Screening of Genes Related to Sex Determination and Differentiation in Mandarin Fish (Siniperca chuatsi)
Abstract
1. Introduction
2. Results
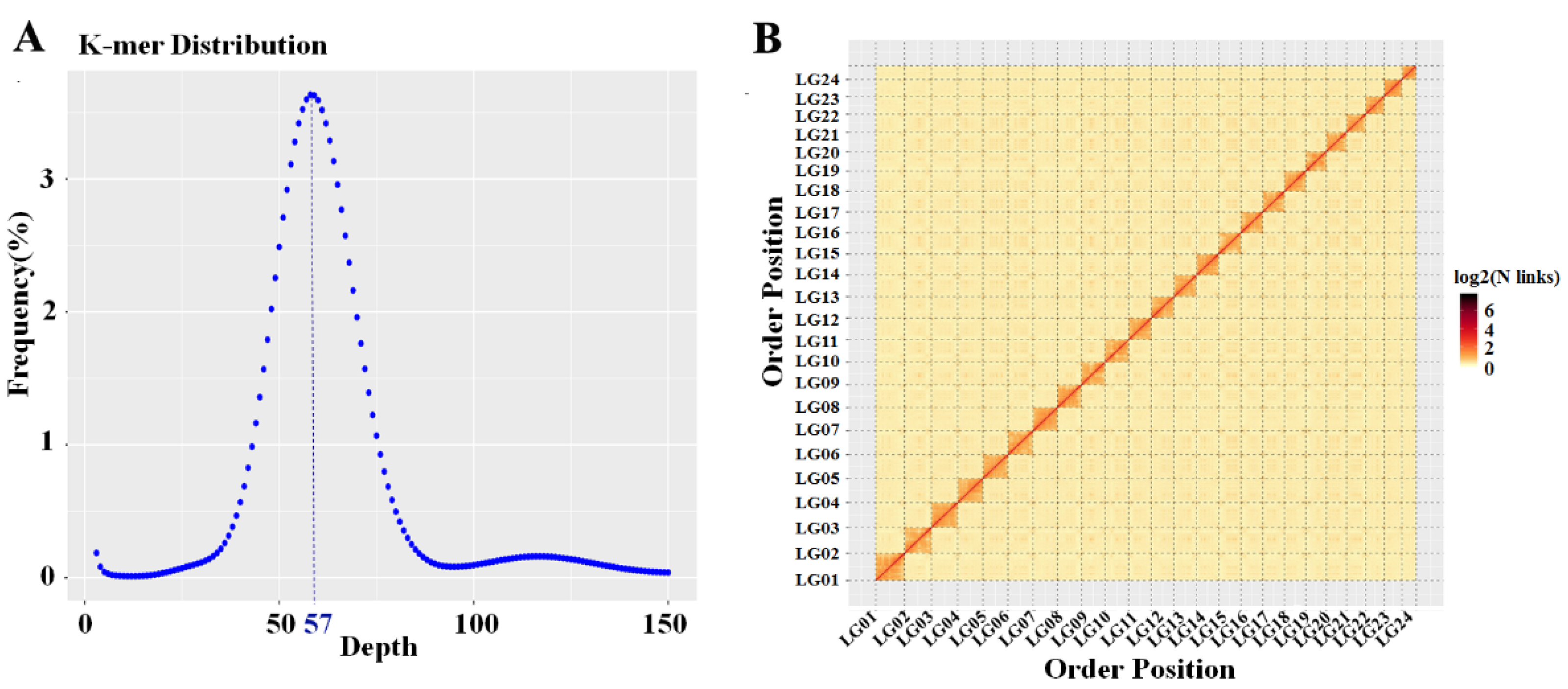
2.1. Genome Assembly and Annotation
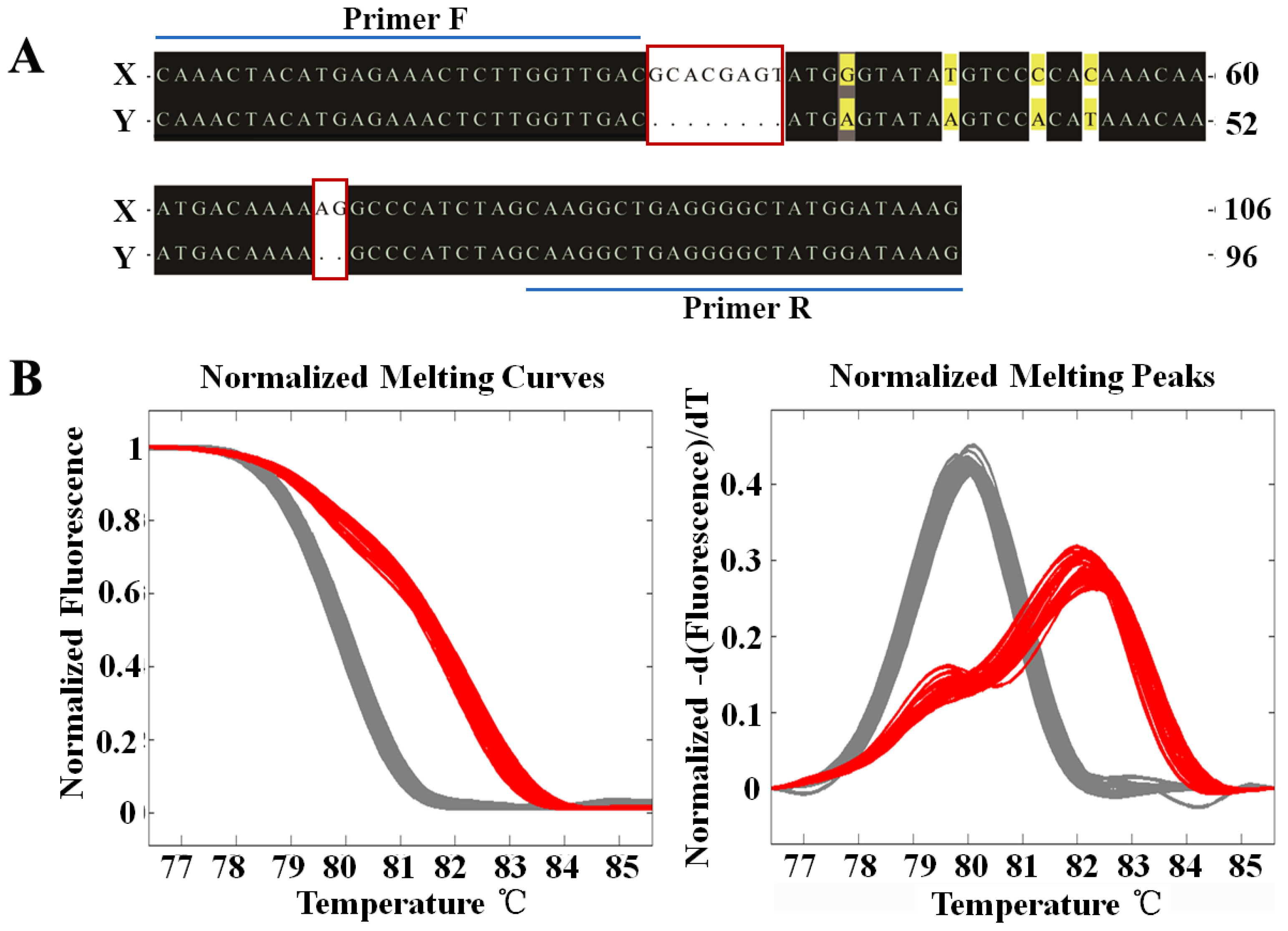
2.2. Sex-Specific Marker Screening and X Chromosome Identification
2.3. Distribution Characteristics of the Coding Genes and Noncoding Genes on the X Chromosome
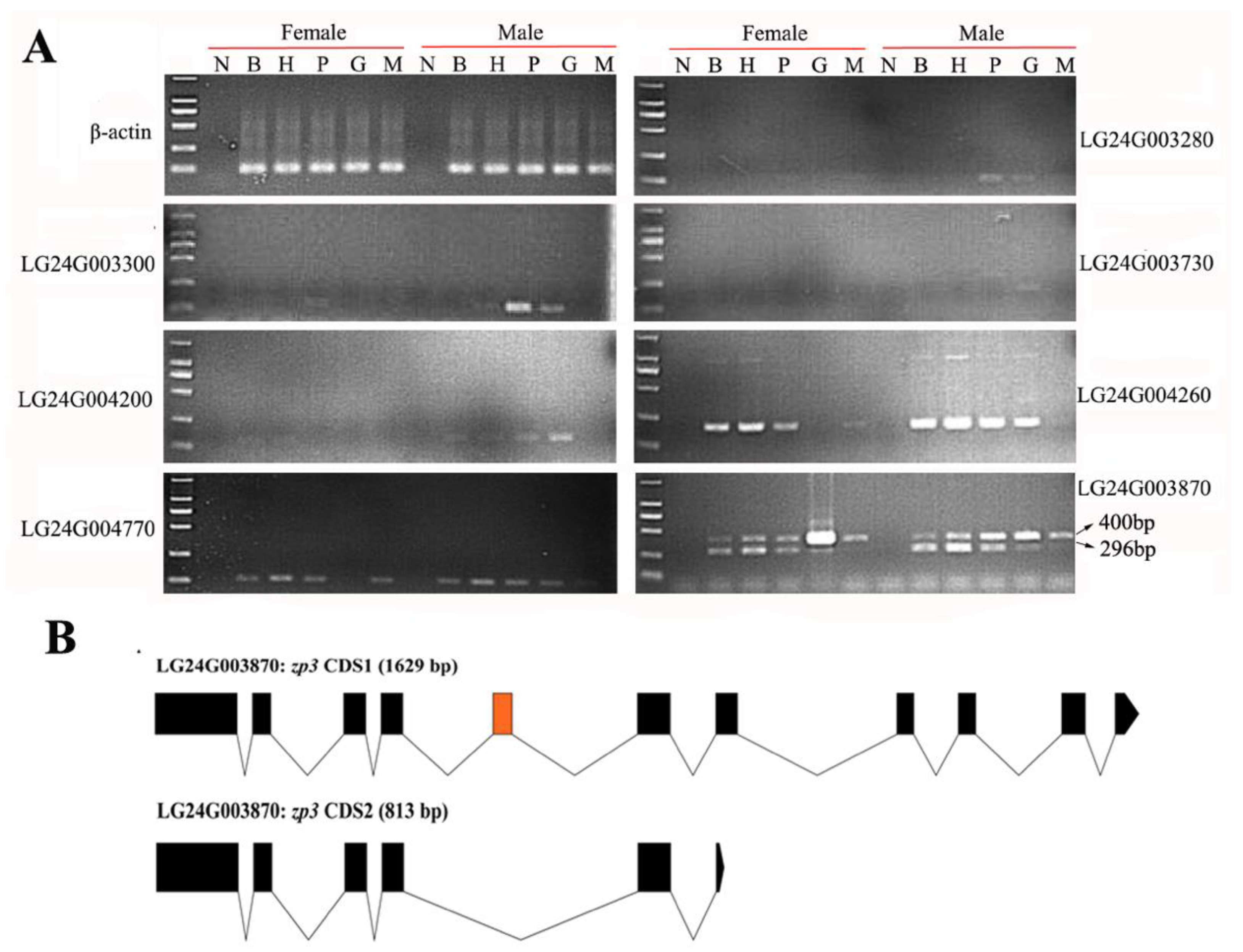
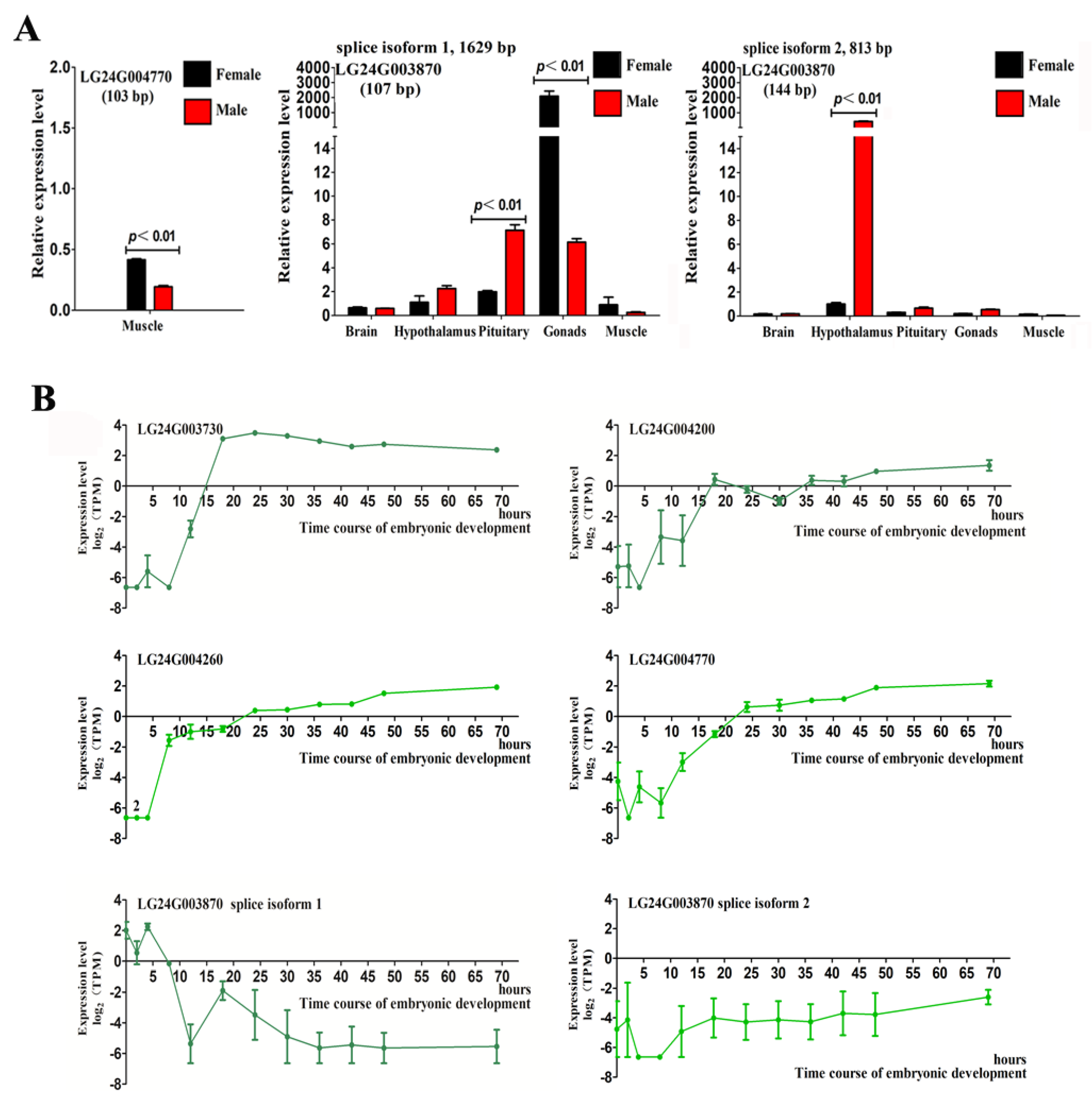
2.4. Gonadal Differentially Expressed Genes in the HPG Axis and Muscle Tissues
2.5. Expression of Gonadal Differentially Expressed Genes during Early Embryonic Development in Mandarin Fish
2.6. Domain Difference Analysis of Two Isomers of LG24G003870 Gene
3. Discussion
4. Materials and Methods
4.1. Source of Tissue and Embryo Samples of S. chuats
4.2. Assembly and Annotation of S. chuatsi Genome
4.3. X Chromosome Identification and Gene Distribution
4.4. Differential Expression Analysis of X Chromosome Gene in the Testis and Ovary
4.5. Expression of Differentially Expressed Genes in the Gonad in HPG Axis and Muscle Tissues
4.6. Expression Analysis of Differentially Expressed Genes in the Gonad during Early Embryonic Development and Structural Analysis of Different Transcripts
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Almeida, T.L.F.; Foresti, F. Morphologically Differentiated Sex Chromosomes in Neotropical Freshwater Fish. Genetica 2001, 111, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, D.; Volff, J.N.; Galiana, D.; Anderson, J.L.; Schartl, M. Transposable Elements and Early Evolution of Sex Chromosomes in Fish. Chromosom. Res. 2015, 23, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.-J.; Li, X.-Y.; Zhou, F.-J.; Qiang, X.-G.; Gui, J.-F. Identification of Sex-Specific Markers Reveals Male Heterogametic Sex Determination in Pseudobagrus Ussuriensis. Mar. Biotechnol. 2015, 17, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xiao, Z.; Ma, D.; Liu, J.; Li, J. Genome Sequence of the Barred Knifejaw Oplegnathus Fasciatus (Temminck & Schlegel, 1844): The First Chromosome-Level Draft Genome in the Family Oplegnathidae. Gigascience 2019, 8, giz013. [Google Scholar] [CrossRef]
- Iturra, P.; Medrano, J.F.; Bagley, M.; Lam, N.; Vergara, N.; Marin, J.C. Identification of Sex Chromosome Molecular Markers Using RAPDs and Fluorescent in Situ Hybridization in Rainbow Trout. Genetica 1997, 101, 209–213. [Google Scholar] [CrossRef]
- Ou, M.; Yang, C.; Luo, Q.; Huang, R.; Zhang, A.-D.; Liao, L.-J.; Li, Y.-M.; He, L.-B.; Zhu, Z.-Y.; Chen, K.-C.; et al. An NGS-Based Approach for the Identification of Sex-Specific Markers in Snakehead (Channa argus). Oncotarget 2017, 8, 98733–98744. [Google Scholar] [CrossRef]
- Chen, S.-L.; Li, J.; Deng, S.-P.; Tian, Y.-S.; Wang, Q.-Y.; Zhuang, Z.-M.; Sha, Z.-X.; Xu, J.-Y. Isolation of Female-Specific AFLP Markers and Molecular Identification of Genetic Sex in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar. Biotechnol. 2007, 9, 273–280. [Google Scholar] [CrossRef]
- Vale, L.; Dieguez, R.; Sánchez, L.; Martínez, P.; Viñas, A. A Sex-Associated Sequence Identified by RAPD Screening in Gynogenetic Individuals of Turbot (Scophthalmus maximus). Mol. Biol. Rep. 2014, 41, 1501–1509. [Google Scholar] [CrossRef]
- Chen, S.L.; Ji, X.S.; Shao, C.W.; Li, W.L.; Yang, J.F.; Liang, Z.; Liao, X.L.; Xu, G.B.; Xu, Y.; Song, W.T. Induction of Mitogynogenetic Diploids and Identification of WW Super-Female Using Sex-Specific SSR Markers in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar. Biotechnol. 2012, 14, 120–128. [Google Scholar] [CrossRef]
- Yang, C.; Huang, R.; Ou, M.; Gui, B.; Zhao, J.; He, L.; Li, Y.; Liao, L.; Chen, K.; Wang, Y. A Rapid Method of Sex-Specific Marker Discovery Based on NGS and Determination of the XX/XY Sex-Determination System in Channa Maculata. Aquaculture 2020, 528, 735499. [Google Scholar] [CrossRef]
- Sun, C.; Niu, Y.; Ye, X.; Dong, J.; Hu, W.; Zeng, Q.; Chen, Z.; Tian, Y.; Zhang, J.; Lu, M. Construction of a High-Density Linkage Map and Mapping of Sex Determination and Growth-Related Loci in the Mandarin Fish (Siniperca chuatsi). BMC Genomics 2017, 18, 446. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.Z.; Qiu, G.F.; Liu, F. Transcriptome Analysis of the Growth Performance of Hybrid Mandarin Fish after Food Conversion. PLoS ONE 2020, 15, 0240308. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhu, Q.; Lu, H.; Wang, C.; Zhou, X.; Peng, C.; Tang, L.; Han, L.; Chen, J.; Li, S.; et al. Screening and Characterization of Sex-Specific Markers Developed by a Simple NGS Method in Mandarin Fish (Siniperca chuatsi). Aquaculture 2020, 527, 735495. [Google Scholar] [CrossRef]
- Khodursky, S.; Svetec, N.; Durki, S.M.; Zhao, L. The Evolution of Sex-Biased Gene Expression in the Drosophila Brain. Genome Res. 2020, 30, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Broquard, C.; Saowaros, S.-a.; Lepoittevin, M.; Degremont, L.; Lamy, J.B.; Morga, B.; Elizur, A.; Martinez, A.S. Gonadal Transcriptomes Associated with Sex Phenotypes Provide Potential Male and Female Candidate Genes of Sex Determination or Early Differentiation in Crassostrea Gigas, a Sequential Hermaphrodite Mollusc. BMC Genomics 2021, 22, 609. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Zhang, Y.; Ning, Z.; Li, Y.; Zhao, Q.; Lu, H.; Huang, R.; Xia, X.; Feng, Q.; et al. The Draft Genome of the Grass Carp (Ctenopharyngodon idellus) Provides Insights into Its Evolution and Vegetarian Adaptation. Nat. Genet. 2015, 47, 625–631. [Google Scholar] [CrossRef]
- Hao, Z.; Lv, D.; Ge, Y.; Shi, J.; Weijers, D.; Yu, G.; Chen, J. RIdeogram: Drawing SVG Graphics to Visualize and Map Genome-Wide Data on the Idiograms. PeerJ Comput. Sci. 2020, 6, e251. [Google Scholar] [CrossRef]
- Cheng, M.K.; Disteche, C.M. A Balancing Act between the X Chromosome and the Autosomes. J. Biol. 2006, 5, 2. [Google Scholar] [CrossRef]
- Zeng, K.; Jackson, B.C.; Barton, H.J. Methods for Estimating Demography and Detecting Between-Locus Differences in the Effective Population Size and Mutation Rate. Mol. Biol. Evol. 2019, 36, 423–433. [Google Scholar] [CrossRef]
- Allen, S.L.; Bonduriansky, R.; Chenoweth, S.F. The Genomic Distribution of Sex-Biased Genes in Drosophila Serrata: X Chromosome Demasculinization, Feminization, and Hyperexpression in Both Sexes. Genome Biol. Evol. 2013, 5, 1986–1994. [Google Scholar] [CrossRef]
- Wise, J.L.; Crout, R.J.; Mcneil, D.W.; Weyant, R.J.; Marazita, M.L. Human Telomere Length Correlates to the Size of the Associated Chromosome Arm. PLoS ONE 2009, 4, 6013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, S.; Li, L.; Lv, L.-Y.; Cai, W.-J.; Dou, Y.-Q.; Li, J.; Tang, S.-L.; Chen, X.; Zhang, Z.; Xu, J.; et al. Mandarin Fish (Sinipercidae) Genomes Provide Insights into Innate Predatory Feeding. Commun. Biol. 2020, 3, 361. [Google Scholar] [CrossRef] [PubMed]
- Mank, J.E.; Avise, J.C. Evolutionary Diversity and Turn-Over of Sex Determination in Teleost Fishes. Sex. Dev. 2009, 3, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gao, Z.; Wang, W. Advancement on Research of Sex Determination and Sex-specific Markers in Fish. Fish. Sci. 2010, 29, 432–437. [Google Scholar]
- Sakai, H.; Aoki, F.; Suzuki, M.G. Identification of the Key Stages for Sex Determination in the Silkworm, Bombyx Mori. Dev. Genes Evol. 2014, 224, 119. [Google Scholar] [CrossRef][Green Version]
- Bink, R.J.; Habuchi, H.; Lele, Z.; Dolk, E.; Joore, J.; Rauch, G.J.; Geisler, R.; Wilson, S.W.; Den Hertog, J.; Kimata, K.; et al. Heparan Sulfate 6-O-Sulfotransferase Is Essential for Muscle Development in Zebrafish. J. Biol. Chem. 2003, 278, 31118–31127. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.V.; Skinner, S.M.; Carino, C.; Wang, N.; Cartwright, J.; Dunbar, B.S. Structure and Function of the Proteins of the Mammalian Zona Pellucida. Cells Tissues Organs 2000, 166, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, H.; Zhang, Q.; Qi, J.; Zhong, Q.; Chen, Y.; Li, C. Molecular Characterization and Expression Pattern of Two Zona Pellucida Genes in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 155, 316–321. [Google Scholar] [CrossRef]
- Chuang-Ju, L.; Qi-Wei, W.; Xi-Hua, C.; Li, Z.; Hong, C.; Fang, G.; Jian-Fang, G. Molecular Characterization and Expression Pattern of Three Zona Pellucida 3 Genes in the Chinese Sturgeon, Acipenser Sinensis. Fish Physiol. Biochem. 2011, 37, 471–484. [Google Scholar] [CrossRef]
- Wang, H.; Gong, Z. Characterization of Two Zebrafish CDNA Clones Encoding Egg Envelope Proteins ZP2 and ZP3. Biochim. Biophys. Acta—Gene Struct. Expr. 1999, 1446, 156–160. [Google Scholar] [CrossRef]
- Del Giacco, L.; Diani, S.; Cotelli, F. Identification and Spatial Distribution of the MRNA Encoding an Egg Envelope Component of the Cyprinid Zebrafish, Danio Rerio, Homologous to the Mammalian ZP3 (ZPC). Dev. Genes Evol. 2000, 210, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Hyllner, S.J.; Westerlund, L.; Olsson, P.E.; Schopen, A. Cloning of Rainbow Trout Egg Envelope Proteins: Members of a Unique Group of Structural Proteins. Biol. Reprod. 2001, 64, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, H.; Gong, Z. Tandem-Repeated Zebrafish Zp3 Genes Possess Oocyte-Specific Promoters and Are Insensitive to Estrogen Induction. Biol. Reprod. 2006, 74, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and Accurate Long-Read Assembly via Adaptive k-Mer Weighting and Repeat Separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Li, H. Fast and Accurate Long-Read Assembly with Wtdbg2. Nat. Methods 2019, 17, 155–158. [Google Scholar] [CrossRef]
- Vaser, R.; Sović, I.; Nagarajan, N.; Šikić, M. Fast and Accurate de Novo Genome Assembly from Long Uncorrected Reads. Genome Res. 2017, 27, 737–746. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, 112963. [Google Scholar] [CrossRef]
- Servant, N.; Varoquaux, N.; Lajoie, B.R.; Viara, E.; Chen, C.J.; Vert, J.P.; Heard, E.; Dekker, J.; Barillot, E. HiC-Pro: An Optimized and Flexible Pipeline for Hi-C Data Processing. Genome Biol. 2015, 16, 259. [Google Scholar] [CrossRef]
- Burton, J.N.; Adey, A.; Patwardhan, R.P.; Qiu, R.; Kitzman, J.O.; Shendure, J. Chromosome-Scale Scaffolding of de Novo Genome Assemblies Based on Chromatin Interactions. Nat. Biotechnol. 2013, 31, 1119–1125. [Google Scholar] [CrossRef]
- Burge, C.; Karlin, S. Prediction of Complete Gene Structures in Human Genomic DNA. J. Mol. Biol. 1997, 268, 78–94. [Google Scholar] [CrossRef]
- Stanke, M.; Waack, S. Gene Prediction with a Hidden Markov Model and a New Intron Submodel. Bioinformatics 2003, 19 (Suppl. S2), ii215–ii225. [Google Scholar] [CrossRef] [PubMed]
- Majoros, W.H.; Pertea, M.; Salzberg, S.L. TigrScan and GlimmerHMM: Two Open Source Ab Initio Eukaryotic Gene-Finders. Bioinformatics 2004, 20, 2878–2879. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Parra, G.; Guigó, R. Using Geneid to Identify Genes. Curr. Protoc. Bioinform. 2007, 18, 4.3.1–4.3.28. [Google Scholar] [CrossRef]
- Korf, I. Gene Finding in Novel Genomes. BMC Bioinform. 2004, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Keilwagen, J.; Hartung, F.; Paulini, M.; Twardziok, S.O.; Grau, J. Combining RNA-Seq Data and Homology-Based Gene Prediction for Plants, Animals and Fungi. BMC Bioinform. 2018, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- Keilwagen, J.; Wenk, M.; Erickson, J.L.; Schattat, M.H.; Grau, J.; Hartung, F. Using Intron Position Conservation for Homology-Based Gene Prediction. Nucleic Acids Res. 2016, 44, e89. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De Novo Transcript Sequence Reconstruction from RNA-Seq Using the Trinity Platform for Reference Generation and Analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Tang, S.; Lomsadze, A.; Borodovsky, M. Identification of Protein Coding Regions in RNA Transcripts. Nucleic Acids Res. 2015, 43, e78. [Google Scholar] [CrossRef]
- Campbell, M.A.; Haas, B.J.; Hamilton, J.P.; Mount, S.M.; Robin, C.R. Comprehensive Analysis of Alternative Splicing in Rice and Comparative Analyses with Arabidopsis. BMC Genomics 2006, 7, 327. [Google Scholar] [CrossRef]
- Haas, B.J.; Salzberg, S.L.; Zhu, W.; Pertea, M.; Allen, J.E.; Orvis, J.; White, O.; Robin, C.R.; Wortman, J.R. Automated Eukaryotic Gene Structure Annotation Using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008, 9, R7. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Moxon, S.; Marshall, M.; Khanna, A.; Eddy, S.R.; Bateman, A. Rfam: Annotating Non-Coding RNAs in Complete Genomes. Nucleic Acids Res. 2005, 33, D121. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. TRNAscan-SE: A Program for Improved Detection of Transfer RNA Genes in Genomic Sequence. Nucleic Acids Res. 1997, 25, 955. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J. BLAT—The BLAST-Like Alignment Tool. Genome Res. 2002, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Birney, E.; Clamp, M.; Durbin, R. GeneWise and Genomewise. Genome Res. 2004, 14, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Han, C.; Peng, C.; Zhou, X.; Wang, C.; Han, L.; Li, S.; Li, G.; Lin, H.; Zhang, Y. Identification of Potential Sex-Related Genes in Siniperca Chuatsi. J. Oceanol. Limnol. 2021, 39, 1500–1512. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Chen, L.; Huang, R.; Gui, B.; Li, Y.; Li, Y.; Li, Y.; Liao, L.; Zhu, Z.; Wang, Y. Screening of Genes Related to Sex Determination and Differentiation in Mandarin Fish (Siniperca chuatsi). Int. J. Mol. Sci. 2022, 23, 7692. https://doi.org/10.3390/ijms23147692
Yang C, Chen L, Huang R, Gui B, Li Y, Li Y, Li Y, Liao L, Zhu Z, Wang Y. Screening of Genes Related to Sex Determination and Differentiation in Mandarin Fish (Siniperca chuatsi). International Journal of Molecular Sciences. 2022; 23(14):7692. https://doi.org/10.3390/ijms23147692
Chicago/Turabian StyleYang, Cheng, Liangming Chen, Rong Huang, Bin Gui, Yangyu Li, Yangyang Li, Yongming Li, Lanjie Liao, Zuoyan Zhu, and Yaping Wang. 2022. "Screening of Genes Related to Sex Determination and Differentiation in Mandarin Fish (Siniperca chuatsi)" International Journal of Molecular Sciences 23, no. 14: 7692. https://doi.org/10.3390/ijms23147692
APA StyleYang, C., Chen, L., Huang, R., Gui, B., Li, Y., Li, Y., Li, Y., Liao, L., Zhu, Z., & Wang, Y. (2022). Screening of Genes Related to Sex Determination and Differentiation in Mandarin Fish (Siniperca chuatsi). International Journal of Molecular Sciences, 23(14), 7692. https://doi.org/10.3390/ijms23147692




