Can Modern Molecular Modeling Methods Help Find the Area of Potential Vulnerability of Flaviviruses?
Abstract
:1. Introduction
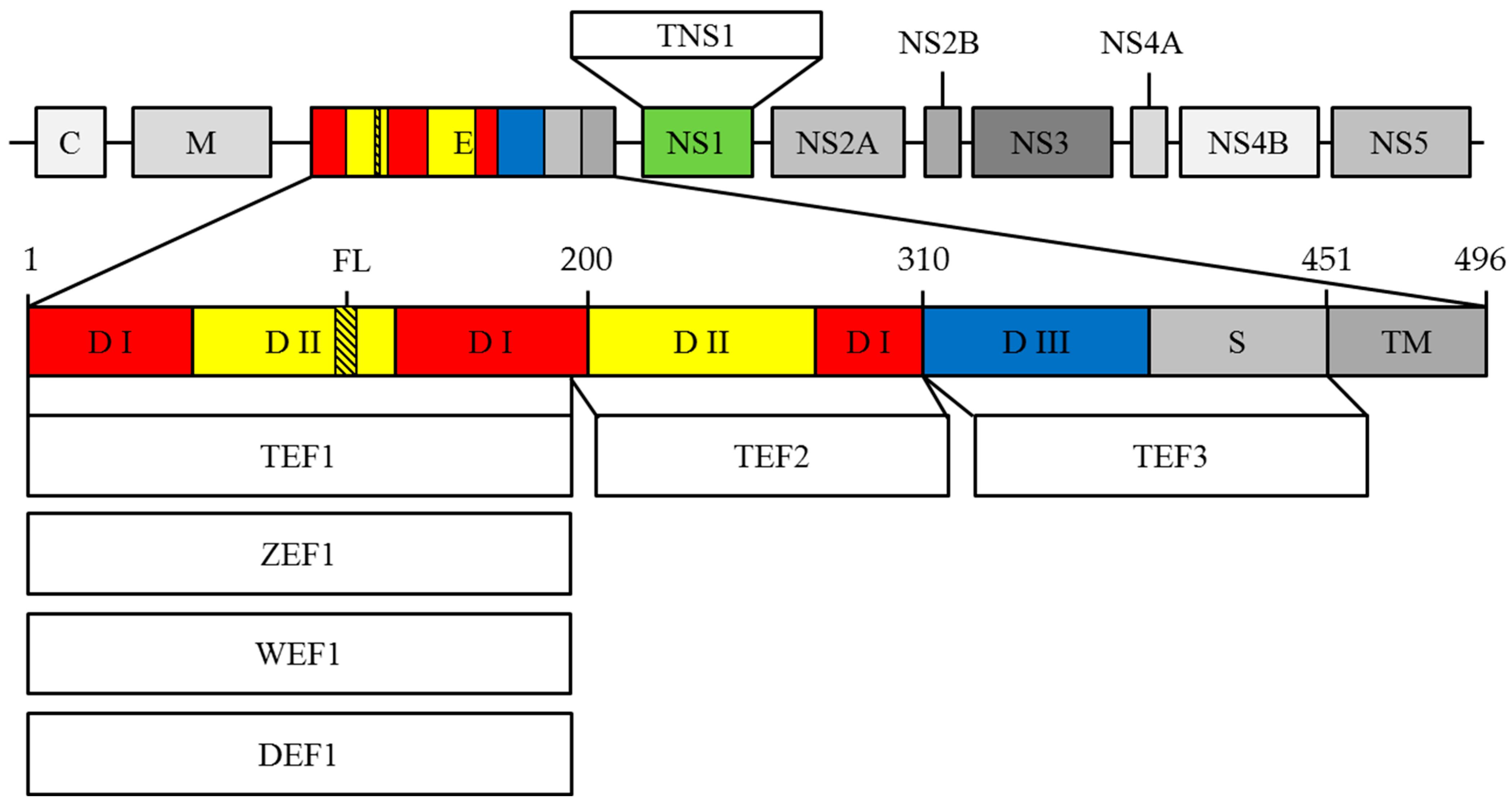
2. Results and Discussion
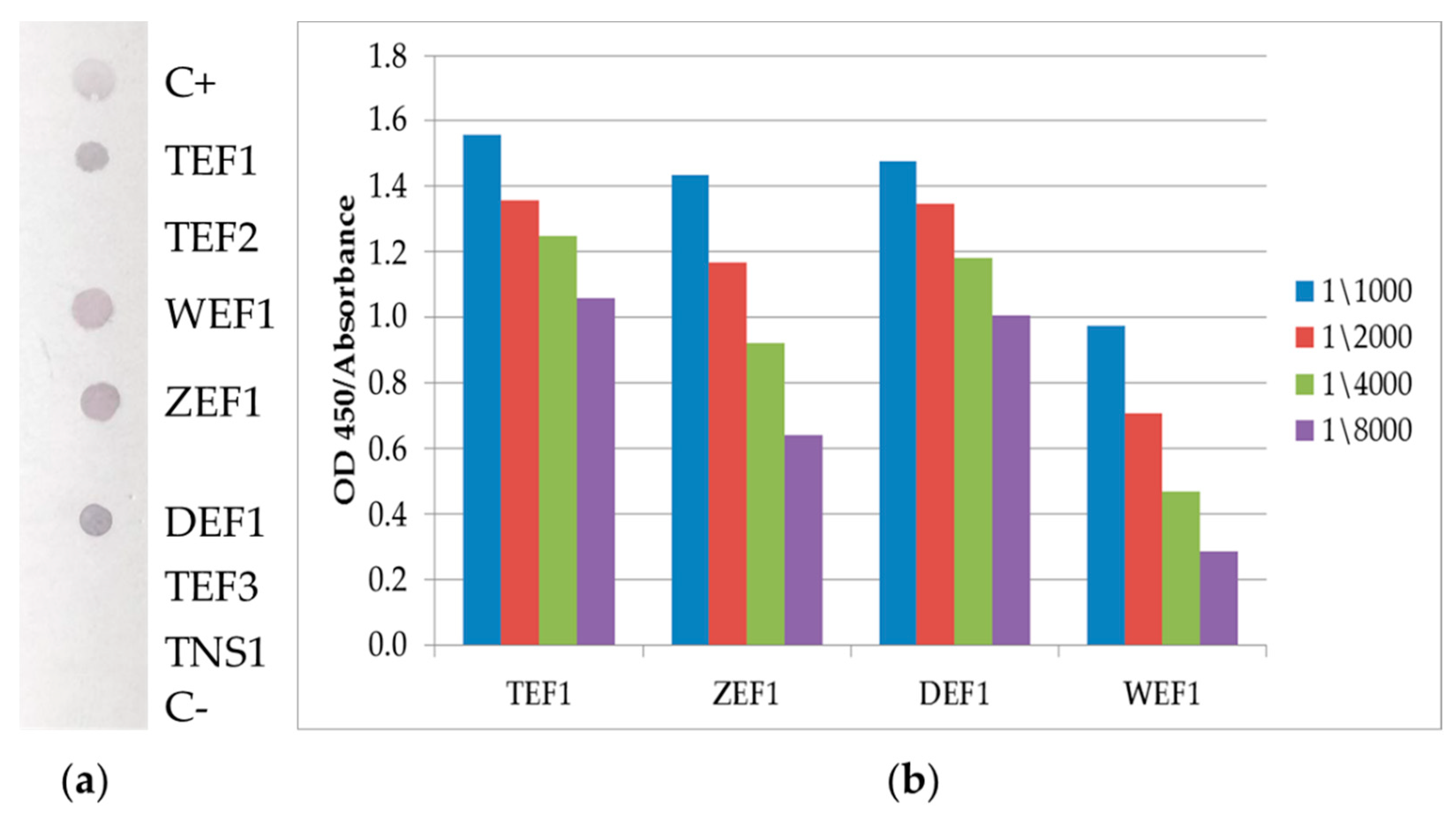
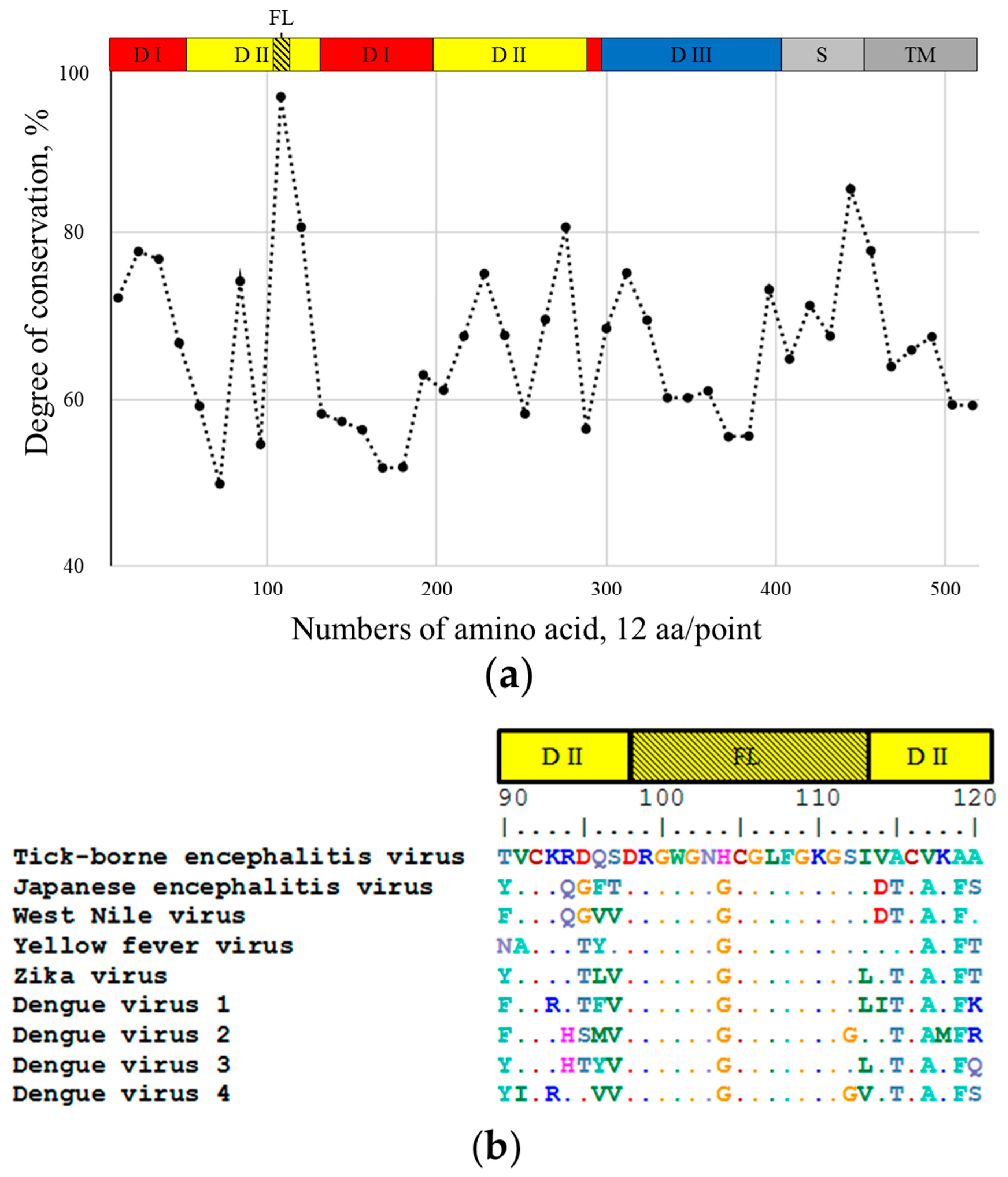
2.1. 10H10 Antibody Epitope Mapping
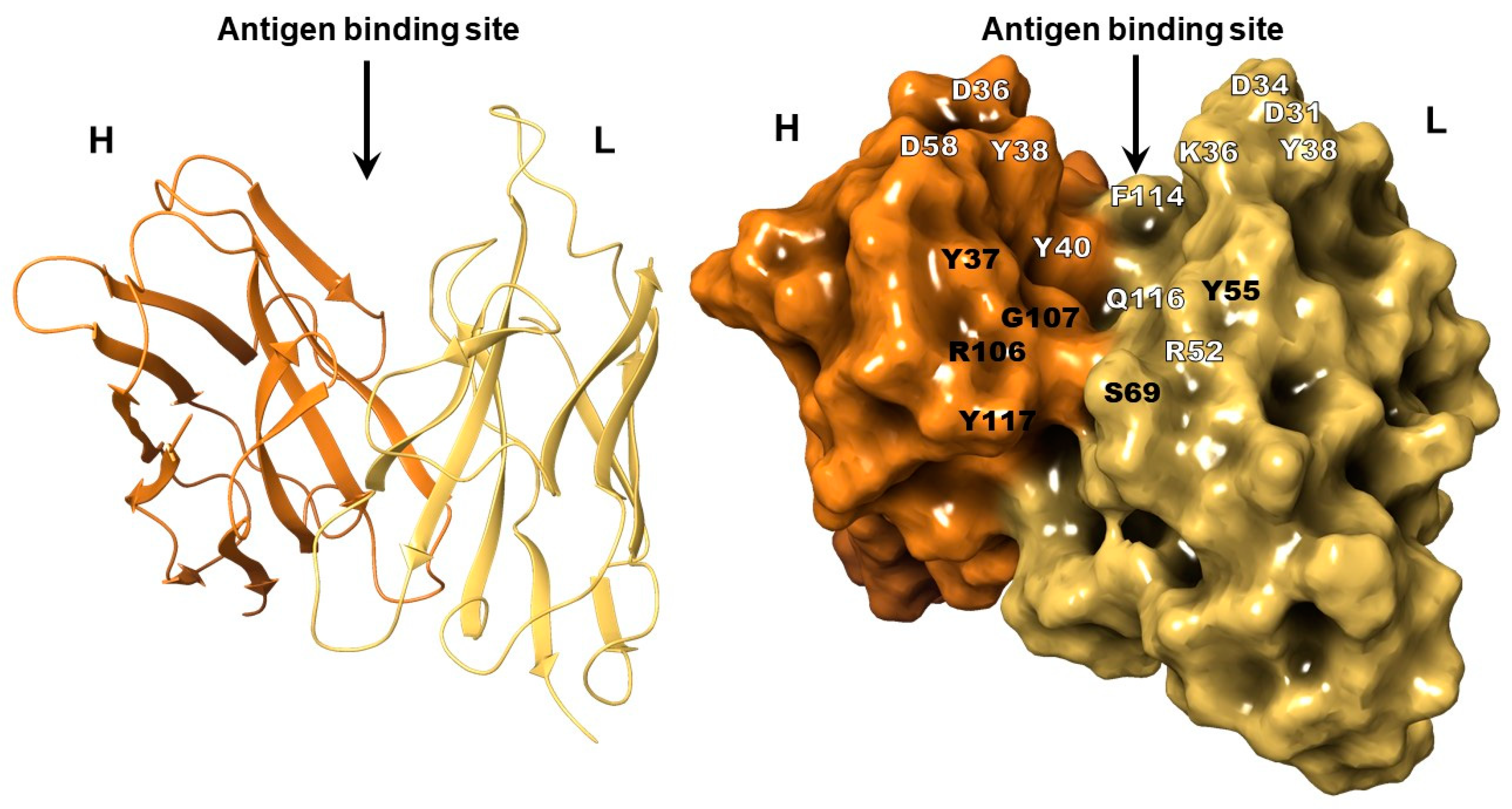
2.2. The Structure of 10H10 Antibody Variable Domains
2.3. Molecular Modeling of 10H10 Variable Domains Binding to E Protein
3. Materials and Methods
3.1. Construction of Recombinant Plasmids
3.2. E. coli Transformation and Recombinant Proteins Production
3.3. Dot-Blot
3.4. ELISA
3.5. Alignment of Amino Acid Sequences
3.6. Sequencing of the VH and VL Sequence of the 10H10 Antibody
3.7. Purification of the 10H10 Monoclonal Antibody Preparation
3.8. Structure of 10H10
3.9. Protein Docking the Structure of Antigens and Antibodies
3.9.1. Protein Preparation
3.9.2. Molecular Docking Procedure
3.9.3. Molecular Dynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sigfrid, L.; Reusken, C.; Eckerle, I.; Nussenblatt, V.; Lipworth, S.; Messina, J.; Kraemer, M.; Ergonul, O.; Papa, A.; Koopmans, M.; et al. Preparing clinicians for (re-)emerging arbovirus infectious diseases in Europe. J. Clin. Med. 2018, 24, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinal, M.A.; Andrus, J.K.; Jauregui, B.; Waterman, S.H.; Morens, D.M.; Santos, J.I.; Horstick, O.; Francis, L.A.; Olson, D. Emerging and (re-)emerging aedes-transmitted arbovirus infections in the region of the americas: Implications for health policy. AJPH 2019, 109, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Twiddy, S.S.; Holmes, E.C. The extent of homologous recombination in members of the genus Flavivirus. J. Gen. Virol. 2003, 842, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Sevvana, M.; Kuhn, R.J. Mapping the diverse structural landscape of the flavivirus antibody repertoire. Curr. Opin. Virol. 2020, 45, 51–64. [Google Scholar] [CrossRef]
- Neufeldt, C.J.; Cortese, M.; Acosta, E.G.; Bartenschlager, R. Rewiring cellular networks by members of the Flaviviridae family. Nat. Rev. Microbiol. 2018, 16, 125–142. [Google Scholar] [CrossRef]
- Begum, F.; Das, S.; Mukherjee, D.; Mal, S.; Ray, U. Insight into the Tropism of Dengue Virus in Humans. Viruses 2019, 11, 1136. [Google Scholar] [CrossRef] [Green Version]
- Kyle, J.L.; Harris, E. Global spread and persistence of dengue. Annu. Rev. Microbiol. 2008, 62, 71–92. [Google Scholar] [CrossRef] [Green Version]
- Basarab, M.; Bowman, C.; Aarons, E.J.; Cropley, I. Zika virus. BMJ 2016, 352, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Aubry, F.; Jacobs, S.; Darmuzey, M.; Lequime, S.; Delang, L.; Fontaine, A.; Jupatanakul, N.; Miot, E.F.; Dabo, S.; Manet, C.; et al. Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asian strains. Nat. Commun. 2021, 12, 916. [Google Scholar] [CrossRef]
- Fox, J.P.; Da Cunha, J.F.; Kossobtjdzki, S.L. Additional observations on the duration of humoral immunity following vaccination with the 17d strain of yellow fever virus. Am. J. Epidemiol. 1948, 47, 64–70. [Google Scholar] [CrossRef]
- Durbin, A.; Wilder-Smith, A. An update on Zika vaccine developments. Expert Rev. Vaccines 2017, 16, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Yellow fever vaccine. Expert Rev. Vaccines 2005, 4, 553–574. [Google Scholar] [CrossRef]
- Baykov, I.K.; Matveev, A.L.; Stronin, O.V.; Ryzhikov, A.B.; Matveev, L.E.; Kasakin, M.F.; Richter, V.A.; Tikunova, N.V. A protective chimeric antibody to tick-borne encephalitis virus. Vaccine 2014, 32, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, R.; Gao, F.; Li, M.; Liu, J.; Wang, J.; Hong, W.; Zhao, L.; Wen, Y.; Yin, C.; et al. Delineating antibody recognition against Zika virus during natural infection. JCI Insight 2017, 2, 93042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, F.A.; Stiasny, K.; Vaney, M.; Dellarole, M.; Heinz, F.X. The bright and the dark side of human antibody responses to flaviviruses: Lessons for vaccine design. EMBO Rep. 2018, 19, 206–224. [Google Scholar] [CrossRef]
- Watterson, D.; Modhiran, N.; Young, P.R. The many faces of the flavivirus NS1 protein offer a multitude of options for inhibitor design. Antivir. Res. 2016, 130, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Slon Campos, J.L.; Mongkolsapaya, J.; Screaton, G.R. The immune response against flaviviruses. Nat.Immunol. 2018, 19, 1189–1198. [Google Scholar] [CrossRef]
- Kotaki, T.; Kurosu, T.; Grinyo-Escuer, A.; Davidson, E.; Churrotin, S.; Okabayashi, T.; Puiprom, O.; Mulyatno, K.C.; Teguh Hari Sucipto, T.H.; Doranz, B.J.; et al. An affinity-matured human monoclonal antibody targeting fusion loop epitope of dengue virus with in vivo therapeutic potency. Sci. Rep. 2021, 11, 12987. [Google Scholar] [CrossRef]
- VanBlargan, L.A.; Errico, J.M.; Kafai, N.M.; Burgomaster, K.E.; Jethva, P.N.; Broeckel, R.M.; Meade-White, K.; Nelson, C.A.; Himansu, S.; Wang, D.; et al. Broadly neutralizing monoclonal antibodies protect against multiple tick-borne flaviviruses. J. Exp. Med. 2021, 218, e20210174. [Google Scholar] [CrossRef]
- Dai, L.; Song, J.; Lu, X.; Deng, Y.Q.; Musyoki, A.M.; Cheng, H.; Zhang, Y.; Yuan, Y.; Song, H.; Haywood, J.; et al. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 2016, 19, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M.V.; Kaufmann, B.; Nybakken, G.E.; Lok, S.M.; Warren, J.T.; Chen, B.R.; Nelson, C.A.; Kostyuchenko, V.A.; Holdaway, H.A.; Chipman, P.R.; et al. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J. 2009, 28, 3269–3276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinski, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murin, C.D.; Bruhn, J.F.; Bornholdt, Z.A.; Copps, J.; Stanfield, R.; Ward, A.B. Structural basis of pan-ebola virus neutralization by an antibody targeting the glycoprotein fusion loop. Cell Rep. 2018, 24, 2723–2732.e4. [Google Scholar] [CrossRef] [Green Version]
- Wec, A.Z.; Herbert, A.S.; Murin, C.D.; Nyakatura, E.K.; Abelson, D.M.; Fels, M.J.; He, S.; James, R.M.; deVega, M.-A.; Zhu, W.; et al. Antibodies from a Human Survivor Define Sites of Vulnerability for Broad Protection against Ebolaviruses. Cell 2017, 169, 878–890.e15. [Google Scholar] [CrossRef]
- Van Gils, M.J.; van den Kerkhof, T.L.; Ozorowski, G.; Cottrell, C.A.; Sok, D.; Pauthner, M.; Pallesen, J.; de Val, N.; Yasmeen, A.; de Taeye, S.W.; et al. An HIV-1 antibody from an elite neutralizer implicates the fusion peptide as a site of vulnerability. Nat. Microbiol. 2016, 2, 423–426. [Google Scholar] [CrossRef]
- Protopopova, Y.V.; Khusainova, A.D.; Konovalova, S.N.; Loktev, V.B. Preparation and study of anti-idiotypical antibodies carrying hemagglutinating paratopes of tick-borne encephalitis virus on their surface. Vopr Virusol. 1996, 41, 50–53. [Google Scholar] [PubMed]
- Bogachek, M.V.; Protopopova, E.V.; Ternovoi, V.A.; Kachko, A.V.; Ivanova, A.V.; Ivanisenko, V.A.; Loktev, V.B. Immunochemical properties of recombinant polypeptides mimicking domains I and II of West Nile virus glycoprotein E. Mol. Biol. 2005, 39, 710–718. [Google Scholar] [CrossRef]
- Wong, S.H. Cloning of Flavin Reductase into pET32a(+) Expression Vector Lacking the Thioredoxin A Tag to Study Solubility of EDTA Monooxygenase A in Overexpression Systems. J. Exp. Microbiol. Immunol. 2005, 8, 59–66. [Google Scholar]
- Dunbar, J.; Krawczyk, K.; Leem, J.; Marks, C.; Nowak, J.; Regep, C.; Georges, G.; Kelm, S.; Popovic, B.; Deane, C.M. Sabpred: A structure-based antibody prediction server. Nucleic Acids Res. 2016, 44, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Bhachoo, J.; Beuming, T. Investigating protein–peptide interactions using the schrödinger computational suite. Methods Mol. Biol. 2017, 1561, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A. PDBsum: Summaries and analyses of PDB structures. Nucleic Acids Res. 2001, 29, 221–222. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Raifu, M.; Howard, M.; Smith, L.; Hansen, D.; Goldsby, R.; Ratner, D. Universal PCR amplification of mouse immunoglobulin gene variable regions: The design of degenerate primers and an assessment of the effect of DNA polymerase 3′ to 5′ exonuclease activity. J. Immunol. Methods 2000, 233, 167–177. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanshin, D.V.; Borisevich, S.S.; Bondar, A.A.; Porozov, Y.B.; Rukhlova, E.A.; Protopopova, E.V.; Ushkalenko, N.D.; Loktev, V.B.; Chapoval, A.I.; Ilyichev, A.A.; et al. Can Modern Molecular Modeling Methods Help Find the Area of Potential Vulnerability of Flaviviruses? Int. J. Mol. Sci. 2022, 23, 7721. https://doi.org/10.3390/ijms23147721
Shanshin DV, Borisevich SS, Bondar AA, Porozov YB, Rukhlova EA, Protopopova EV, Ushkalenko ND, Loktev VB, Chapoval AI, Ilyichev AA, et al. Can Modern Molecular Modeling Methods Help Find the Area of Potential Vulnerability of Flaviviruses? International Journal of Molecular Sciences. 2022; 23(14):7721. https://doi.org/10.3390/ijms23147721
Chicago/Turabian StyleShanshin, Daniil V., Sophia S. Borisevich, Alexander A. Bondar, Yuri B. Porozov, Elena A. Rukhlova, Elena V. Protopopova, Nikita D. Ushkalenko, Valery B. Loktev, Andrei I. Chapoval, Alexander A. Ilyichev, and et al. 2022. "Can Modern Molecular Modeling Methods Help Find the Area of Potential Vulnerability of Flaviviruses?" International Journal of Molecular Sciences 23, no. 14: 7721. https://doi.org/10.3390/ijms23147721
APA StyleShanshin, D. V., Borisevich, S. S., Bondar, A. A., Porozov, Y. B., Rukhlova, E. A., Protopopova, E. V., Ushkalenko, N. D., Loktev, V. B., Chapoval, A. I., Ilyichev, A. A., & Shcherbakov, D. N. (2022). Can Modern Molecular Modeling Methods Help Find the Area of Potential Vulnerability of Flaviviruses? International Journal of Molecular Sciences, 23(14), 7721. https://doi.org/10.3390/ijms23147721








