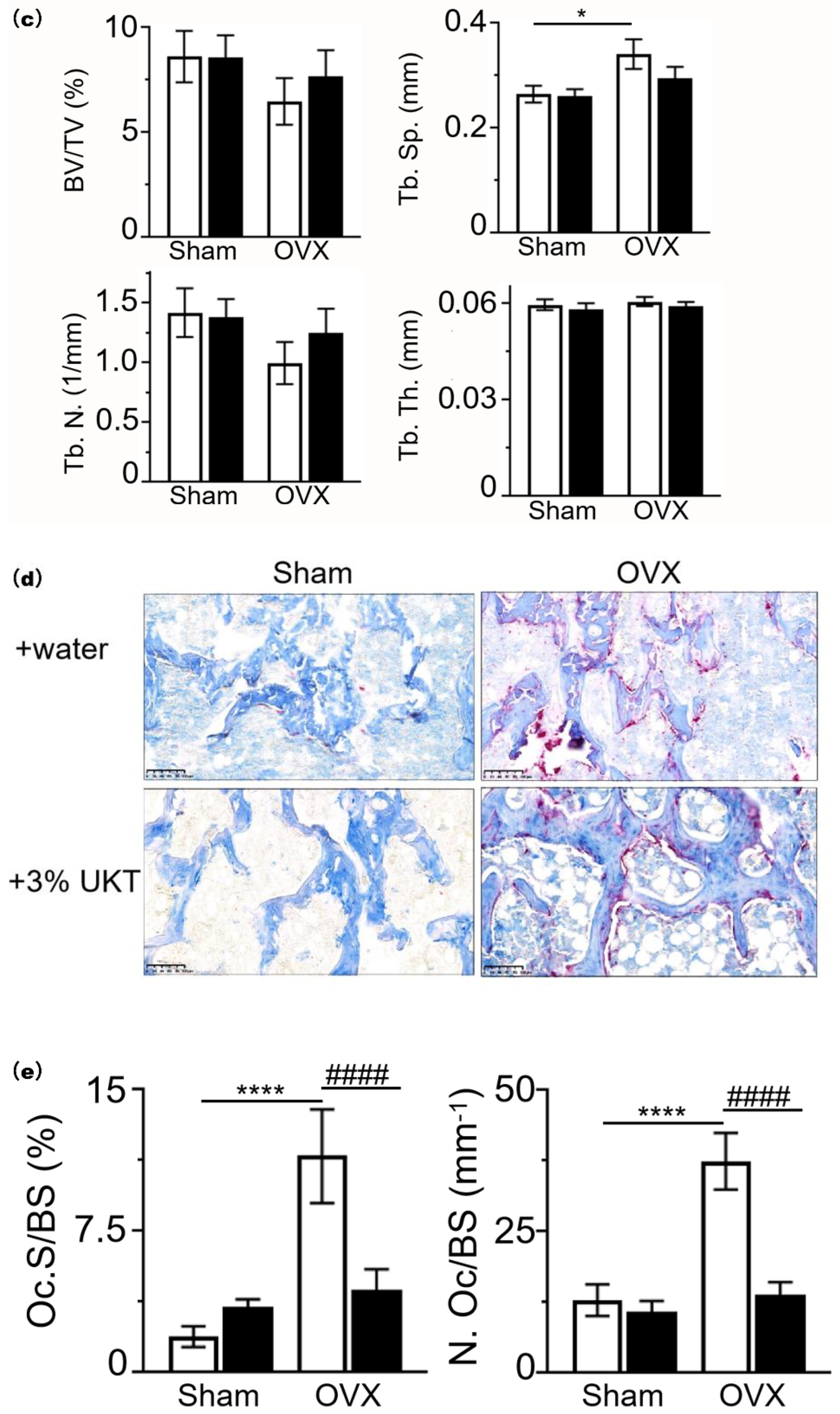
Postmenopausal osteoporosis is primarily caused by estrogen deficiency, a critical concern in aging societies. We found 10 chemical components that are all active estrogenic components, which are shown in the red boxes of
Figure 7. Previous studies indicated that 17β-estradiol can directly inhibit RANKL-induced osteoclast differentiation and enhance osteoclast apoptosis through caspase-3 activation in RAW 264 cells [
17,
18]. These reports established that estrogen-protecting effects against postmenopausal osteoporosis are partly mediated through the estrogen receptor. We have previously demonstrated that UKT exhibited strong estrogen receptor beta (ERβ)-dependent estrogenic activity through the cell proliferation bioassay and yeast two-hybrid assay [
19]. The herb
Paeonia lactiflora, which is one of the constituents of UKT, can increase the expression of ERβ in vivo [
20]. UKT was also reported to be as effective as 17β-estradiol at preventing OVX-induced bone loss in rats [
21]. In this study, we verified that UKT suppressed RANKL-induced osteoclast differentiation and enhanced osteoclast apoptosis in vitro. Moreover, UKT prevented OVX-induced bone loss in a short period of administration in vivo. Both the surface area and number of osteoclasts in bone sections from OVX mice administered with UKT were smaller than those administered with water. Thus, UKT may act on osteoclasts through estrogen-like effects. However, the uterus of UKT-treated OVX mice/sham mice was not altered in terms of either weight gain or enlargement compared to water-treated OVX mice/sham mice. Selective estrogen receptor modulators, which can prevent and treat postmenopausal osteoporosis, can bind the estrogen receptor (ER) and act as ER agonists or antagonists depending on the target tissue [
22]. UKT may have this agonistic effect on bone tissue and an antagonistic effect on the uterus, although further investigations are warranted to confirm this.
In the 3D-HPLC analysis of Unkeito (UKT), we found that one of the largest peaks was paeoniflorin (
Figure 7 green box). Wang et al. showed that paeoniflorin can suppress osteoclastogenesis and bone resorption by inhibiting the NF-κB signaling pathway in vitro and in vivo [
23]. Similarly, UKT contains rutaecarpine, a major alkaloid that can inhibit osteoclast differentiation in bone marrow-derived macrophages in vitro via the NF-κB signaling pathway [
24]. Our data showed that UKT suppressed osteoclastogenesis via the NF-κB signaling pathway. UKT may act on osteoclasts not only through estrogen-like effects, but also in a manner similar to that exhibited by other substances, such as paeoniflorin; however, this requires further investigation.
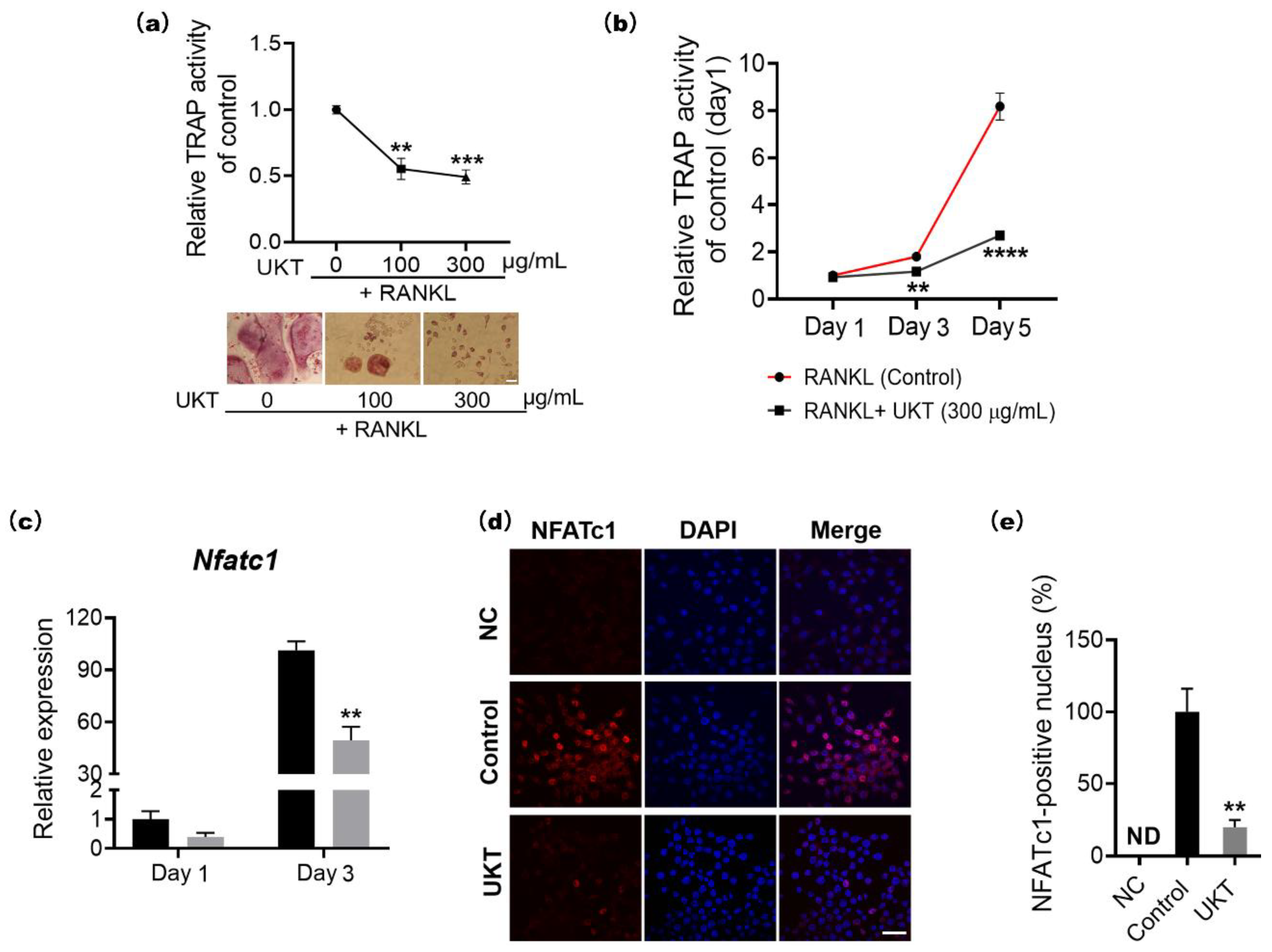
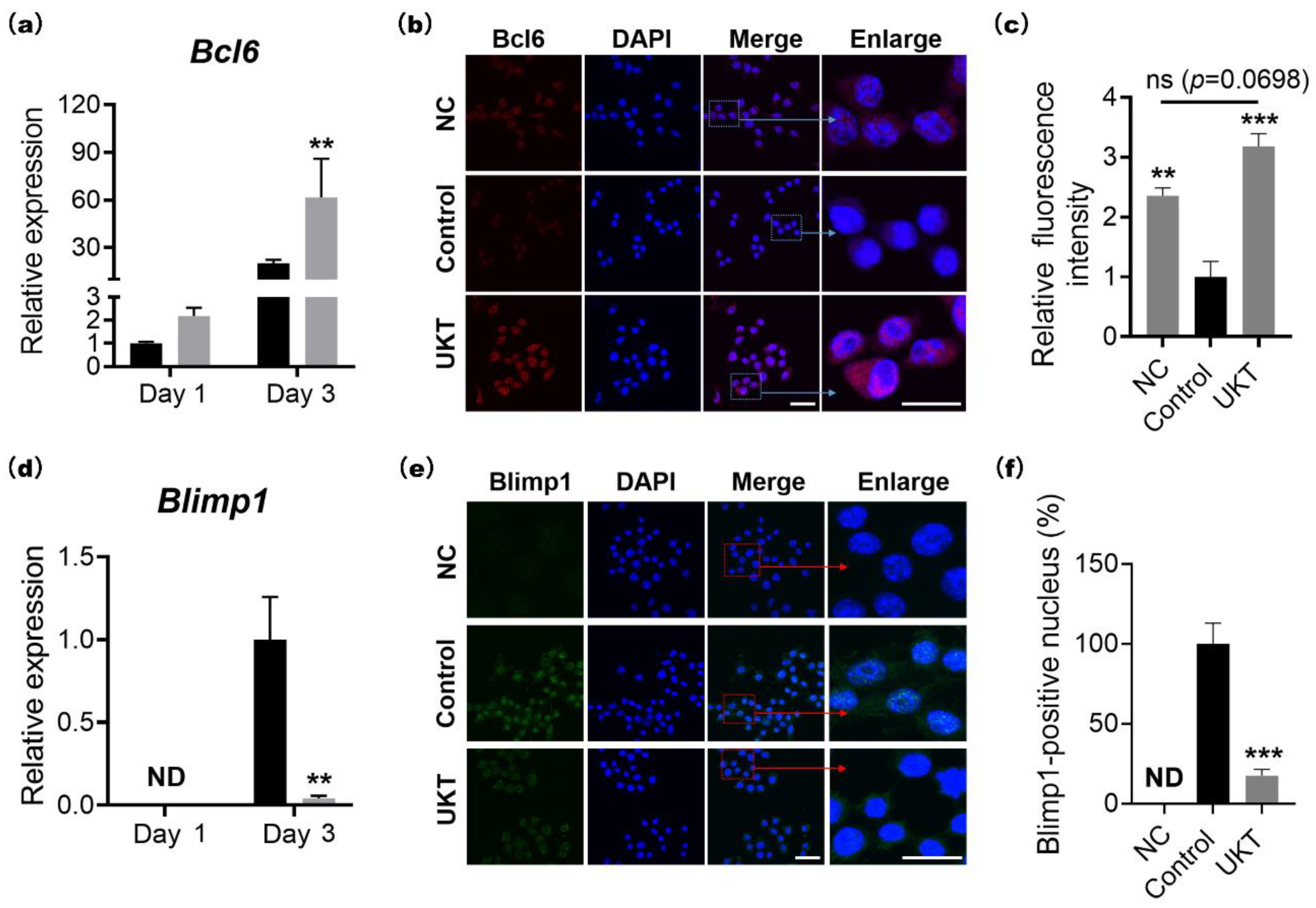
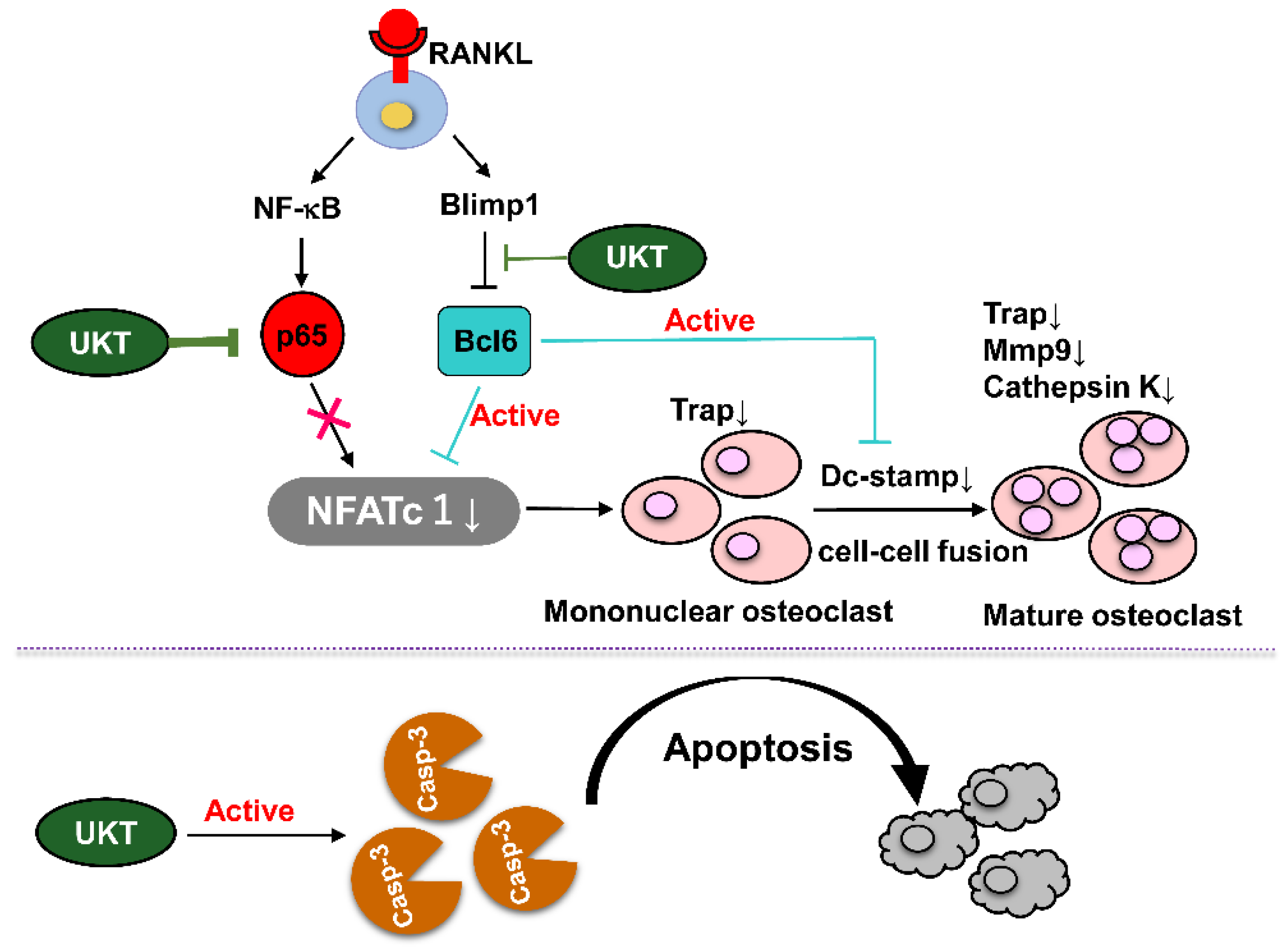
At the molecular level, the homeostasis of bone metabolism is a delicate balance between positive and negative regulation induced by NFATc1 and Bcl6 in osteoclast differentiation, respectively. Based on previous reports, Bcl6 directly binds to NFATc1, negatively regulating its expression. Furthermore, Bcl6 also negatively regulates the cell fusion factor Dc-stamp and functional gene for bone matrix degradation
Ctsk through direct binding [
25]. In other words, activation of Bcl6 expression is required for the inhibition of osteoclast differentiation, multi-nucleation, and functional expression. Our results indicated that the mRNA and protein expression of NFATc1 were decreased by UKT, while those of Bcl6 were increased. Correspondingly, the mRNA and protein expression of Blimp1, the upstream regulator of Bcl6, decreased. Moreover, the gene expression of
Dcstamp and
Ctsk was also decreased by UKT. Other research has indicated that Sirt6 expressed by RANKL-induced NFATc1 forms a complex with Blimp1 that negatively regulates the expression of anti-osteoclastogenic genes, such as
Mafb [
26]. However, the mRNA expression levels of
Mafb did not differ between the UKT and control groups in our study (
Figure S3a). These results suggest that UKT not only suppressed osteoclastogenesis but also inhibited multinuclear maturation and the resorptive function of osteoclasts on bone through the Blimp1–Bcl6 axis.
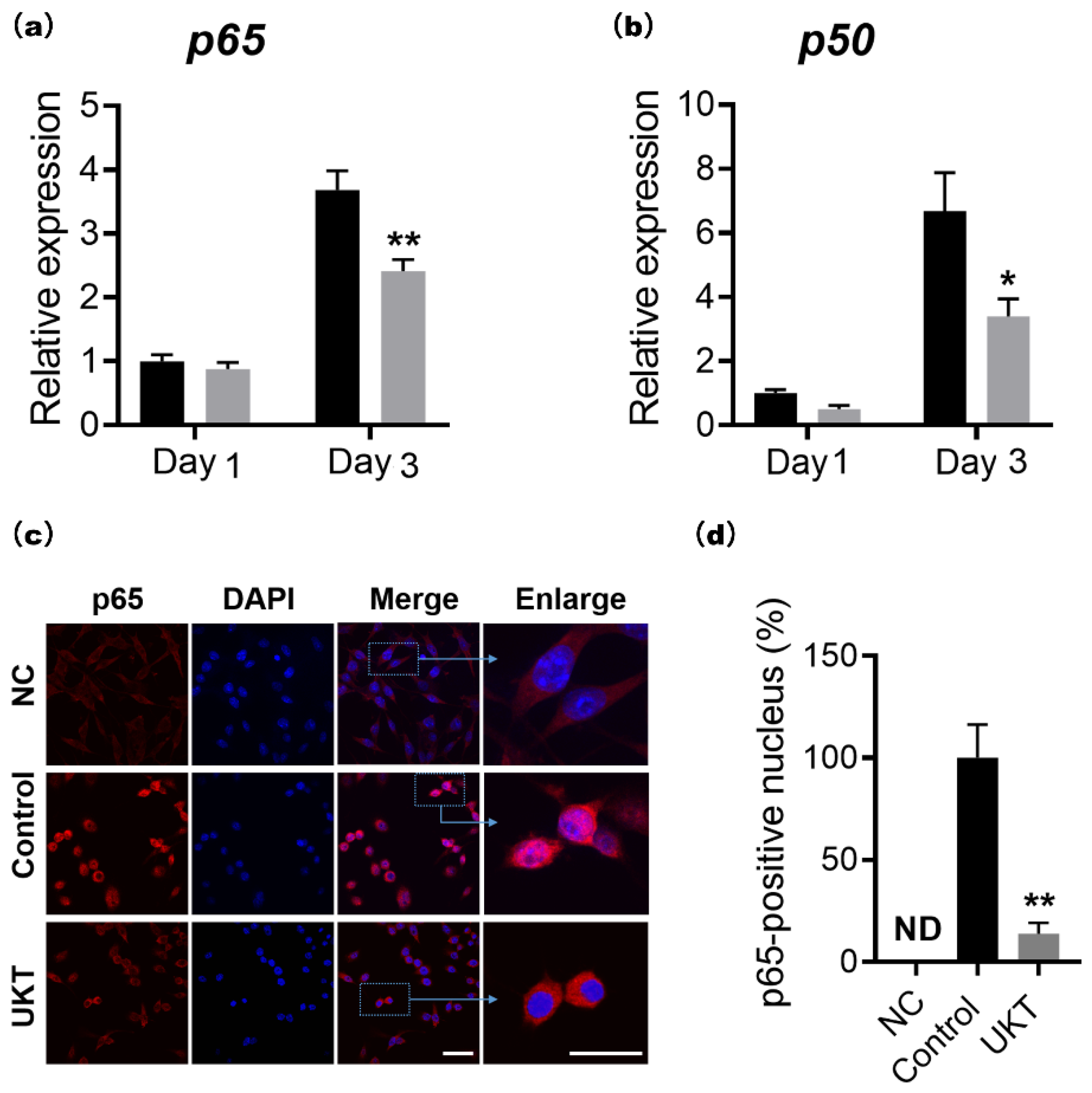
In addition, the NF-κB signaling pathway is a highly sensitive method for the differentiation of RANK-expressing osteoclast precursors into TRAP-positive osteoclasts in response to RANKL. Therefore, upon stimulation by RANKL, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα), is rapidly phosphorylated by the IκB kinase complex leading to ubiquitination and subsequent degradation of IκBα by the proteasome, followed by nuclear translocation of the p65/p50 heterodimer. When the p65/p50 heterodimer enters the nucleus, it can induce transient auto-amplification of NFATc1 expression [
6,
7,
27]. The mRNA and protein expression levels of IκBα did not differ between the UKT and control groups (data not shown). The nucleus translocation factor p65 could directly and positively regulate NFATc1, as described previously [
6,
7]. In this study, both mRNA and protein expression levels of p65 were decreased by UKT, and p65 expression was also reduced in the nucleus. This leads to the inhibition of the downstream promoter NFATc1, which directly affects the expression of genes during osteoclast differentiation, such as
Trap,
Mmp9, and
Ctsk, all of which show reduced expression with UKT. Further, the MAPK signaling pathway is also an important pathway in response to RANKL-RANK, classified into ERK, p38, and JNK based on the homology of primary sequences, and c-Fos is the transcription factor downstream of MAPK [
8,
9,
10]. We therefore examined the mRNA expression levels of ERK, p38, JNK, and c-Fos and found no significant differences following treatment with UKT (
Figure S3b). This is, to the best of our knowledge, the first validation of the effectiveness of UKT to inhibit osteoclast differentiation through both negative and positive signaling pathways.
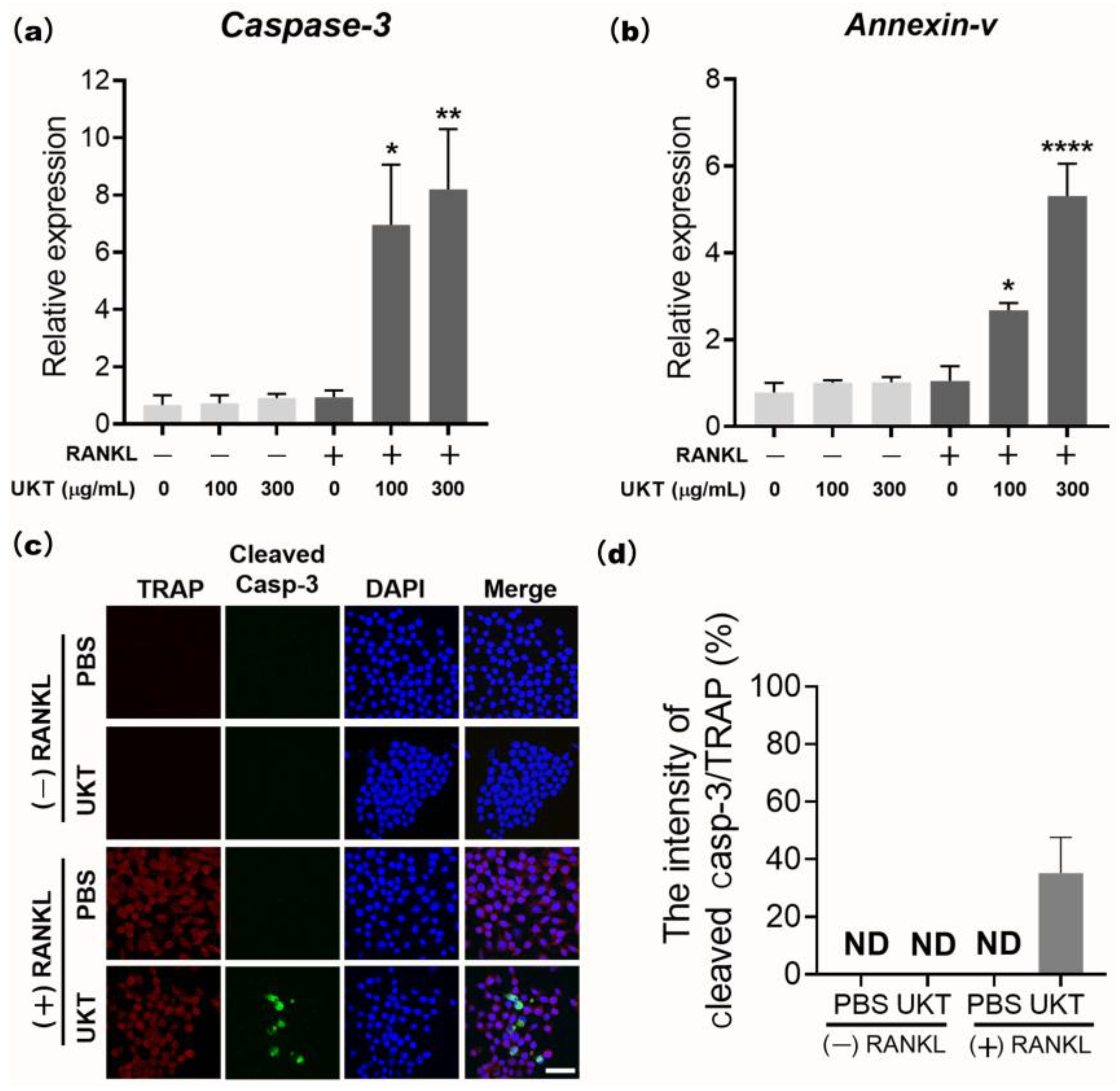
UKT was the most effective at preventing not only the differentiation of precursor osteoclasts into mononuclear osteoclasts during the early stage, but also in preventing fusion into mature osteoclasts during the late stage of differentiation. Thus, apoptosis of osteoclasts is regulated by caspase-3 [
28]. The cleaved form of caspase-3 is responsible for much of the proteolysis during apoptosis; hence, its detection is considered a reliable marker for cells that are dying or have died [
29]. Our data found that UKT enhanced RANKL-induced apoptosis of mononuclear osteoclasts by the activation of caspase-3, which may play an inhibitory role at the late stage of RANKL-induced osteoclast differentiation. This may be another reason for the inhibition of osteoclast maturation. However, we found that mature osteoclasts did not undergo apoptosis following treatment with UKT for 24 h (data not shown). UKT may have an apoptosis-inducing effect only on mononuclear osteoclasts formed during early differentiation, but not on mature and functional osteoclasts. In recent studies, osteoclast-derived apoptotic bodies played a bridging role in osteoclast–osteoblast coupling in bone remodeling [
30]. In osteoclastogenesis, osteoclast-derived exosomes were transferred to osteoblasts, inhibiting bone formation [
31,
32]. Therefore, osteoclast differentiation inhibition and apoptosis not only affect bone resorption, but also have a direct impact on osteoblasts and bone formation. To further elucidate the overall role of UKT in the dynamic balance of bone metabolism, the effects and mechanisms of UKT on osteoblasts warrant further investigation.
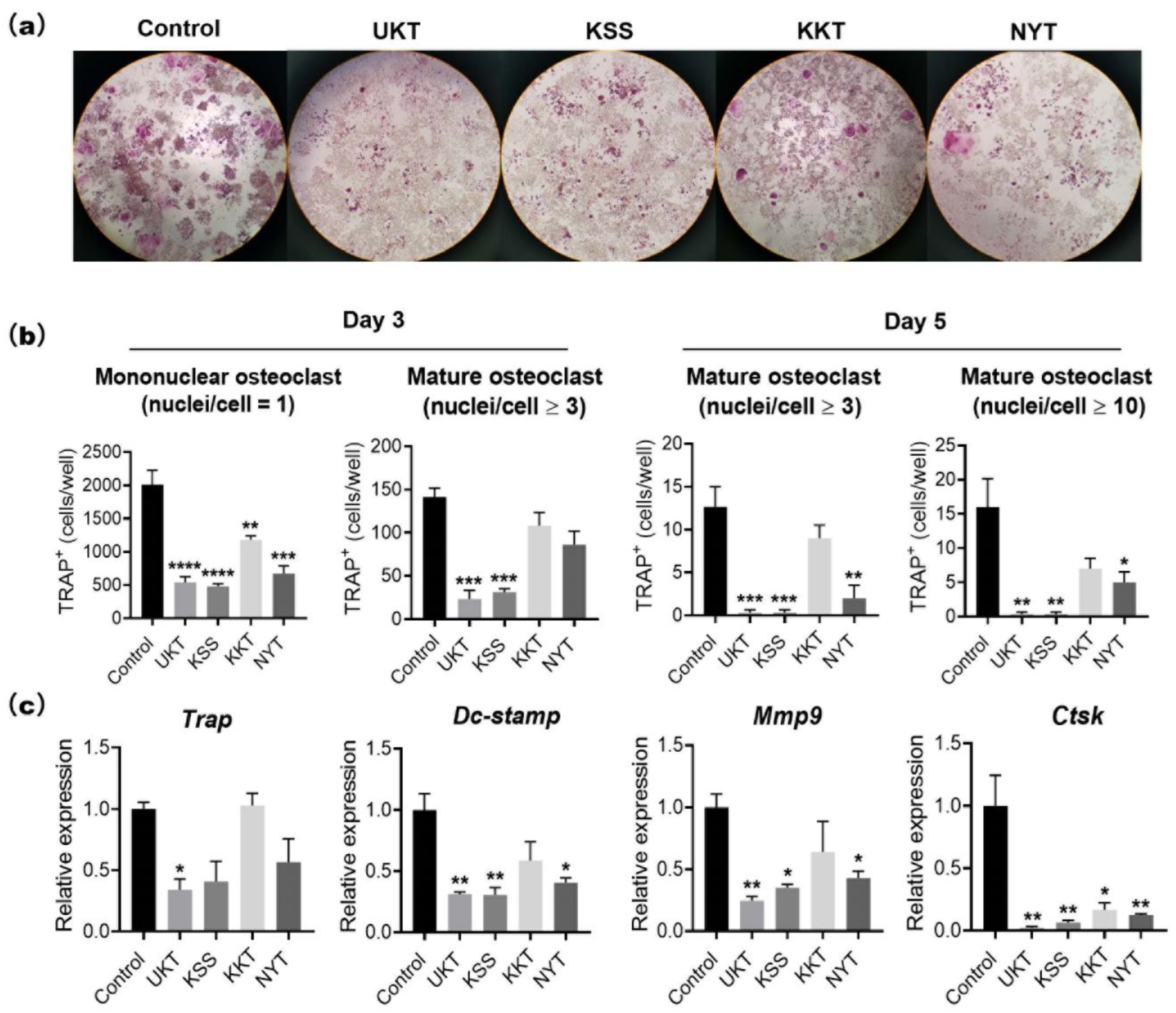
Based on the characteristics of UKT in the clinical treatment of menopausal symptoms, we screened KSS, KKT, and NYT. These Kampo medicines suppressed osteoclast differentiation without cytotoxicity. In particular, UKT and KSS effectively inhibited osteoclast differentiation from the early stage and further inhibited the formation of mature osteoclasts in the mid-differentiation stage, although the effect of KSS was slightly weaker than that of UKT. However, KKT and NYT were effective in the early stage; the formation of mature osteoclasts in the middle stage was slightly affected. We described above that paeoniflorin, which is a major component involved in UKT, may inhibit osteoclastogenesis. Paeoniflorin was also involved in KSS and NYT, but few in KTT. The peak of paeoniflorin in UKT was higher than KSS, meaning that the amount of paeoniflorin in UKT was much more than in KSS. On the other hand, the amount of paeoniflorin in NYT was lower than both UKT and KSS (
Figure 7 and
Figure S4). It is possible that this is one of the reasons that UKT is more effective for the inhibitory activity in osteoclastogenesis than the other three. In addition, liquiritin and liquiritin apioside were common estrogenic components involved in these four, and the amount of them was higher than the other estrogenic components in these four from 3D-HPLC data (
Figure 7 and
Figure S4). To date there has been no study about the effects of liquiritin and liquiritin apioside on osteoclastogenesis. We hypothesize that liquiritin and liquiritin apioside may have a role in inhibiting osteoclasts for the protection of osteoporosis, which is potentially a new subject to be explored.
We found that UKT can suppress osteoclast differentiation and induces apoptosis of mononuclear osteoclasts (
Figure 8). However, the active ingredients from UKT extracts that block the NF-κB signaling pathway and Blimp1–Bcl6 axis have not been identified. We would like to analyze and screen the active ingredients of UKT in the future to investigate the possible direct mechanisms.
4. Materials and Methods
4.1. Reagents
Recombinant human soluble RANKL (sRANKL) was purchased from Oriental Yeast Co., Ltd. (Tokyo, Japan).
4.2. Extraction of Active Ingredients from Kampo Medicines
The extract powders of UKT (TJ-106), KSS (TJ-24), KKT (TJ-137), and NYT (TJ-108) were purchased from Tsumura & Co. (Tokyo, Japan). The extract powders of Kampo medicines were prepared by hot water extraction (decoction) of the crude drugs, which are listed in
Table S1. Each of the extracted powders was mixed 1:10 with 99.5% ethanol, and the supernatant was filter sterilized using 0.45-µm membrane filters (Toyo Roshi Kaisha, Ltd., Tokyo, Japan). The liquid was dehydrated in a vacuum and then resuspended using dimethyl sulfoxide (DMSO; Nacalai Tesque. Inc., Kyoto, Japan) to completely dissolve the concentrate.
4.3. Osteoclast Differentiation
The mouse leukemic monocyte–macrophage cell line RAW 264 was purchased from the Japan Riken BioResource Research Center (RCB0535) (Ibaraki, Japan). RAW264 cells (1 × 103 cells/well) were seeded into 96-well plates and cultured in α-minimal essential medium (Nacalai Tesque Inc., Kyoto, Japan) containing 10% fetal bovine serum (HyClone, Logan, UT, USA) and 100 units/mL penicillin–streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). Kampo medicines at the concentration of 300 µg/mL in 0.1% DMSO or 0.1% DMSO (control) were added to the wells. Cells were incubated at 37 °C in a 5% CO2 atmosphere for three to five days with 100 ng/mL sRANKL to induce osteoclast formation. Osteoclasts were confirmed by TRAP staining. TRAP-positive mononuclear (nuclei/cell = 1) and multinucleated osteoclasts (nuclei/cell ≥ 3) were counted under a microscope.
4.4. TRAP Activity Assay
The TRAP activity assay was performed using 10 mM pNPP (p-nitrophenyl phosphate, disodium salt) (Nacalai Tesque Inc.), which was mixed with tartrate-containing buffer with 0.2 M sodium chloride (Sigma-Aldrich, St. Louis, MO, USA), 1 mM L(+)-ascorbic acid (FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan), 10 mM sodium(+)-tartrate dehydrate (Nacalai Tesque Inc.), and 0.1% Triton X-100 (Sigma-Aldrich), which was further mixed with 0.1 M sodium acetate (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) buffer (pH 5.2). The supernatant of the cell culture was mixed with the same volume of tartrate-containing buffer and incubated at 37 °C for 1 h. The reaction was stopped using 0.3 N sodium hydroxide solution and measured at OD405 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
4.5. TRAP Staining
TRAP staining was performed using 0.23 mM naphthol AS-MX phosphate (Nacalai Tesque Inc.) and 1.3 mM fast red violet LB salt (Sigma-Aldrich), which were dissolved by N,N-dimethylformamide (FUJIFILM Wako Pure Chemical Corporation), and then mixed with 50 mM tartrate-containing buffer (pH 5.0). The tartrate-containing buffer was composed of sodium acetate, 50 mM sodium tartrate dehydrate (Nacalai Tesque Inc.), and 1% acetic acid (FUJIFILM Wako Pure Chemical Corporation). Samples were fixed with 4% paraformaldehyde on ice for 10 min, stained with TRAP staining reagent, and incubated at 37 °C for 1 h. The TRAP-stained cells were observed using a microscope (Nikon ECLIPSE Ts2). Image analysis was performed with NIS-Elements Analysis Software (Nikon Solutions Co., Ltd., Tokyo, Japan). The numbers of TRAP-positive mononuclear osteoclasts (nuclei/cell = 1) and mature osteoclasts (nuclei/cell ≥ 3 or 10) were counted and calculated by averaging the two 96-well field of views from three independent experiments.
4.6. Quantitative Real-Time PCR Analysis of Gene Expression
To evaluate the expression of osteoclast markers, RAW 264 cells were seeded at 1 × 10
3 per well in a 96-well plate and induced using 100 ng/mL sRANKL with or without Kampo medicine to differentiate into osteoclasts for one, three, or five days. qPCR analysis was performed using the CellAmp™ Direct TB Green
® RT-qPCR kit (TaKaRa, Kusatsu, Japan) and Rotor-Gene Q real-time PCR system (QIAGEN, Dusseldorf, Germany) according to the manufacturer’s instructions. To verify the specificity of each primer, a melting-curve analysis was performed by fluorescence every 0.5 °C from 60 °C to 95 °C. The absence of contamination from either genomic DNA amplification or primer dimer formation was ensured with a Milli-Q water template control. Gene expression values of markers were normalized to
Gapdh. The sequences of the gene-specific primer pairs can be found in
Table S2.
4.7. Immunocytochemistry Staining
RAW 264 cells (1 × 10
3) were seeded into an 8-well chamber slide (Thermo Fisher Scientific), stimulated by RANKL to differentiate into osteoclasts, and incubated with UKT. After culturing for three days, the cells were fixed with 4% paraformaldehyde-phosphate buffer solution for 20 min, permeabilized with 0.3% Triton X-100 for 20 min, and blocked with Blocking One for 1 h. Chamber slides were incubated with rabbit anti-NFATc1 (1:100; D15F1, Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit anti-NF-κB p65 (1:100; Poly6226, BioLegend, Inc., San Diego, CA, USA), or Blimp1 (1:100; C14A4, Cell Signaling Technology, Inc.) overnight at 4 °C, followed by incubation with secondary antibody Cy3 conjugated donkey anti-rabbit-IgG (1:200; BioLegend, Inc.) or Alexa Fluor 488-conjugated anti-rabbit-IgG (1:500, Jackson ImmunoResearch, Baltimore, MD, USA), and Texas red conjugated anti-mouse-IgG (1:200) for 1 h. All the secondary antibodies were confirmed to be undetected in the absence of primer antibodies, as shown in
Figure S5. Finally, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Dojindo, Kumamoto, Japan) for nuclear staining and observed by confocal laser scanning microscopy using an FV3000 system (Olympus, Tokyo, Japan). The images were analyzed using cellSens Dimension software (Olympus).
4.8. Apoptosis Assay
RAW 264 cells (1 × 103) were seeded into 96-well plates and stimulated with 125 ng/mL sRANKL to differentiate into osteoclasts. After culturing for three days, the cells were treated with 100 and 300 µg/mL UKT for 24 h. The cell lysates were analyzed using qPCR. RAW 264 cells (1 × 103) were then seeded into 8-well chamber slides, stimulated by 125 ng/mL sRANKL for three days, and treated with 100 µg/mL UKT for 6 h to induce osteoclast apoptosis. Immunocytochemical staining was performed with anti-cleaved caspase-3 (1:200; Cell Signaling Technology, Inc.) and anti-TRAP (1:100; Santa Cruz Biotechnology Inc., Dallas, TX, USA) antibodies overnight at 4 °C, followed by incubation with secondary antibody Alexa Fluor 488-conjugated anti-rabbit-IgG (1:500, Jackson ImmunoResearch Inc., West Grove, PA, USA) and Texas red conjugated anti-mouse-IgG (1:200) for 1 h at room temperature (24 °C). Cells nuclei were stained with DAPI and observed by confocal laser scanning microscopy using an FV3000 system. Images were analyzed using cellSens Dimension software.
4.9. Animals
Eight-week-old specific pathogen-free (SPF) grade female CD-1 mice were obtained from Japan SLC, Inc. (Hamamatsu, Japan). Forty-four mice were divided into 4 experimental groups: (1) sham + normal drinking water, (2) sham + 3% UKT, (3) OVX + normal drinking water, and (4) OVX + 3% UKT. They were subjected to bilateral ovariectomy surgery (OVX) under anesthesia with 2% isoflurane (FUJIFILM Wako Pure Chemical Corporation), and maintained using 0.5–1% isoflurane. Immediately after the surgery, the mice were given daily oral supplementation of normal diet with tap water or 3% UKT in drinking water and weighed every week. Food intake and drinking volume were checked every 3 days during the experiment period.
On day 28 post-surgery, the mice were euthanized under anesthesia for collection of blood and tissues. The animal experiments were carried out in strict accordance with the guidelines of the Japanese Society for Pharmacology and were approved by the Committee of Animal Care at Kansai Medical University (approval number: #21-042).
4.10. Bone Analysis
At the end of the experiments, the mice were sacrificed under anesthesia. Their uteruses were removed and weighed. Both femurs were fixed with 4% paraformaldehyde phosphate buffer solution for bone histomorphometric analyses. The right side of the distal femoral trabecular bone was analyzed using micro-computed tomography (SkyScan 1174; SkyScan, Aartselaar, Belgium) for 3D image reconstruction. Images were acquired using the following settings: 50 kV voltage, 770 mA current, and 6.5 µm voxel resolution. Analysis of bone volume per tissue volume ratio (BV/TV), trabecular number (Tb.N), Tb.Sp, and trabecular thickness (Tb.Th) was made at an area within 1 mm from 0.8 mm under the growth plate peak. The left side of the digital femoral embedded block was sliced at 5 µm thickness. Osteoclast parameters were analyzed via staining with aniline blue and TRAP. Analyses were performed with HistoMorph open source software developed by the Institute of Ageing and Chronic Disease, University of Liverpool [
33].
4.11. Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 8.4.2. All data were presented as mean ± standard error of the mean. For comparisons of in vitro experiments, data were analyzed using Tukey’s multiple comparisons tests. For comparisons of in vivo experiments, two-way analysis of variance was used, followed by the Tukey’s multiple comparison tests. Statistical significance was set at p < 0.05.