Trypsin-like Inhibitor Domain (TIL)-Harboring Protein Is Essential for Aedes aegypti Reproduction
Abstract
:1. Introduction
2. Results
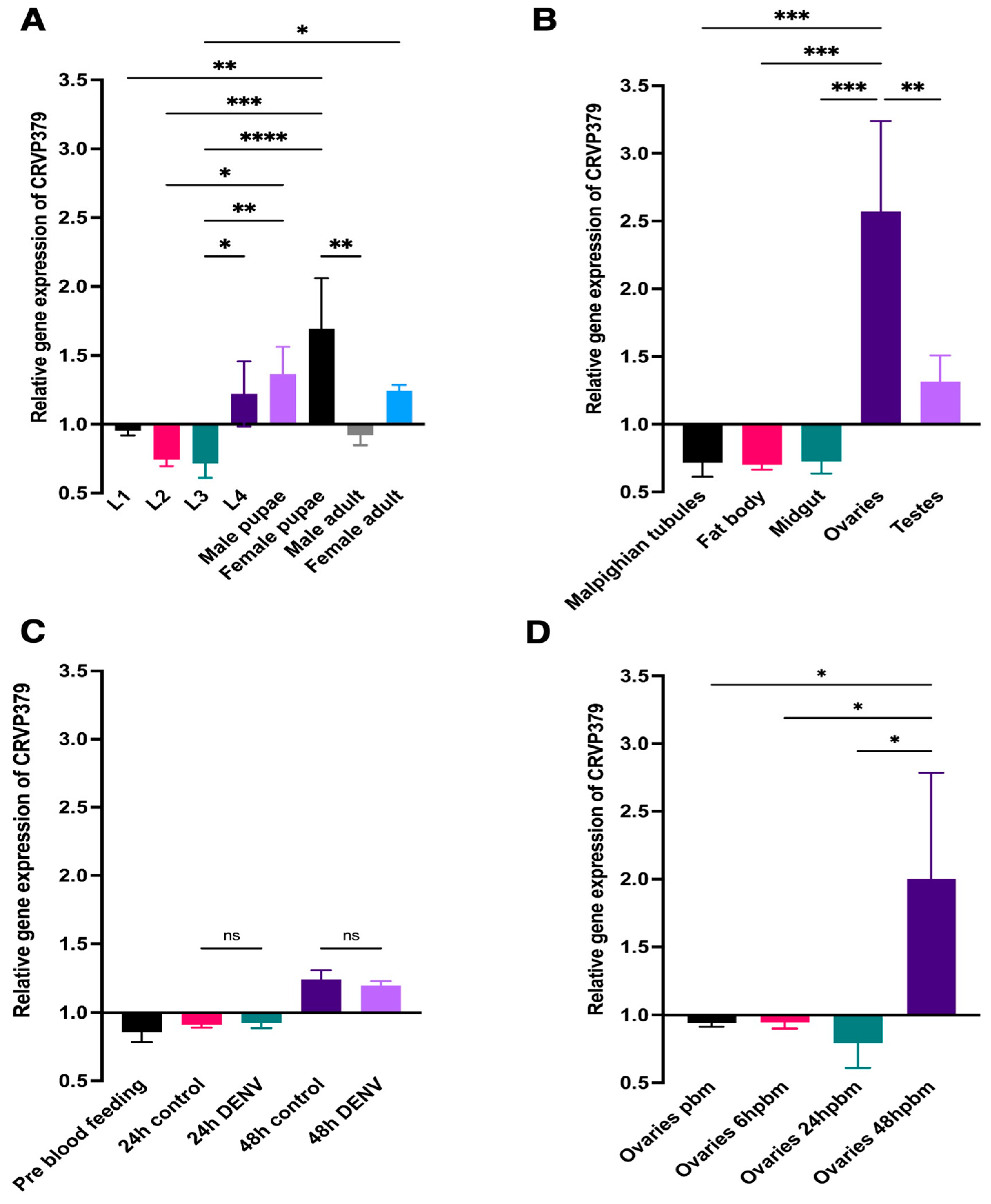
2.1. CRVP379 mRNA Abundance
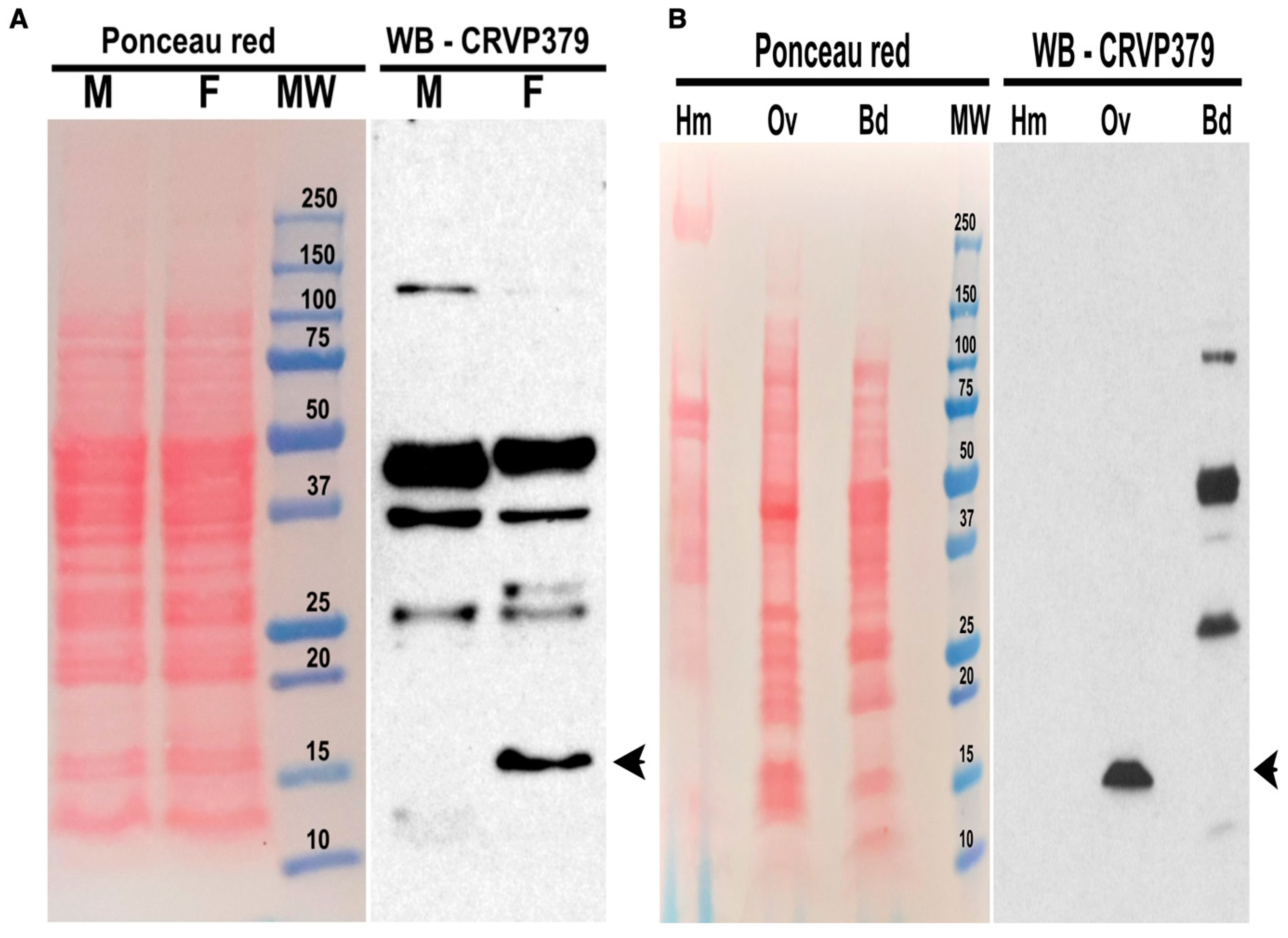
2.2. CRVP379 Is Present in Ae. aegypti Ovaries but Not Secreted in the Hemolymph
2.3. CRVP379 Gene Silencing Does Not Influence DENV Infection in the Midgut
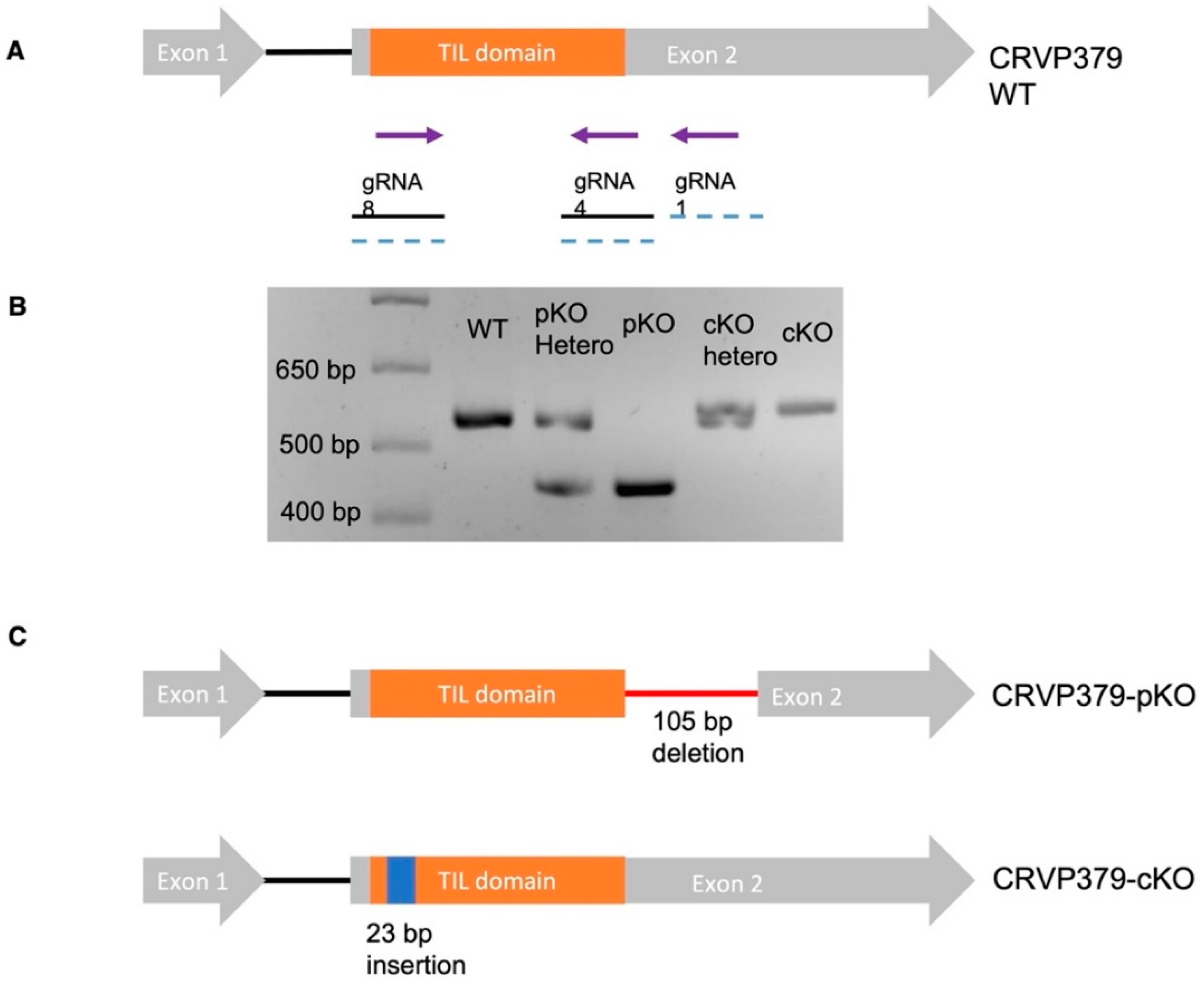
2.4. Generation of CRVP379 Knockout Lines Using the CRISPR-Cas9 System
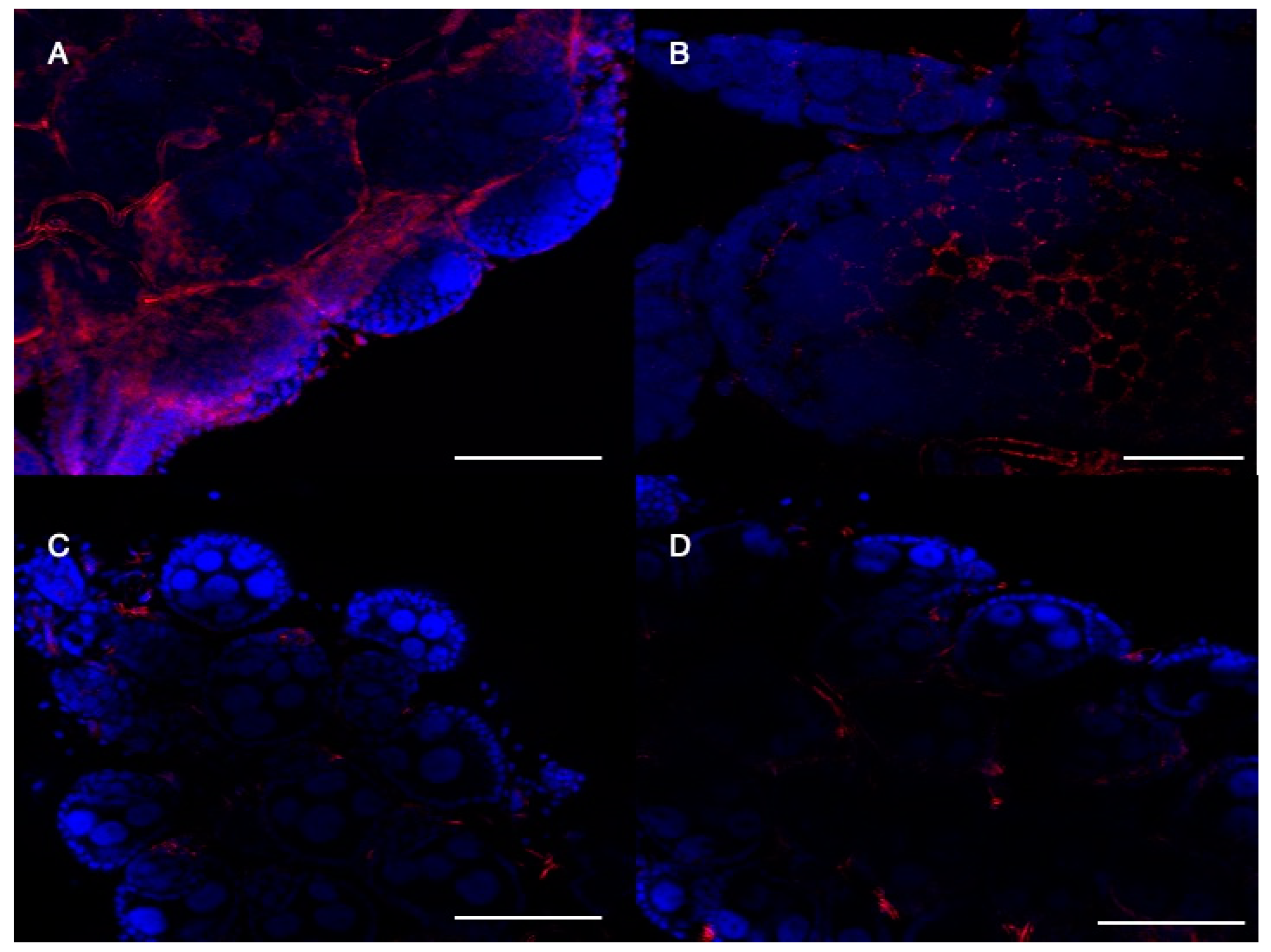
2.5. CRVP379 Is Important for Follicular Morphology in Ae. aegypti Ovaries
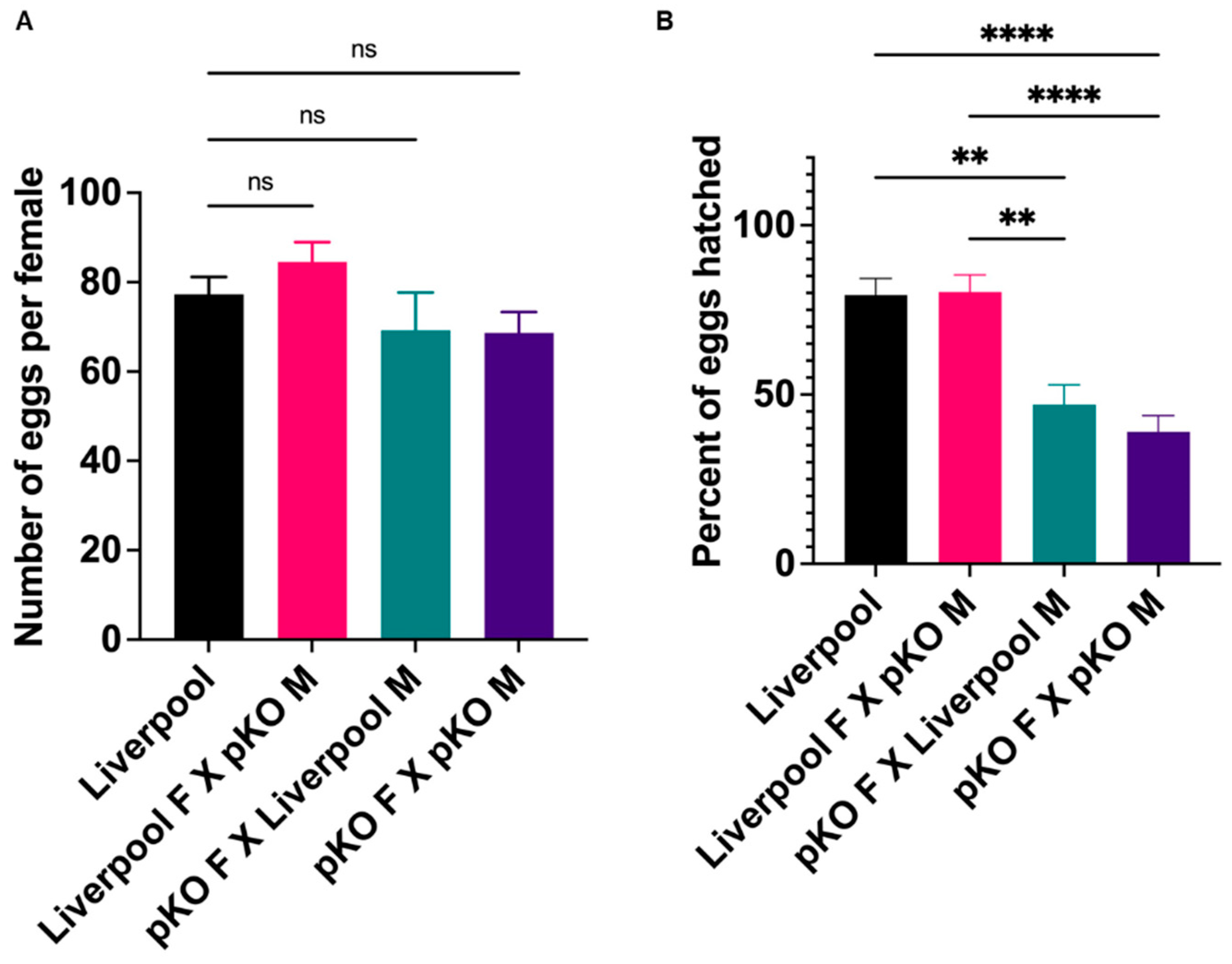
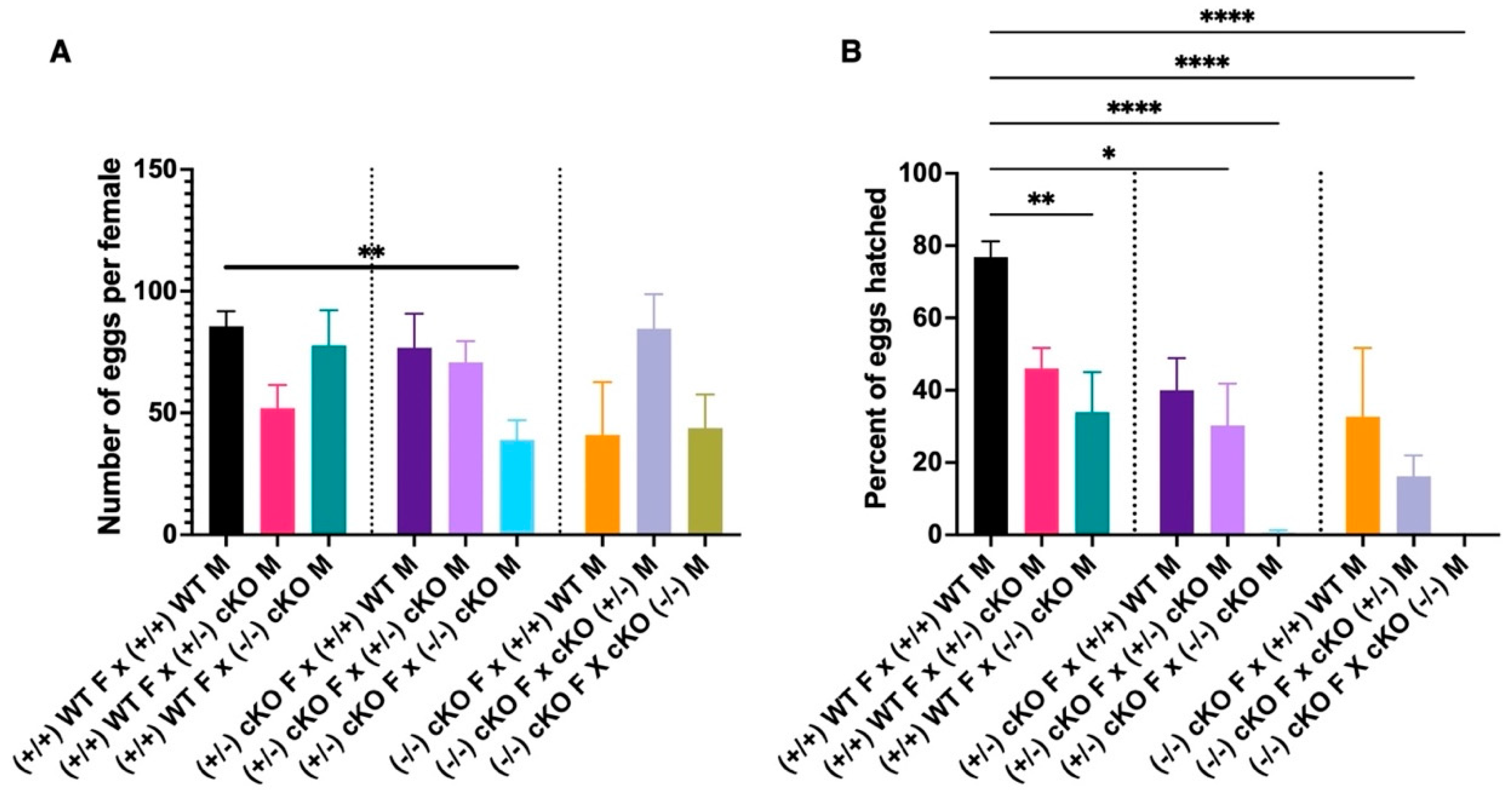
2.6. CRVP379 Plays an Important Role in the Male and Female Reproductive Systems
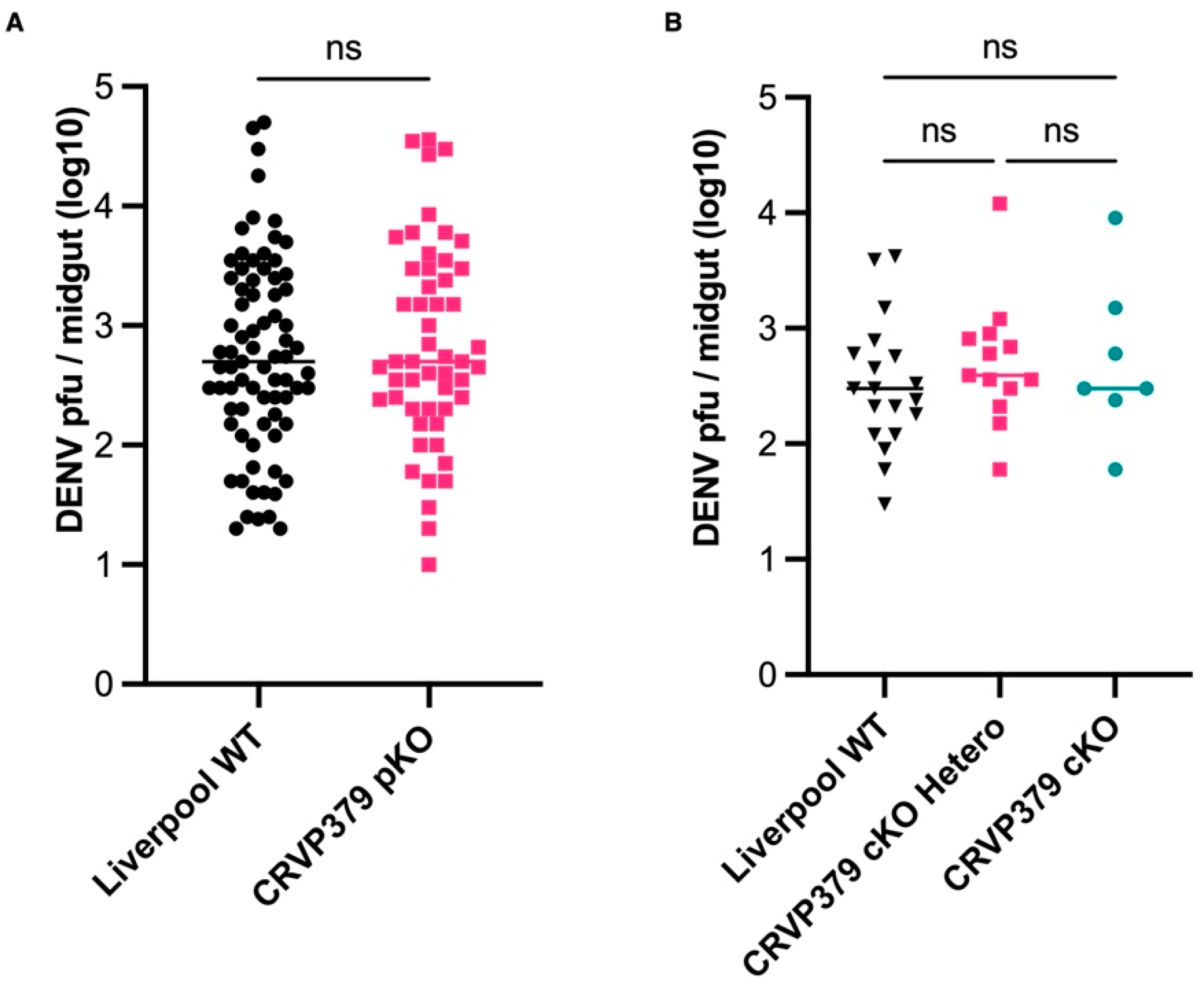
2.7. CRVP379 Does Not Influence DENV Infection in the Midgut
2.8. CRVP-pKO Females Have a Significantly Reduced Lifespan
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Mosquito Rearing
4.3. Cell Culture
4.4. Dengue Virus
4.5. dsRNA Synthesis
4.6. Gene Silencing
4.7. Gene Expression
4.8. Generation of CRVP379 Knockout Lines
4.9. Embryo Injections
4.10. Leg PCR
4.11. Line Maintenance, Fecundity, and Life Span
4.12. Immunoblotting
4.13. Immunohistochemical Staining and Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Dengue Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 4 July 2022).
- Gubler, D.J. The Economic Burden of Dengue. Am. J. Trop. Med. Hyg. 2012, 86, 743. [Google Scholar] [CrossRef] [Green Version]
- Shepard, D.S.; Coudeville, L.; Halasa, Y.A.; Zambrano, B.; Dayan, G.H. Economic Impact of Dengue Illness in the Americas. Am. J. Trop. Med. Hyg. 2011, 84, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.P. Dengue Drug Discovery: Progress, Challenges and Outlook. Antiviral Res. 2019, 163, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A. Dengue Vaccine Development by the Year 2020: Challenges and Prospects. Curr. Opin. Virol. 2020, 43, 71–78. [Google Scholar] [CrossRef]
- Badolo, A.; Sombié, A.; Pignatelli, P.M.; Sanon, A.; Yaméogo, F.; Wangrawa, D.W.; Sanon, A.; Kanuka, H.; McCall, P.J.; Weetman, D. Insecticide Resistance Levels and Mechanisms in Aedes Aegypti Populations in and around Ouagadougou, Burkina Faso. PLoS Negl. Trop. Dis. 2019, 13, e0007439. [Google Scholar] [CrossRef] [PubMed]
- Marcombe, S.; Fustec, B.; Cattel, J.; Chonephetsarath, S.; Thammavong, P.; Phommavanh, N.; David, J.P.; Corbel, V.; Sutherland, I.W.; Hertz, J.C.; et al. Distribution of Insecticide Resistance and Mechanisms Involved in the Arbovirus Vector Aedes Aegypti in Laos and Implication for Vector Control. PLoS Negl. Trop. Dis. 2019, 13, e0007852. [Google Scholar] [CrossRef] [Green Version]
- Sene, N.M.; Mavridis, K.; Ndiaye, E.H.; Diagne, C.T.; Gaye, A.; Ngom, E.H.M.; Ba, Y.; Diallo, D.; Vontas, J.; Dia, I.; et al. Insecticide Resistance Status and Mechanisms in Aedes Aegypti Populations from Senegal. PLoS Negl. Trop. Dis. 2021, 15, e0009393. [Google Scholar] [CrossRef]
- Vontas, J.; Kioulos, E.; Pavlidi, N.; Morou, E.; della Torre, A.; Ranson, H. Insecticide Resistance in the Major Dengue Vectors Aedes Albopictus and Aedes Aegypti. Pestic. Biochem. Physiol. 2012, 104, 126–131. [Google Scholar] [CrossRef]
- Mukherjee, D.; Das, S.; Begum, F.; Mal, S.; Ray, U. The Mosquito Immune System and the Life of Dengue Virus: What We Know and Do Not Know. Pathogens 2019, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Simões, M.L.; Caragata, E.P.; Dimopoulos, G. Diverse Host and Restriction Factors Regulate Mosquito-Pathogen Interactions. Trends Parasitol. 2018, 34, 603–616. [Google Scholar] [CrossRef]
- Tikhe, C.V.; Dimopoulos, G. Mosquito Antiviral Immune Pathways. Dev. Comp. Immunol. 2021, 116, 103964. [Google Scholar] [CrossRef]
- Londono-Renteria, B.; Troupin, A.; Conway, M.J.; Vesely, D.; Ledizet, M.; Roundy, C.M.; Cloherty, E.; Jameson, S.; Vanlandingham, D.; Higgs, S.; et al. Dengue Virus Infection of Aedes Aegypti Requires a Putative Cysteine Rich Venom Protein. PLoS Pathog. 2015, 11, e1005202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, Y.; Morita, T. Structure and Function of Snake Venom Cysteine-Rich Secretory Proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, G.M.; Roelants, K.; O’Bryan, M.K. The CAP Superfamily: Cysteine-Rich Secretory Proteins, Antigen 5, and Pathogenesis-Related 1 Proteins—Roles in Reproduction, Cancer, and Immune Defense. Endocr. Rev. 2008, 29, 865–897. [Google Scholar] [CrossRef] [PubMed]
- Koppers, A.J.; Reddy, T.; O’Bryan, M.K. The Role of Cysteine-Rich Secretory Proteins in Male Fertility. Asian J. Androl. 2011, 13, 111. [Google Scholar] [CrossRef] [Green Version]
- Maróti, G.; Downie, J.A.; Kondorosi, É. Plant Cysteine-Rich Peptides That Inhibit Pathogen Growth and Control Rhizobial Differentiation in Legume Nodules. Curr. Opin. Plant Biol. 2015, 26, 57–63. [Google Scholar] [CrossRef]
- Lavergne, V.; JTaft, R.J.; Alewood, P.F. Cysteine-Rich Mini-Proteins in Human Biology. Curr. Top. Med. Chem. 2012, 12, 1514–1533. [Google Scholar] [CrossRef]
- Srivastava, S.; Dashora, K.; Ameta, K.L.; Singh, N.P.; El-Enshasy, H.A.; Pagano, M.C.; Hesham, A.E.-L.; Sharma, G.D.; Sharma, M.; Bhargava, A. Cysteine-Rich Antimicrobial Peptides from Plants: The Future of Antimicrobial Therapy. Phyther. Res. 2021, 35, 256–277. [Google Scholar] [CrossRef]
- Zeng, X.C.; Liu, Y.; Shi, W.; Zhang, L.; Luo, X.; Nie, Y.; Yang, Y. Genome-Wide Search and Comparative Genomic Analysis of the Trypsin Inhibitor-like Cysteine-Rich Domain-Containing Peptides. Peptides 2014, 53, 106–114. [Google Scholar] [CrossRef]
- Dong, S.; Ye, Z.; Tikhe, C.V.; Tu, Z.J.; Zwiebel, L.J.; Dimopoulos, G. Pleiotropic Odorant-Binding Proteins Promote Aedes Aegypti Reproduction and Flavivirus Transmission. MBio 2021, 12, e0253121. [Google Scholar] [CrossRef]
- Dong, Y.; Simões, M.L.; Marois, E.; Dimopoulos, G. CRISPR/Cas9 -Mediated Gene Knockout of Anopheles Gambiae FREP1 Suppresses Malaria Parasite Infection. PLoS Pathog. 2018, 14, e1006898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, M.L.; Dong, Y.; Mlambo, G.; Dimopoulos, G. C-Type Lectin 4 Regulates Broad-Spectrum Melanization-Based Refractoriness to Malaria Parasites. PLoS Biol. 2022, 20, e3001515. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bui, M.; Yang, T.; Bowman, C.S.; White, B.J.; Akbari, O.S. Germline Cas9 Expression Yields Highly Efficient Genome Engineering in a Major Worldwide Disease Vector, Aedes Aegypti. Proc. Natl. Acad. Sci. USA 2017, 114, E10540–E10549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isoe, J.; Koch, L.E.; Isoe, Y.E.; Rascón, A.A.; Brown, H.E.; Massani, B.B.; Miesfeld, R.L. Identification and Characterization of a Mosquito-Specific Eggshell Organizing Factor in Aedes Aegypti Mosquitoes. PLoS Biol. 2019, 17, e3000068. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.-J.; Zhou, S.-M.; Zhou, Y.-N.; Huang, J.-H.; Shi, M.; Chen, X.-X. A Trypsin Inhibitor-like Protein Secreted by Cotesia Vestalis Teratocytes Inhibits Hemolymph Prophenoloxidase Activation of Plutella Xylostella. J. Insect Physiol. 2019, 116, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Lee, K.S.; Lee, K.Y.; Yoon, H.J.; Jin, B.R. Anti-Fibrinolytic Activity of a Metalloprotease Inhibitor from Bumblebee (Bombus Ignitus) Venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 245, 109042. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, P.; Liu, S.; Dong, Z.; Chen, J.; Xiang, Z.; Xia, Q. A Novel Protease Inhibitor in Bombyx Mori Is Involved in Defense against Beauveria Bassiana. Insect Biochem. Mol. Biol. 2012, 42, 766–775. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zhu, R.; Xia, Q.; Zhao, P. Loss of Second and Sixth Conserved Cysteine Residues from Trypsin Inhibitor-like Cysteine-Rich Domain-Type Protease Inhibitors in Bombyx Mori May Induce Activity against Microbial Proteases. Peptides 2016, 86, 13–23. [Google Scholar] [CrossRef]
- Mojzisch, A.; Brehm, M.A. The Manifold Cellular Functions of von Willebrand Factor. Cells 2021, 10, 2351. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Eng, E.T.; Zhu, J.; Lu, C.; Walz, T.; Springer, T.A. Sequence and Structure Relationships within von Willebrand Factor. Blood 2012, 120, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.W.; Tan, J.M.; Du, C.W.; Luan, N.; Yan, X.W.; Lai, R.; Lu, Q.M. A Novel Trypsin Inhibitor-Like Cysteine-Rich Peptide from the Frog Lepidobatrachus Laevis Containing Proteinase-Inhibiting Activity. Nat. Products Bioprospect. 2015, 5, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakeel, M.; Zafar, J. Molecular Identification, Characterization, and Expression Analysis of a Novel Trypsin Inhibitor-like Cysteine-Rich Peptide from the Cotton Bollworm, Helicoverpa Armigera (Hübner) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2020, 30, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mesquita-Rodrigues, C.; Saboia-Vahia, L.; Cuervo, P.; Masini d’Avila Levy, C.; Alves Honorio, N.; Domont, G.B.; Batista de Jesus, J. Expression of Trypsin-like Serine Peptidases in Pre-Imaginal Stages of Aedes Aegypti (Diptera: Culicidae). Arch. Insect Biochem. Physiol. 2011, 76, 223–235. [Google Scholar] [CrossRef]
- Saboia-Vahia, L.; Borges-Veloso, A.; Mesquita-Rodrigues, C.; Cuervo, P.; Dias-Lopes, G.; Britto, C.; De Barros Silva, A.P.; De Jesus, J.B. Trypsin-like Serine Peptidase Profiles in the Egg, Larval, and Pupal Stages of Aedes Albopictus. Parasites Vectors 2013, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.J.; Davies, D.M. Trypsin and Chymotrypsin during Metamorphosis in Aedes Aegypti and Properties of the Chymotrypsin. J. Insect Physiol. 1971, 17, 117–131. [Google Scholar] [CrossRef]
- Akbari, O.S.; Antoshechkin, I.; Amrhein, H.; Williams, B.; Diloreto, R.; Sandler, J.; Hay, B.A. The Developmental Transcriptome of the Mosquito Aedes Aegypti, an Invasive Species and Major Arbovirus Vector. G3 (Bethesda) 2013, 3, 1493–1509. [Google Scholar] [CrossRef] [Green Version]
- Hixson, B.; Bing, X.-L.; Yang, X.; Bonfini, A.; Nagy, P.; Buchon, N. A Transcriptomic Atlas of Aedes Aegypti Reveals Detailed Functional Organization of Major Body Parts and Gut Regional Specializations in Sugar-Fed and Blood-Fed Adult Females. Elife 2022, 11, e76132. [Google Scholar] [CrossRef]
- Ling, L.; Raikhel, A.S. Cross-Talk of Insulin-like Peptides, Juvenile Hormone, and 20-Hydroxyecdysone in Regulation of Metabolism in the Mosquito Aedes Aegypti. Proc. Natl. Acad. Sci. USA 2021, 118, e2023470118. [Google Scholar] [CrossRef]
- Clifton, M.E.; Noriega, F.G. The Fate of Follicles after a Blood Meal Is Dependent on Previtellogenic Nutrition and Juvenile Hormone in Aedes Aegypti. J. Insect Physiol. 2012, 58, 1007–1019. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Martínez, S.; Cardoso-Jaime, V.; Nouzova, M.; Michalkova, V.; Ramirez, C.E.; Fernandez-Lima, F.; Noriega, F.G. Juvenile Hormone Controls Ovarian Development in Female Anopheles Albimanus Mosquitoes. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bian, G.; Raikhel, A.S.; Zhu, J. Characterization of a Juvenile Hormone-Regulated Chymotrypsin-like Serine Protease Gene in Aedes Aegypti Mosquito. Insect Biochem. Mol. Biol. 2008, 38, 190–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.-L.; Han, S.-J.; Zhang, R.-J.; Yu, J.-B.; Li, Y.-B.; Yu, X.-P.; Liu, G.-F.; Xu, Y.-P. Inter-Alpha-Trypsin Inhibitor Heavy Chain 4 Plays an Important Role in the Development and Reproduction of Nilaparvata Lugens. Insects 2022, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Labun, K.; Montague, T.G.; Krause, M.; Torres Cleuren, Y.N.; Tjeldnes, H.; Valen, E. CHOPCHOP v3: Expanding the CRISPR Web Toolbox beyond Genome Editing. Nucleic Acids Res. 2019, 47, W171–W174. [Google Scholar] [CrossRef] [Green Version]
- Maya-Maldonado, K.; Cardoso-Jaime, V.; Hernández-Martínez, S.; Vázquez-Calzada, C.; Hernández-Hernández, F.C.; Lanz-Mendoza, H. DNA Synthesis Increases during the First Hours Post-Emergence in Anopheles Albimanus Mosquito Midgut. Dev. Comp. Immunol. 2020, 112, 103753. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhe, C.V.; Cardoso-Jaime, V.; Dong, S.; Rutkowski, N.; Dimopoulos, G. Trypsin-like Inhibitor Domain (TIL)-Harboring Protein Is Essential for Aedes aegypti Reproduction. Int. J. Mol. Sci. 2022, 23, 7736. https://doi.org/10.3390/ijms23147736
Tikhe CV, Cardoso-Jaime V, Dong S, Rutkowski N, Dimopoulos G. Trypsin-like Inhibitor Domain (TIL)-Harboring Protein Is Essential for Aedes aegypti Reproduction. International Journal of Molecular Sciences. 2022; 23(14):7736. https://doi.org/10.3390/ijms23147736
Chicago/Turabian StyleTikhe, Chinmay Vijay, Victor Cardoso-Jaime, Shengzhang Dong, Natalie Rutkowski, and George Dimopoulos. 2022. "Trypsin-like Inhibitor Domain (TIL)-Harboring Protein Is Essential for Aedes aegypti Reproduction" International Journal of Molecular Sciences 23, no. 14: 7736. https://doi.org/10.3390/ijms23147736
APA StyleTikhe, C. V., Cardoso-Jaime, V., Dong, S., Rutkowski, N., & Dimopoulos, G. (2022). Trypsin-like Inhibitor Domain (TIL)-Harboring Protein Is Essential for Aedes aegypti Reproduction. International Journal of Molecular Sciences, 23(14), 7736. https://doi.org/10.3390/ijms23147736








