Celiac Disease and Targeting the Molecular Mechanisms of Autoimmunity in COVID Pandemic
Abstract
:1. Introduction
2. Celiac Disease in Children—General Aspects
3. Celiac Disease in Children during COVID Pandemic
4. Molecular Mechanisms of SARS-CoV-2 Infection and How CD Led to an Adjuvant Drug for MIS-C
5. Discussions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Angiotensin-converting enzyme 2 | ACE-2 |
| α-amylase/trypsin | ATI |
| Anti-endomysium antibodies IgA | anti-EMA-IgA |
| Antigen-presenting cell | APC |
| Anti-tissue transglutaminase IgA antibodies | anti-tTG IgA |
| Aspartate transaminase/Alanine transaminase | AST/ALT |
| Autoimmune | AI |
| Before Common Era | BCE |
| Casein kinase 1, alpha 1 | CSNK1A1 |
| Casein kinase 2, alpha 1 | CSNK2A1 |
| Casein kinase 1, epsilon 1 | CSNK1E1 |
| Celiac disease | CD |
| Chemokine (C-C motif) ligand 3 | CCL3 |
| Chemokine (C-C motif) ligand 4 | CCL4 |
| Chemokine (C-C motif) ligand 20 | CCL20 |
| Chemokine (C-C motif) ligand 28 | CCL28 |
| Chemokine (C-X-C Motif) Receptor 3 | CXCR3 |
| Complement | C |
| Coronavirus Disease 2019 | COVID-19 |
| Creatine phosphokinase | CPK |
| Crypt base columnar | CBC |
| C-reactive protein | CRP |
| C-type lectins | CLEC |
| CUB domain-containing protein 1 | CDCP1 |
| Damage-associated molecular pattern | DAMP |
| Deamidated gliadin peptide | DGP |
| Deamidated gliadin peptide antibodies | DGP-AGA |
| Delta-like canonical Notch ligand 1 | DLL1 |
| Delta-like canonical Notch ligand 4 | DLL4 |
| Dendritic cell | DC |
| Epidermal growth factor | EGF |
| Epidermal growth factor receptor | EGFR |
| Erythrocyte sedimentation rate | ESR |
| European Society for Paediatric Gastroenterology Hepatology and Nutrition | ESPGHAN |
| Food and Drug Administration | FDA |
| Forkhead box P3—also known as Scurfin | FOXP3 |
| Gastrointestinal | GI |
| Gluten-free | GF |
| Gluten-free diet | GFD |
| Pre-haptoglobin | HP |
| High-mobility group protein 1 | HMGB1 |
| Human leukocyte antigen | HLA |
| IgA tissue transglutaminase | tTGA |
| IgA anti-endomysium antibodies | EMA-IgA |
| IgA anti-intestinal transglutaminase 2 antibodies | TGA-IgA |
| IgG against deamidate gliadin peptide | DGP-IgG |
| Immunoglobulin type A | IgA |
| Immunoglobulin type G | IgG |
| Immunoglobulin type M | IgM |
| Inflammatory bowel disease | IBD |
| Institutional Review Board | IRB |
| Interferon-γ | IFN-γ |
| Interleukin | IL- |
| Intraepithelial CD8+ lymphocytes | IEL |
| Intravenous immune globulins | IVIG |
| Junctional adhesion molecules | JAM |
| Kawasaki disease | KD |
| Lactate dehydrogenase | LDH or LD |
| Matrix metalloproteinase-1 | MMP-1 |
| Membrane-associated guanylate kinase homologs | MAGUK |
| Dual specificity mitogen-activated protein kinase kinase 2 | MAP2K2 |
| Microbial associated molecular pattern | MAMPs |
| Middle East Respiratory Syndrome | MERS |
| Monocyte | Mo |
| Monocyte chemoattractant protein-1 | MCP-1 |
| Multisystem inflammatory syndrome in children | MIS-C |
| Myeloid differentiation primary response 88 | MYD88 |
| Myosin 1C | MYO1C |
| Natural killer | NK |
| Neutrophil extracellular traps | NETs |
| NET activation and release | NETosis |
| (NOD)-like receptor | NLR |
| N-terminal prohormone of brain natriuretic peptide | NT-proBNP |
| Pathogen-associated molecular pattern | PAMP |
| Pattern recognition receptors | PRRs |
| Pediatric inflammatory multisystem syndrome | PIMS |
| Phospholipase C | PLC |
| Polymorphonuclear leukocyte | PMN |
| Protease-activated receptor 2 | PAR2 |
| Protein kinase C alpha | PKCα |
| Protein Wnt-3a | WNT3a |
| Quality of life | QOL |
| Reactive oxygen species | ROS |
| Real-time polymerase chain reaction | Real-time PCR or RT-PCR |
| Receptor Binding Domain | RBD |
| Regulatory T lymphocytes | Tregs |
| Ribonucleic acid | RNA |
| Secretory IgA | SIgA |
| Severe Acute Respiratory Syndrome | SARS |
| Severe acute respiratory syndrome coronavirus 2 | SARS-CoV-2 |
| Soluble C5b-9 | sC5b-9 |
| Systemic inflammatory syndrome in COVID19 | SISCoV |
| Systemic juvenile idiopathic arthritis | sJIA |
| Temporally associated with SARS-CoV-2 infection | PIMS-TS |
| T-helper 1 or T helper type 1 | Th1 |
| T-helper 2 or T helper type 2 | Th2 |
| T-helper 17 or T helper type 17 | Th17 |
| Tight junctions | TJs |
| Tissue transglutaminase or tissue transglutaminase autoantigen | tTG/tTG2 |
| Toll-like receptor 2 | TLR2 |
| Toll-like receptor 4 | TLR 4 |
| Transforming growth factor alpha | TGF-α |
| Transglutaminase 2 | TG2 |
| Transmembrane serine protease 2 | TMPRSS2 |
| Tumor necrosis factor alpha | TNF-α |
| Type-1 diabetes mellitus | T1DM |
| Upper limit of normal | ULN |
| Wnt receptor frizzled 5 | FZD5 |
| World Health Organization | WHO |
| Wnt Family Member 3 | WNT3a |
| Zonula occludens | ZO |
| Zonula occludens-1 | ZO-1 |
| Zonula occludens toxin | Zot |
| ↑ | Increased |
| ↓ | Decreased |
References
- Mallah, S.I.; Ghorab, O.K.; Al-Salmi, S.; Abdellatif, O.S.; Tharmaratnam, T.; Iskandar, M.A.; Sefen, J.; Sidhu, P.; Atallah, B.; El-Lababidi, R.; et al. COVID-19: Breaking down a global health crisis. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Stefanolo, J.P.; Temprano, M.P.; Tedesco, S.; Seiler, C.; Caminero, A.F.; de-Madaria, E.; Huguet, M.M.; Vivas, S.; Niveloni, S.I.; et al. The Risk of Contracting COVID-19 Is Not Increased in Patients With Celiac Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 391–393. [Google Scholar] [CrossRef] [PubMed]
- WHO. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 12 May 2022).
- Domingo, J.L. What we know and what we need to know about the origin of SARS-CoV-2. Environ. Res. 2021, 200, 111785. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 12 May 2022).
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O.=. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [Green Version]
- Proctor, L. The NIH Human Microbiome Project: Catalyst for an Emerging Field in Biomedical Research. 2018. Available online: https://www.genome.gov/Pages/About/NACHGR/February2018AgendaDocuments/HMP_talk_Feb_Council_final_020618.pdf (accessed on 23 June 2022).
- The Editors of Encyclopaedia Britannica. Small Intestine. Encyclopedia Britannica. Available online: https://www.britannica.com/science/small-intestine (accessed on 23 June 2022).
- Children’s Hospital of Pittsburgh. Difference between Small and Large Intestine. Available online: https://www.chp.edu/our-services/transplant/intestine/education/about-small-large-intestines (accessed on 23 June 2022).
- Zuo, L.; Kuo, W.T.; Turner, J.R. Tight Junctions as Targets and Effectors of Mucosal Immune Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 327–340. [Google Scholar] [CrossRef]
- Sahin, Y. Celiac disease in children: A review of the literature. World J. Clin. Pediatr. 2021, 10, 53–71. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European society Paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [Green Version]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Prim. 2019, 5, 3. [Google Scholar] [CrossRef]
- Laurikka, P.; Nurminen, S.; Kivelä, L.; Kurppa, K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients 2018, 10, 1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebwohl, B.; Murray, J.A.; Verdú, E.F.; Crowe, S.E.; Dennis, M.; Fasano, A.; Green, P.H.; Guandalini, S.; Khosla, C. Gluten Introduction, Breastfeeding, and Celiac Disease: Back to the Drawing Board. Am. J. Gastroenterol. 2016, 111, 12–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.L.; Zhang, B.L.; Liu, Y.S. Is adult celiac disease really uncommon in Chinese? J. Zhejiang Univ. Sci. B 2009, 10, 168–171. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; Liu, W.; Xu, C.D.; Mei, H.; Gao, Y.; Peng, H.M.; Yuan, L.; Xu, J.J. Celiac disease in children with diarrhea in 4 cities in China. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kong, W.J.; Feng, Y.; Lu, J.J.; Hui, W.J.; Liu, W.D.; Li, Z.Q.; Shi, T.; Cui, M.; Sun, Z.Z.; et al. Epidemiological, clinical, and histological presentation of celiac disease in Northwest China. World J. Gastroenterol. 2022, 28, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Y.; Liu, X.Y.; Wang, M.M.; Liang, L.P.; Liu, L.; Zheng, K.; Silvester, J.A.; Ma, W.J.; Wu, W.; Ji, G.Y.; et al. Prevalence of celiac disease in China: Meta-analysis and serological survey in high-risk populations. J. Dig. Dis. 2021, 22, 645–655. [Google Scholar] [CrossRef]
- Tarar, Z.I.; Zafar, M.U.; Farooq, U.; Basar, O.; Tahan, V.; Daglilar, E. The Progression of Celiac Disease, Diagnostic Modalities, and Treatment Options. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211053702. [Google Scholar] [CrossRef]
- Abenavoli, L.; Dastoli, S.; Bennardo, L.; Boccuto, L.; Passante, M.; Silvestri, M.; Proietti, I.; Potenza, C.; Luzza, F.; Nisticò, S.P. The Skin in Celiac Disease Patients: The Other Side of the Coin. Medicina 2019, 55, 578. [Google Scholar] [CrossRef] [Green Version]
- Hadithi, M.; von Blomberg, B.M.; Crusius, J.B.; Bloemena, E.; Kostense, P.J.; Meijer, J.W.; Mulder, C.J.; Stehouwer, C.D.; Peña, A.S. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann. Intern. Med. 2007, 147, 294–302. [Google Scholar] [CrossRef]
- Hoffmanová, I.; Sánchez, D.; Szczepanková, A.; Tlaskalová-Hogenová, H. The Pros and Cons of Using Oat in a Gluten-Free Diet for Celiac Patients. Nutrients 2019, 11, 2345. [Google Scholar] [CrossRef] [Green Version]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maglio, M.; Troncone, R. Intestinal Anti-tissue Transglutaminase2 Autoantibodies: Pathogenic and Clinical Implications for Celiac Disease. Front. Nutr. 2020, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Castellaneta, S.; Francavilla, R.; Pulvirenti, A.; Catassi, C. SIGENP Working Group of Weaning and CD Risk. Mode of Delivery and Risk of Celiac Disease: Risk of Celiac Disease and Age at Gluten Introduction Cohort Study. J. Pediatr. 2017, 184, 81–86.e2. [Google Scholar] [CrossRef]
- Koletzko, S.; Lee, H.S.; Beyerlein, A.; Aronsson, C.A.; Hummel, M.; Liu, E.; Simell, V.; Kurppa, K.; Lernmark, Å.; Hagopian, W.; et al. TEDDY Study Group. Cesarean Section on the Risk of Celiac Disease in the Offspring: The Teddy Study. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, G.; Navone, R.; Lunardi, C.; Tridente, G.; Bason, C.; Sivori, S.; Beri, R.; Dolcino, M.; Valletta, E.; Corrocher, R.; et al. In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med. 2006, 3, e358. [Google Scholar] [CrossRef]
- Silvester, J.A.; Leffler, D.A. Is Autoimmunity Infectious? The Effect of Gastrointestinal Viral Infections and Vaccination on Risk of Celiac Disease Autoimmunity. Clin. Gastroenterol. Hepatol. 2017, 15, 703–705. [Google Scholar] [CrossRef] [Green Version]
- Hemming-Harlo, M.; Lähdeaho, M.L.; Mäki, M.; Vesikari, T. Rotavirus vaccination does not increase type 1 diabetes and may decrease celiac disease in children and adolescents. Pediatr. Infect. Dis. J. 2019, 38, 539–541. [Google Scholar] [CrossRef]
- Bouziat, R.; Hinterleitner, R.; Brown, J.J.; Stencel-Baerenwald, J.E.; Ikizler, M.; Mayassi, T.; Meisel, M.; Kim, S.M.; Discepolo, V.; Pruijssers, A.J.; et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017, 356, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Thomas, K.E.; Sapone, A.; Fasano, A.; Vogel, S.N. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: Role of the innate immune response in Celiac disease. J. Immunol. 2006, 176, 2512–2521. [Google Scholar] [CrossRef] [Green Version]
- Neil, J.A.; Cadwell, K. The Intestinal Virome and Immunity. J. Immunol. 2018, 201, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, D.; Hoffmanová, I.; Szczepanková, A.; Hábová, V.; Tlaskalová-Hogenová, H. Contribution of Infectious Agents to the Development of Celiac Disease. Microorganisms 2021, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Scott-Sheldon, L.A.J.; Risech-Neyman, Y.; Moss, S.F.; Ludvigsson, J.F.; Green, P.H.R. Celiac disease and increased risk of pneumococcal infection: A systematic review and meta-analysis. Am. J. Med. 2018, 131, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Canova, C.; Ludvigsson, J.; Baldo, V.; Barbiellini Amidei, C.; Zanier, L.; Zingone, F. Risk of bacterial pneumonia and pneumococcal infection in youths with celiac disease-A population-based study. Dig. Liver Dis. 2019, 51, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Mårild, K.; Kahrs, C.R.; Tapia, G.; Stene, L.C.; Størdal, K. Infections and risk of celiac disease in childhood: A prospective nationwide cohort study. Am. J. Gastroenterol. 2015, 110, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Van De Kamer, J.H.; Weijers, H.A.; Dicke, W.K. Coeliac disease. IV. An investigation into the injurious constituents of wheat in connection with their action on patients with coeliac disease. Acta Paediatr. 1953, 42, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, S.; De Ritis, G.; De Vincenzi, M.; Silano, V. Toxicity mechanisms of wheat and other cereals in celiac disease and related enteropathies. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 923–930. [Google Scholar] [CrossRef] [PubMed]
- De Re, V.; Caggiari, L.; Tabuso, M.; Cannizzaro, R. The versatile role of gliadin peptides in celiac disease. Clin. Biochem. 2013, 46, 552–560. [Google Scholar] [CrossRef]
- De Re, V.; Magris, R.; Cannizzaro, R. New Insights into the Pathogenesis of Celiac Disease. Front. Med. 2017, 4, 137. [Google Scholar] [CrossRef] [Green Version]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Silano, M.; Vincentini, O.; De Vincenzi, M. Toxic, immunostimulatory and antagonist gluten peptides in celiac disease. Curr. Med. Chem. 2009, 16, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Valenti, S.; Corica, D.; Ricciardi, L.; Romano, C. Gluten-related disorders: Certainties, questions and doubts. Ann. Med. 2017, 11, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Cebolla, Á.; Moreno, M.L.; Coto, L.; Sousa, C. Gluten Immunogenic Peptides as Standard for the Evaluation of Potential Harmful Prolamin Content in Food and Human Specimen. Nutrients 2018, 10, 1927. [Google Scholar] [CrossRef] [Green Version]
- Clemente, M.G.; De Virgiliis, S.; Kang, J.S.; Macatagney, R.; Musu, M.P.; Di Pierro, M.R.; Drago, S.; Congia, M.; Fasano, A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 2003, 52, 218–223. [Google Scholar] [CrossRef]
- Moreno, M.L.; Cebolla, Á.; Muñoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, Á.; León, F.; Rodríguez-Herrera, A.; Sousa, C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Palanski, B.A.; Weng, N.; Zhang, L.; Hilmer, A.J.; Fall, L.A.; Swaminathan, K.; Jabri, B.; Sousa, C.; Fernandez-Becker, N.Q.; Khosla, C.; et al. An efficient urine peptidomics workflow identifies chemically defined dietary gluten peptides from patients with celiac disease. Nat. Commun. 2022, 13, 888. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.R.; Byrne, G.; Chirdo, F.G.; Feighery, C. Coeliac Disease Pathogenesis: The Uncertainties of a Well-Known Immune Mediated Disorder. Front. Immunol. 2020, 11, 1374. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Russel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017, 152, 1100–1113.e12. [Google Scholar] [CrossRef] [Green Version]
- Schumann, M.; Siegmund, B.; Schulzke, J.D.; Fromm, M. Celiac disease: Role of the epithelial barrier. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.; Robert, M.E. Frontiers in Celiac Disease: Where Autoimmunity and Environment Meet. Am. J. Surg. Pathol. 2022, 46, e43–e54. [Google Scholar] [CrossRef]
- Kupfer, S.S.; Jabri, B. Pathophysiology of celiac disease. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 639–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parzanese, I.; Qehajaj, D.; Patrinicola, F.; Aralica, M.; Chiriva-Internati, M.; Stifter, S.; Elli, L.; Grizzi, F. Celiac disease: From pathophysiology to treatment. World J. Gastrointest. Pathophysiol. 2017, 8, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Stamnaes, J.; Sollid, L.M. Celiac disease: Autoimmunity in response to food antigen. Semin. Immunol. 2015, 27, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Palová-Jelínková, L.; Dáňová, K.; Drašarová, H.; Dvořák, M.; Funda, D.P.; Fundová, P.; Fundová, P.; Kotrbová-Kozak, A.; Černá, M.; Kamanová, J.; et al. Pepsin Digest of Wheat Gliadin Fraction Increases Production of IL-1β via TLR4/MyD88/TRIF/MAPK/NF-κB Signaling Pathway and an NLRP3 Inflammasome Activation. PLoS ONE 2013, 8, e62426. [Google Scholar] [CrossRef] [Green Version]
- Araya, R.E.; Gomez Castro, M.F.; Carasi, P.; McCarville, J.L.; Jury, J.; Mowat, A.M.; Verdu, E.F.; Chirdo, F.G. Mechanisms of innate immune activation by gluten peptide p31-43 in mice. Am. J. Physiol. Liver Physiol. 2016, 311, G40–G49. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556. [Google Scholar] [CrossRef]
- Perez, F.; Ruera, C.N.; Miculan, E.; Carasi, P.; Chirdo, F.G. Programmed Cell Death in the Small Intestine: Implications for the Pathogenesis of Celiac Disease. Int. J. Mol. Sci. 2021, 22, 7426. [Google Scholar] [CrossRef]
- Kuja-Halkola, R.; Lebwohl, B.; Halfvarson, J.; Wijmenga, C.; Magnusson, P.K.; Ludvigsson, J.F. Heritability of non-HLA genetics in coeliac disease: A population-based study in 107000 twins. Gut 2016, 65, 1793–1798. [Google Scholar] [CrossRef] [Green Version]
- Dubois, P.C.; Trynka, G.; Franke, L.; Hunt, K.A.; Romanos, J.; Curtotti, A.; Zhernakova, A.; Heap, G.A.; Adány, R.; Aromaa, A.; et al. Multiple common variants for celiac disease influencing immune gene expression. Nat. Genet. 2010, 42, 295–302. [Google Scholar] [CrossRef]
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-Free Diet in Celiac Disease-Forever and for All? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crehuá-Gaudiza, E.; Barrés Fernández, A.; Jovaní Casano, C.; Latorre Tejerina, M.; Largo Blanco, E.M.; Moreno Ruiz, M.A.; Berghezan Suárez, A.; García-Peris, M.; Gil Piquer, R.; Coret Sinisterra, A.; et al. Diagnóstico de enfermedad celiaca en la práctica clínica: Presente y futuro. Diagnosis of celiac disease in clinical practice: Present and future. An. Pediatría 2021, 94, 223–229. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Parisi, P.; Pietropaoli, N.; Ferretti, A.; Nenna, R.; Mastrogiorgio, G.; Del Pozzo, M.; Principessa, L.; Bonamico, M.; Villa, M.P. Role of the gluten-free diet on neurological-EEG findings and sleep disordered breathing in children with celiac disease. Seizure 2015, 25, 181–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Houmich, T.; Admou, B. Celiac disease: Understandings in diagnostic, nutritional, and medicinal aspects. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211008709. [Google Scholar] [CrossRef]
- Sabino, L.; Marinot, S.; Falsaperla, R.; Pisani, F.; Massimino, C.; Pavone, P. Celiac disease and headache in children: A narrative state of the art. Acta Biomed. 2020, 91, e2020056. [Google Scholar] [CrossRef]
- Bao, F.; Green, P.H.; Bhagat, G. An update on celiac disease histopathology and the road ahead. Arch. Pathol. Lab. Med. 2012, 136, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Granito, A.; Parisi, C.; Fabbri, A.; Fiorini, E.; Piscaglia, M.; Tovoli, F.; Grasso, V.; Muratori, P.; Pappas, G.; et al. Deamidated gliadin peptide antibodies as a routine test for celiac disease: A prospective analysis. J. Clin. Gastroenterol. 2010, 44, 186–190. [Google Scholar] [CrossRef]
- Maheshwari, A.; He, Z.; Weidner, M.N.; Lin, P.; Bober, R.; Del Rosario, F.J. Influence of Age and Type 1 Diabetes Mellitus on Serological Test for Celiac Disease in Children. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 218–229. [Google Scholar] [CrossRef]
- Kulkarni, A.; Patel, S.; Khanna, D.; Parmar, M.S. Current pharmacological approaches and potential future therapies for Celiac disease. Eur. J. Pharmacol. 2021, 909, 174434. [Google Scholar] [CrossRef]
- Pais, W.P.; Duerksen, D.R.; Pettigrew, N.M.; Bernstein, C.N. How many duodenal biopsy specimens are required to make a diagnosis of celiac disease? Gastrointest. Endosc. 2008, 67, 1082–1087. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Villanacci, V.; Zambelli, C.; Milione, M.; Luinetti, O.; Vindigni, C.; Chioda, C.; Albarello, L.; Bartolini, D.; Donato, F. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin. Gastroenterol. Hepatol. 2007, 5, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fasano, A. Celiac disease diagnosis: Simple rules are better than complicated algorithms. Am. J. Med. 2010, 123, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, A.; Granito, A.; Giamperoli, A.; Catenaro, T.; Negrini, G.; Tovoli, F. Current guidelines for the management of celiac disease: A systematic review with comparative analysis. World J. Gastroenterol. 2022, 28, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mearint, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160, Erratum in J. Pediatr. Gastroenterol. Nutr. 2012, 54, 572. [Google Scholar] [CrossRef]
- Calado, J.; Machado, M.V. Celiac Disease Revisited. GE Port. J. Gastroenterol. 2021, 29, 111–124. [Google Scholar] [CrossRef]
- Wysocka-Mincewicz, M.; Groszek, A.; Ambrozkiewicz, F.; Paziewska, A.; Dąbrowska, M.; Rybak, A.; Konopka, E.; Ochocińska, A.; Żeber-Lubecka, N.; Karczmarski, J.; et al. Combination of HLA-DQ2/-DQ8 Haplotypes and a Single MSH5 Gene Variant in a Polish Population of Patients with Type 1 Diabetes as a First Line Screening for Celiac Disease? J. Clin. Med. 2022, 11, 2223. [Google Scholar] [CrossRef]
- Valitutti, F.; Trovato, C.M.; Montuori, M.; Cucchiara, S. Pediatric celiac disease: Follow-up in the spotlight. Adv. Nutr. 2017, 8, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef]
- Fueyo-Díaz, R.; Montoro, M.; Magallón-Botaya, R.; Gascón-Santos, S.; Asensio-Martínez, Á.; Palacios-Navarro, G.; Sebastián-Domingo, J.J. Influence of Compliance to Diet and Self-Efficacy Expectation on Quality of Life in Patients with Celiac Disease in Spain. Nutrients 2020, 12, 2672. [Google Scholar] [CrossRef]
- Wagner, G.; Berger, G.; Sinnreich, U.; Grylli, V.; Schober, E.; Huber, W.D.; Karwautz, A. Quality of life in adolescents with treated coeliac disease: Influence of compliance and age at diagnosis. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Dowhaniuk, J.K.; Mileski, H.; Saab, J.; Tutelman, P.; Thabane, L.; Brill, H. The Gluten Free Diet: Assessing Adherence in a Pediatric Celiac Disease Population. J. Can. Assoc. Gastroenterol. 2020, 3, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Ruiz-Carnicer, Á.; Segura, V.; Comino, I.; Sousa, C. Challenges of Monitoring the Gluten-Free Diet Adherence in the Management and Follow-Up of Patients with Celiac Disease. Nutrients 2021, 13, 2274. [Google Scholar] [CrossRef] [PubMed]
- Stasi, E.; Marafini, I.; Caruso, R.; Soderino, F.; Angelucci, E.; Del Vecchio Blanco, G.; Paoluzi, O.A.; Calabrese, E.; Sedda, S.; Zorzi, F.; et al. Frequency and Cause of Persistent Symptoms in Celiac Disease Patients on a Long-term Gluten-free Diet. J. Clin. Gastroenterol. 2016, 50, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Truitt, K.E.; Daveson, A.J.M.; Ee, H.C.; Goel, G.; MacDougall, J.; Neff, K.; Anderson, R.P. Randomised clinical trial: A placebo-controlled study of subcutaneous or intradermal NEXVAX2, an investigational immunomodulatory peptide therapy for coeliac disease. Aliment. Pharmacol. Ther. 2019, 50, 547–555. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Kelly, D.G.; Lahr, B.D.; Dogan, A.; Wu, T.T.; Murray, J.A. Clinical staging and survival in refractory celiac disease: A single center experience. Gastroenterology 2009, 136, 99–353. [Google Scholar] [CrossRef] [Green Version]
- Galli, G.; Carabotti, M.; Pilozzi, E.; Lahner, E.; Annibale, B.; Conti, L. Relationship between Persistent Gastrointestinal Symptoms and Duodenal Histological Findings after Adequate Gluten-Free Diet: A Gray Area of Celiac Disease Management in Adult Patients. Nutrients 2021, 13, 600. [Google Scholar] [CrossRef]
- Sparks, B.; Hill, I.; Ediger, T. Going Beyond Gluten-Free: A Review of Potential Future Therapies for Celiac Disease. Curr. Treat. Options Pediatr. 2021, 7, 17–31. [Google Scholar] [CrossRef]
- Syage, J.A.; Green, P.H.R.; Khosla, C.; Adelman, D.C.; Sealey-Voyksner, J.A.; Murray, J.A. Latiglutenase Treatment for Celiac Disease: Symptom and Quality of Life Improvement for Seropositive Patients on a Gluten-Free Diet. GastroHep 2019, 1, 293–301. [Google Scholar] [CrossRef]
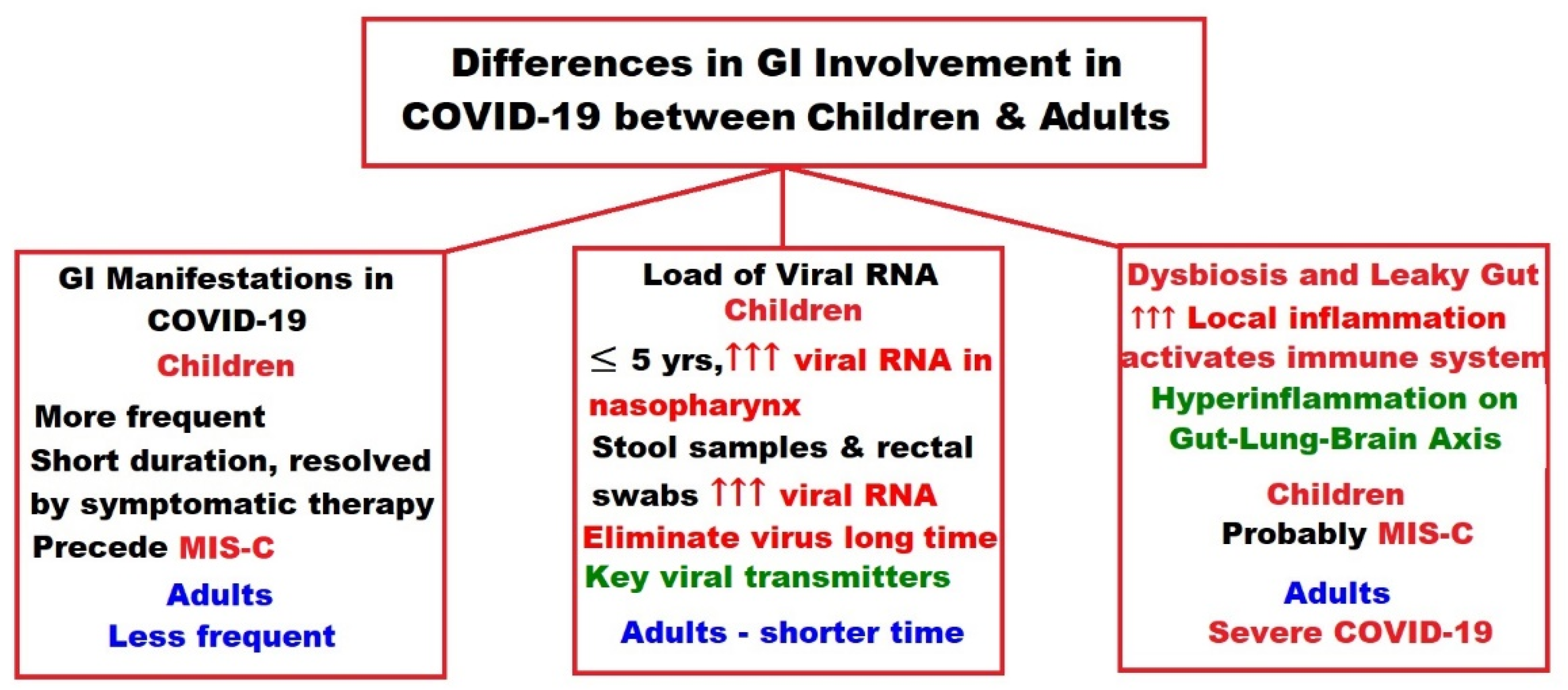
- Calitri, C.; Fumi, I.; Ignaccolo, M.G.; Banino, E.; Benetti, S.; Lupica, M.M.; Fantone, F.; Pace, M.; Garofalo, F. Gastrointestinal involvement in paediatric COVID-19-from pathogenesis to clinical management: A comprehensive review. World J. Gastroenterol. 2021, 27, 3303–3316. [Google Scholar] [CrossRef]
- Cakir, M.; Guven, B.; Issi, F.; Ozkaya, E. New-onset celiac disease in children during COVID-19 pandemic. Acta Paediatr. 2022, 111, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Trovato, C.M.; Montuori, M.; Pietropaoli, N.; Oliva, S. COVID-19 and celiac disease: A pathogenetic hypothesis for a celiac outbreak. Int. J. Clin. Pract. 2021, 75, e14452. [Google Scholar] [CrossRef] [PubMed]
- Asri, N.; Nazemalhosseini Mojarad, E.; Mirjalali, H.; Mohebbi, S.R.; Baghaei, K.; Rostami-Nejad, M.; Yadegar, A.; Rezaei-Tavirani, M.; Asadzadeh Aghdaei, H.; Rostami, K.; et al. Toward finding the difference between untreated celiac disease and COVID-19 infected patients in terms of CD4, CD25 (IL-2 Rα), FOXP3 and IL-6 expressions as genes affecting immune homeostasis. BMC Gastroenterol. 2021, 21, 462. [Google Scholar] [CrossRef] [PubMed]
- Renzo, S.; Scarallo, L.; Antoniello, L.M.; Bramuzzo, M.; Chiaro, A.; Cisarò, F.; Contini, A.C.I.; De Angelis, G.L.; Angelis, P.; Nardo, G.D.; et al. Impact of COVID-19 pandemic on pediatric endoscopy: A multicenter study on behalf of the SIGENP Endoscopy Working Group. Dig. Liver Dis. 2022, 54, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Concas, G.; Barone, M.; Francavilla, R.; Cristofori, F.; Dargenio, V.N.; Giorgio, R.; Dargenio, C.; Fanos, V.; Marcialis, M.A. Twelve Months with COVID-19: What Gastroenterologists Need to Know. Dig. Dis. Sci. 2022, 67, 2771–2791. [Google Scholar] [CrossRef] [PubMed]
- Uche-Anya, E.; Husby, S.; Kaplan, G.G.; Underwood, F.E.; Green, P.H.R.; Lebwohl, B. An International Reporting Registry of Patients With Celiac Disease and COVID-19: Initial Results From SECURE-CELIAC. Clin. Gastroenterol. Hepatol. 2021, 19, 2435–2437.e4. [Google Scholar] [CrossRef]
- Mehtab, W.; Chauhan, A.; Agarwal, A.; Singh, A.; Rajput, M.S.; Mohta, S.; Jindal, V.; Banyal, V.; Ahmed, A.; Pramanik, A.; et al. Impact of Corona Virus Disease 2019 pandemic on adherence to gluten-free diet in Indian patients with celiac disease. Indian J. Gastroenterol. 2021, 40, 613–620. [Google Scholar] [CrossRef]
- Falcomer, A.L.; Farage, P.; Pratesi, C.B.; Pratesi, R.; Gandolfi, L.; Nakano, E.Y.; Raposo, A.; Zandonadi, R.P. Health-Related Quality of Life and Experiences of Brazilian Celiac Individuals over the Course of the Sars-Cov-2 Pandemic. Nutrients 2021, 13, 1582. [Google Scholar] [CrossRef]
- Monzani, A.; Lionetti, E.; Felici, E.; Fransos, L.; Azzolina, D.; Rabbone, I.; Catassi, C. Adherence to the Gluten-Free Diet during the Lockdown for COVID-19 Pandemic: A Web-Based Survey of Italian Subjects with Celiac Disease. Nutrients 2020, 12, 3467. [Google Scholar] [CrossRef]
- Temsah, M.H.; Aljamaan, F.; Alhaboob, A.; Almosned, B.; Alsebail, R.; Temsah, R.; Senjab, A.; Alarfaj, A.; Aljudi, T.; Jamal, A.; et al. Enhancing parental knowledge of childhood and adolescence safety: An interventional educational campaign. Medicine 2022, 101, e28649. [Google Scholar] [CrossRef]
- Barschkett, M.; Koletzko, B.; Spiess, C.K. COVID-19 Associated Contact Restrictions in Germany: Marked Decline in Children’s Outpatient Visits for Infectious Diseases without Increasing Visits for Mental Health Disorders. Children 2021, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Dipasquale, V.; Passanisi, S.; Cucinotta, U.; Cascio, A.; Romano, C. Implications of SARS-COV-2 infection in the diagnosis and management of the pediatric gastrointestinal disease. Ital. J. Pediatr. 2021, 47, 71. [Google Scholar] [CrossRef] [PubMed]
- Bükülmez, A.; Baş, M.T.; Çiftci, E. Evaluation of anti-COVID-19 measures taken by the parents of children with celiac disease: A cross-sectional study. Sao Paulo Med. J. 2021, 139, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Fabbrizi, A.; Catassi, C. Prevalence of COVID-19 in Italian Children With Celiac Disease: A Cross-Sectional Study. Clin. Gastroenterol. Hepatol. 2021, 19, 1075. [Google Scholar] [CrossRef]
- Catassi, G.N.; Vallorani, M.; Cerioni, F.; Lionetti, E.; Catassi, C. A negative fallout of COVID-19 lockdown in Italy: Life-threatening delay in the diagnosis of celiac disease. Dig. Liver. Dis. 2020, 52, 1092–1093. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Overcoming COVID-19 Investigators. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef]
- Fremed, M.A.; Farooqi, K.M. Longitudinal Outcomes and Monitoring of Patients With Multisystem Inflammatory Syndrome in Children. Front. Pediatr. 2022, 10, 820229. [Google Scholar] [CrossRef]
- Belhadjer, Z.; Méot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Cheung, E.W.; Zachariah, P.; Gorelik, M.; Boneparth, A.; Kernie, S.G.; Orange, J.S.; Milner, J.D. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA 2020, 324, 294–296. [Google Scholar] [CrossRef]
- Nakra, N.A.; Blumberg, D.A.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Cattalini, M.; Della Paolera, S.; Zunica, F.; Bracaglia, C.; Giangreco, M.; Verdoni, L.; Meini, A.; Sottile, R.; Caorsi, R.; Zuccotti, G.; et al. Defining Kawasaki disease and pediatric inflammatory multisystem syndrome-temporally associated to SARS-CoV-2 infection during SARS-CoV-2 epidemic in Italy: Results from a national, multicenter survey. Pediatr. Rheumatol. 2021, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020, 183, 968–981.e7. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, J.; Thurner, B.; Kessel, C.; Fadle, N.; Kheiroddin, P.; Regitz, E.; Hoffmann, M.C.; Kos, I.A.; Preuss, K.D.; Fischer, Y.; et al. Autoantibodies against interleukin-1 receptor antagonist in multisystem inflammatory syndrome in children: A multicentre, retrospective, cohort study. Lancet Rheumatol. 2022, 4, e329–e337. [Google Scholar] [CrossRef]
- Dhaliwal, M.; Tyagi, R.; Malhotra, P.; Barman, P.; Loganathan, S.K.; Sharma, J.; Sharma, K.; Mondal, S.; Rawat, A.; Singh, S. Mechanisms of Immune Dysregulation in COVID-19 Are Different From SARS and MERS: A Perspective in Context of Kawasaki Disease and MIS-C. Front. Pediatr. 2022, 10, 790273. [Google Scholar] [CrossRef]
- Gruber, C.; Patel, R.; Trachman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef]
- Diorio, C.; Henrickson, S.E.; Vella, L.A.; McNerney, K.O.; Chase, J.; Burudpakdee, C.; Lee, J.H.; Jasen, C.; Balamuth, F.; Barrett, D.M.; et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J. Clin. Investig. 2020, 130, 5967–5975. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Wang, C.; Zohar, T.; Fischinger, S.; Atyeo, C.; Burke, J.S.; Kang, J.; Edlow, A.G.; Fasano, A.; Baden, L.R.; et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 2021, 27, 454–462. [Google Scholar] [CrossRef]
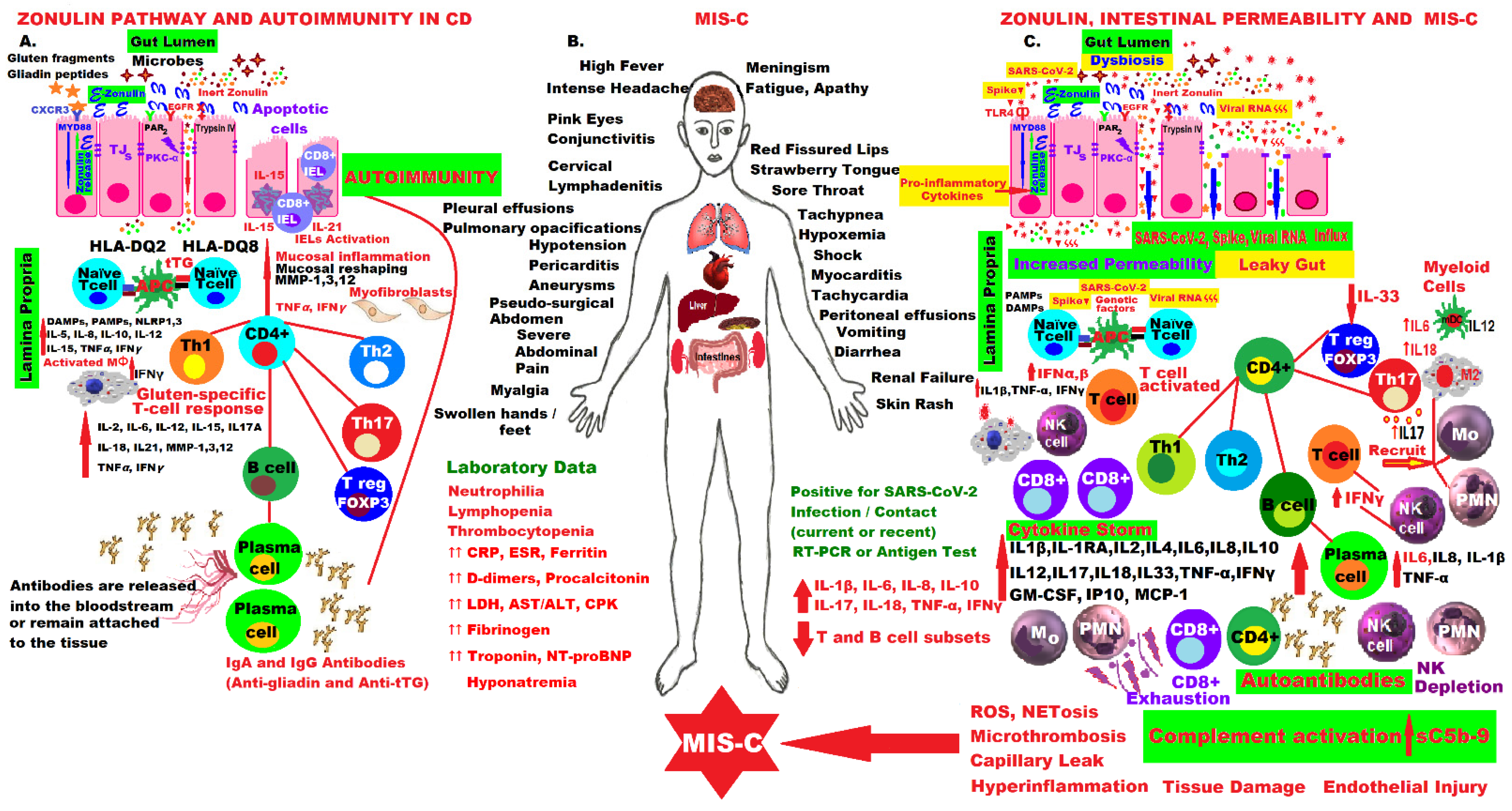
- Yonker, L.M.; Gilboa, T.; Ogata, A.F.; Senussi, Y.; Lazarovits, R.; Boribong, B.P.; Bartsch, Y.C.; Loiselle, M.; Rivas, M.N.; Porritt, R.A.; et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J. Clin. Investig. 2021, 131, e149633. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Ashida, T.; Kohgo, Y.; Kono, T. The role of Paneth cells and their antimicrobial peptides in innate host defense. Trends Microbiol. 2004, 12, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Heyman, M.; Abed, J.; Lebreton, C.; Cerf-Bensussan, N. Intestinal permeability in coeliac disease: Insight into mechanisms and relevance to pathogenesis. Gut 2012, 61, 1355–1364. [Google Scholar] [CrossRef]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Günzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [Green Version]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Wood Heickman, L.K.; DeBoer, M.D.; Fasano, A. Zonulin as a potential putative biomarker of risk for shared type 1 diabetes and celiac disease autoimmunity. Diabetes/Metab. Res. Rev. 2020, 36, e3309. [Google Scholar] [CrossRef]
- Jian, C.; Kanerva, S.; Qadri, S.; Yki-Järvinen, H.; Salonen, A. In vitro Effects of Bacterial Exposure on Secretion of Zonulin Family Peptides and Their Detection in Human Tissue Samples. Front. Microbiol. 2022, 13, 848128. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Campbell, M. Tight junction modulation at the blood-brain barrier: Current and future perspectives. Biochim. Biophys. Acta (BBA)–Biomembr. 2020, 1862, 183298. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, M.; Lu, R.; Uzzau, S.; Wang, W.; Margaretten, K.; Pazzani, C.; Maimone, F.; Fasano, A. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 2001, 276, 19160–19165. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [Green Version]
- Martinez, E.E.; Lan, J.; Konno, T.; Miranda-Ribera, A.; Fiorentino, M.; Mehta, N.M.; Fasano, A. Novel role of zonulin in the pathophysiology of gastro-duodenal transit: A clinical and translational study. Sci. Rep. 2021, 11, 22462. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Memon, A.A.; Palmér, K.; Hedelius, A.; Sundquist, J.; Sundquist, K. The association of zonulin-related proteins with prevalent and incident inflammatory bowel disease. BMC Gastroenterol. 2022, 22, 3. [Google Scholar] [CrossRef]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl. Acad. Sci. USA 2009, 106, 16799–16804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturgeon, C.; Lan, J.; Fasano, A.; Ann, N.Y. Zonulin transgenic mice show altered gut permeability and increased morbidity/mortality in the DSS colitis model. Acad. Sci. 2017, 1397, 130–142. [Google Scholar] [CrossRef]
- Mashaqi, S.; Kallamadi, R.; Matta, A.; Quan, S.F.; Patel, S.I.; Combs, D.; Estep, L.; Lee-Iannotti, J.; Smith, C.; Parthasarathy, S.; et al. Obstructive Sleep Apnea as a Risk Factor for COVID-19 Severity—The Gut Microbiome as a Common Player Mediating Systemic Inflammation via Gut Barrier Dysfunction. Cells 2022, 11, 1569. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Gilboa, T.; Senussi, Y.; Kenyon, V.; Papadakis, L.; Boribong, B.P.; Carroll, R.W.; Walt, D.R.; Fasano, A. Zonulin Antagonist, Larazotide (AT1001), As an Adjuvant Treatment for Multisystem Inflammatory Syndrome in Children: A Case Series. Crit. Care Explor. 2022, 10, e0641. [Google Scholar] [CrossRef]
- American Academy of Pediatrics/Home/Children and COVID-19: State-Level Data Report. Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ (accessed on 25 May 2022).
- Centers for Disease Control and Prevention (CDC). COVID Data Tracker. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. Available online: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance (accessed on 25 May 2022).
- Abrams, J.Y.; Godfred-Cato, S.E.; Oster, M.E.; Chow, E.J.; Koumans, E.H.; Bryant, B.; Leung, J.W.; Belay, E.D. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review. J. Pediatr. 2020, 226, 45–54.e1. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Qiu, Y.; He, J.S.; Tan, J.Y.; Li, X.H.; Liang, J.; Shen, J.; Zhu, L.R.; Chen, Y.; Iacucci, M.; et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- Sultan, S.; Altayar, O.; Siddique, S.M.; Davitkov, P.; Feuerstein, J.D.; Lim, J.K.; Falck-Ytter, Y.; El-Serag, H.B. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020, 159, 320–334.e27. [Google Scholar] [CrossRef] [PubMed]
- Bankaitis, E.D.; Ha, A.; Kuo, C.J.; Magness, S.T. Reserve stem cells in intestinal homeostasis and injury. Gastroenterology 2018, 155, 1348–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belcher, J.D.; Zhang, P.; Nguyen, J.; Kiser, Z.M.; Nath, K.A.; Hu, J.; Trent, J.O.; Vercellotti, G.M. Identification of a Heme Activation Site on the MD-2/TLR4 Complex. Front. Immunol. 2020, 11, 1370. [Google Scholar] [CrossRef] [PubMed]
- Santaolalla, R.; Abreu, M.T. Innate immunity in the small intestine. Curr. Opin. Gastroenterol. 2012, 28, 124–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alibeik, N.; Pishgar, E.; Bozorgmehr, R.; Aghaaliakbari, F.; Rahimian, N. Potential role of gut microbiota in patients with COVID-19, its relationship with lung axis, central nervous system (CNS) axis, and improvement with probiotic therapy. Iran. J. Microbiol. 2022, 14, 1–9. [Google Scholar] [CrossRef]
- Llorens, S.; Nava, E.; Muñoz-López, M.; Sánchez-Larsen, Á.; Segura, T. Neurological Symptoms of COVID-19: The Zonulin Hypothesis. Front. Immunol. 2021, 12, 665300. [Google Scholar] [CrossRef]
- Giron, L.B.; Dweep, H.; Yin, X.; Wang, H.; Damra, M.; Goldman, A.R.; Gorman, N.; Palmer, C.S.; Tang, H.Y.; Shaikh, M.W.; et al. Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front. Immunol. 2021, 12, 686240, Erratum in Front. Immunol. 2021, 12, 779064. [Google Scholar] [CrossRef]
- Trottein, F.; Sokol, H. Potential Causes and Consequences of Gastrointestinal Disorders during a SARS-CoV-2 Infection. Cell Rep. 2020, 32, 107915. [Google Scholar] [CrossRef]
- Campisi, L.; Barbet, G.; Ding, Y.; Esplugues, E.; Flavell, R.A.; Blander, J.M. Apoptosis in response to microbial infection induces autoreactive TH17 cells. Nat. Immunol. 2016, 17, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Hensley-McBain, T.; Manuzak, J.A. Zonulin as a biomarker and potential therapeutic target in multisystem inflammatory syndrome in children. J. Clin. Investig. 2021, 131, e151467. [Google Scholar] [CrossRef] [PubMed]
- Mønsted, M.Ø.; Falck, N.D.; Pedersen, K.; Buschard, K.; Holm, L.J.; Haupt-Jorgensen, M. Intestinal permeability in type 1 diabetes: An updated comprehensive overview. J. Autoimmun. 2021, 122, 102674. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.M.; Liu, E. Celiac disease: Pathophysiology, clinical manifestations, and associated autoimmune conditions. Adv. Pediatr. 2008, 55, 349–365. [Google Scholar] [CrossRef] [Green Version]
- del Servicio Canario, S.D.E.; de la Salud (SESCS). Protocolo Para El Diagnóstico Precoz de la Enfermedad Celíaca. Madrid: Ministerio de Sanidad, Servicios Sociales E Igualdad. 2018. Available online: https://www.sanidad.gob.es/profesionales/prestacionesSanitarias/publicaciones/Celiaquia/enfermedadCeliaca.pdf (accessed on 29 April 2022).
- Lebeaux, R.M.; Madan, J.C.; Nguyen, Q.P.; Coker, M.O.; Dade, E.F.; Moroishi, Y.; Palys, T.J.; Ross, B.D.; Pettigrew, M.M.; Morrison, H.G.; et al. Impact of antibiotics on off-target infant gut microbiota and resistance genes in cohort studies. Pediatr. Res. 2022. Advance online publication. [Google Scholar] [CrossRef]
- Dydensborg Sander, S.; Nybo Andersen, A.M.; Murray, J.A.; Karlstad, Ø.; Husby, S.; Størdal, K. Association between antibiotics in the first year of life and celiac disease. Gastroenterology 2019, 156, 2217–2229. [Google Scholar] [CrossRef] [Green Version]
- Caminero, A.; Verdu, E.F. Celiac disease: Should we care about microbes? Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G161–G170. [Google Scholar] [CrossRef]
- Verdu, E.F.; Schuppan, D. Co-factors, Microbes, and Immunogenetics in Celiac Disease to Guide Novel Approaches for Diagnosis and Treatment. Gastroenterology 2021, 161, 1395–1411.e4. [Google Scholar] [CrossRef]
- Alavi Moghaddam, M.; Rostami Nejad, M.; Shalmani, H.M.; Rostami, K.; Nazemalhosseini Mojarad, E.; Aldulaimi, D.; Zali, M.R. The effects of gluten-free diet on hypertransaminasemia in patients with celiac disease. Int. J. Prev. Med. 2013, 4, 700–704. [Google Scholar]
- Mostarica-Stojković, M. Mechanisms of the induction of autoimmunity. Srp. Arh. za Celok. Lek. 2005, 133, 9–15. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Shi, H.; Yang, M.; Wang, Y.; Sun, Z.; Xu, S. Identification of key genes implicated in the suppressive function of human FOXP3+CD25+CD4+ regulatory T cells through the analysis of time-series data. Mol. Med. Rep. 2018, 17, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Barartabar, Z.; Nikzamir, A.; Sirati-Sabet, M.; Aghamohammadi, E.; Chaleshi, V.; Rostami Nejad, M.; Asadzadeh-Aghdaei, H.; Reza, Z.M. The relationship between 174 G/C and -572 G/C of IL-6 gene polymorphisms and susceptibility of celiac disease in the Iranian population. Prz. Gastroenterol. 2018, 13, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Nasserinejad, M.; Shojaee, S.; Ghobakhlou, M.; Lak, E.; Eslami, P.; Pourhoseingholi, M.A. The effects of IL-8, IL- 6, and IL-1 on the risk of celiac disease: A Bayesian regression analysis. Gastroenterol. Hepatol. Bed Bench 2019, 12, S117–S122. [Google Scholar] [CrossRef]
- Grifoni, E.; Valoriani, A.; Cei, F.; Lamanna, R.; Gelli, A.M.G.; Ciambottit, B.; Vannucchi, V.; Moroni, F.; Pelagatti, L.; Tarquini, R.; et al. Interleukin-6 as prognosticator in patients with COVID-19. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef]
- Nasonov, E.; Samsonov, M. The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomed. Pharmacother. 2020, 131, 110698. [Google Scholar] [CrossRef]
- Mojtabavi, H.; Saghazadeh, A.; Rezaei, N. Interleukin-6 and severe COVID-19: A systematic review and meta-analysis. Eur. Cytokine Netw. 2020, 31, 44–49. [Google Scholar] [CrossRef]
- Wang, C.; Fei, D.; Li, X.; Zhao, M.; Yu, K. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Med. 2020, 46, 1475–1476. [Google Scholar] [CrossRef]
- Ouyang, W.; Kolls, J.K.; Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008, 28, 454–467. [Google Scholar] [CrossRef] [Green Version]
- Shekhawat, J.; Gauba, K.; Gupta, S.; Purohit, P.; Mitra, P.; Garg, M.; Misra, S.; Sharma, P.; Banerjee, M. Interleukin-6 Perpetrator of the COVID-19 Cytokine Storm. Indian J. Clin. Biochem. 2021, 36, 1–11. [Google Scholar] [CrossRef]
- Pecora, F.; Persico, F.; Gismondi, P.; Fornaroli, F.; Iuliano, S.; de’Angelis, G.L. Esposito, S. Gut Microbiota in Celiac Disease: Is There Any Role for Probiotics? Front. Immunol. 2020, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Mårild, K.; Fredlund, H.; Ludvigsson, J.F. Increased risk of hospital admission for influenza in patients with celiac disease: A nationwide cohort study in Sweden. Am. J. Gastroenterol. 2010, 105, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Magazzù, G.; Aquilina, S.; Barbara, C.; Bondin, R.; Brusca, I.; Bugeja, J.; Camilleri, M.; Cascio, D.; Costa, S.; Cuzzupè, C.; et al. Recognizing the Emergent and Submerged Iceberg of the Celiac Disease: ITAMA Project—Global Strategy Protocol. Pediatr. Rep. 2022, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Barisani, D.; Vaira, V.; Bardella, M.T.; Topa, M.; Vecchi, M.; Doneda, L.; Scricciolo, A.; Lombardo, V.; Roncoroni, L. How to manage celiac disease and gluten-free diet during the COVID-19 era: Proposals from a tertiary referral center in a high-incidence scenario. BMC Gastroenterol. 2020, 20, 387, Erratum in BMC Gastroenterol. 2022, 22, 86. [Google Scholar] [CrossRef]
- Hadi, Y.B.; Sohail, A.H.; Lakhani, D.A.; Naqvi, S.F.; Kupec, J.T.; Pervez, A. Outcomes of SARS-CoV-2 infection in patients with celiac disease: A multicenter research network study. Ann. Gastroenterol. 2022, 35, 164–168. [Google Scholar] [CrossRef]
- Greco, N.; Meacci, A.; Mora, B.; Vestri, A.; Picarelli, A. Coeliac disease in the COVID-19 pandemic: Does HLA have a protective effect? Ann. Med. 2022, 54, 617–621. [Google Scholar] [CrossRef]
- Samasca, G.; Lerner, A. Celiac disease in the COVID-19 pandemic. J. Transl. Autoimmun. 2021, 4, 100120. [Google Scholar] [CrossRef]
- Lefthériotis, G.; Wray, S.; Girardi, A.C.C.; Vidal-Petiot, E.; Bailey, M.A.; Schechtman, D.; Ravi, N.; Noble, D. Editorial: The Tribute of Physiology for the Understanding of COVID-19 Disease. Front. Physiol. 2021, 12, 761644. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Implications of SARS-CoV-2 Infection in Systemic Juvenile Idiopathic Arthritis. Int. J. Mol. Sci. 2022, 23, 4268. [Google Scholar] [CrossRef]
- Costantino, A.; Topa, M.; Roncoroni, L.; Doneda, L.; Lombardo, V.; Stocco, D.; Gramegna, A.; Costantino, C.; Vecchi, M.; Elli, L. COVID-19 Vaccine: A Survey of Hesitancy in Patients with Celiac Disease. Vaccines 2021, 9, 511. [Google Scholar] [CrossRef]
- Zhen, J.; Stefanolo, J.P.; Temprano, M.P.; Seiler, C.L.; Caminero, A.; de-Madaria, E.; Huguet, M.M.; Santiago, V.; Niveloni, S.I.; Smecuol, E.G.; et al. Risk perception and knowledge of COVID-19 in patients with celiac disease. World J. Gastroenterol. 2021, 27, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Aaron Lerner. The COVID-19 Vaccination Debate: CoV-2 in Celiac Disease: A Pathogen or just along for the Ride? Int. J. Celiac Dis. 2021, 9, 6–9. [Google Scholar] [CrossRef]
- Cascini, F.; Pantovic, A.; Al-Ajlouni, Y.; Failla, G.; Ricciardi, W. Attitudes, acceptance and hesitancy among the general population worldwide to receive the COVID-19 vaccines and their contributing factors: A systematic review. eClinicalMedicine 2021, 40, 101113. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailioaie, L.M.; Ailioaie, C.; Litscher, G.; Chiran, D.A. Celiac Disease and Targeting the Molecular Mechanisms of Autoimmunity in COVID Pandemic. Int. J. Mol. Sci. 2022, 23, 7719. https://doi.org/10.3390/ijms23147719
Ailioaie LM, Ailioaie C, Litscher G, Chiran DA. Celiac Disease and Targeting the Molecular Mechanisms of Autoimmunity in COVID Pandemic. International Journal of Molecular Sciences. 2022; 23(14):7719. https://doi.org/10.3390/ijms23147719
Chicago/Turabian StyleAilioaie, Laura Marinela, Constantin Ailioaie, Gerhard Litscher, and Dragos Andrei Chiran. 2022. "Celiac Disease and Targeting the Molecular Mechanisms of Autoimmunity in COVID Pandemic" International Journal of Molecular Sciences 23, no. 14: 7719. https://doi.org/10.3390/ijms23147719
APA StyleAilioaie, L. M., Ailioaie, C., Litscher, G., & Chiran, D. A. (2022). Celiac Disease and Targeting the Molecular Mechanisms of Autoimmunity in COVID Pandemic. International Journal of Molecular Sciences, 23(14), 7719. https://doi.org/10.3390/ijms23147719








