Current Insights into the Molecular Mode of Action of Seaweed-Based Biostimulants and the Sustainability of Seaweeds as Raw Material Resources
Abstract
1. Introduction
2. Resources, Sustainability and Regulation of Seaweed-Based Biostimulants
2.1. Chemodiversity
2.2. Seaweed as a Raw Material for Plant Biostimulants and Other Products
- (a)
- Global seaweed production from harvesting wild stock sources.
- (b)
- Seaweed aquaculture.
- (c)
- Beach/storm-cast seaweeds.
2.3. Regulatory and Sustainability Aspects of Harvesting Wild Seaweed Resources
- (a)
- Regulatory aspects of harvesting seaweed in Europe.
- (b)
- Growth rates of seaweeds post-harvesting.
- (c)
- Carbon sequestration
- (d)
- Hydrodynamics
- (e)
- Flora and fauna
2.4. Regulatory Aspects Governing Classification and Registration of Plant Biostimulants
3. Stimulation of Growth and Increase in Yield Induced by Seaweed Biostimulants
3.1. Seaweed Biostimulants and Plant Hormones
3.2. Seaweed Derived Polysaccharides Induce Molecular Level Changes That Influence Plant Growth
4. Transcriptome and Metabolome Changes in Plants Treated with Seaweed Extracts
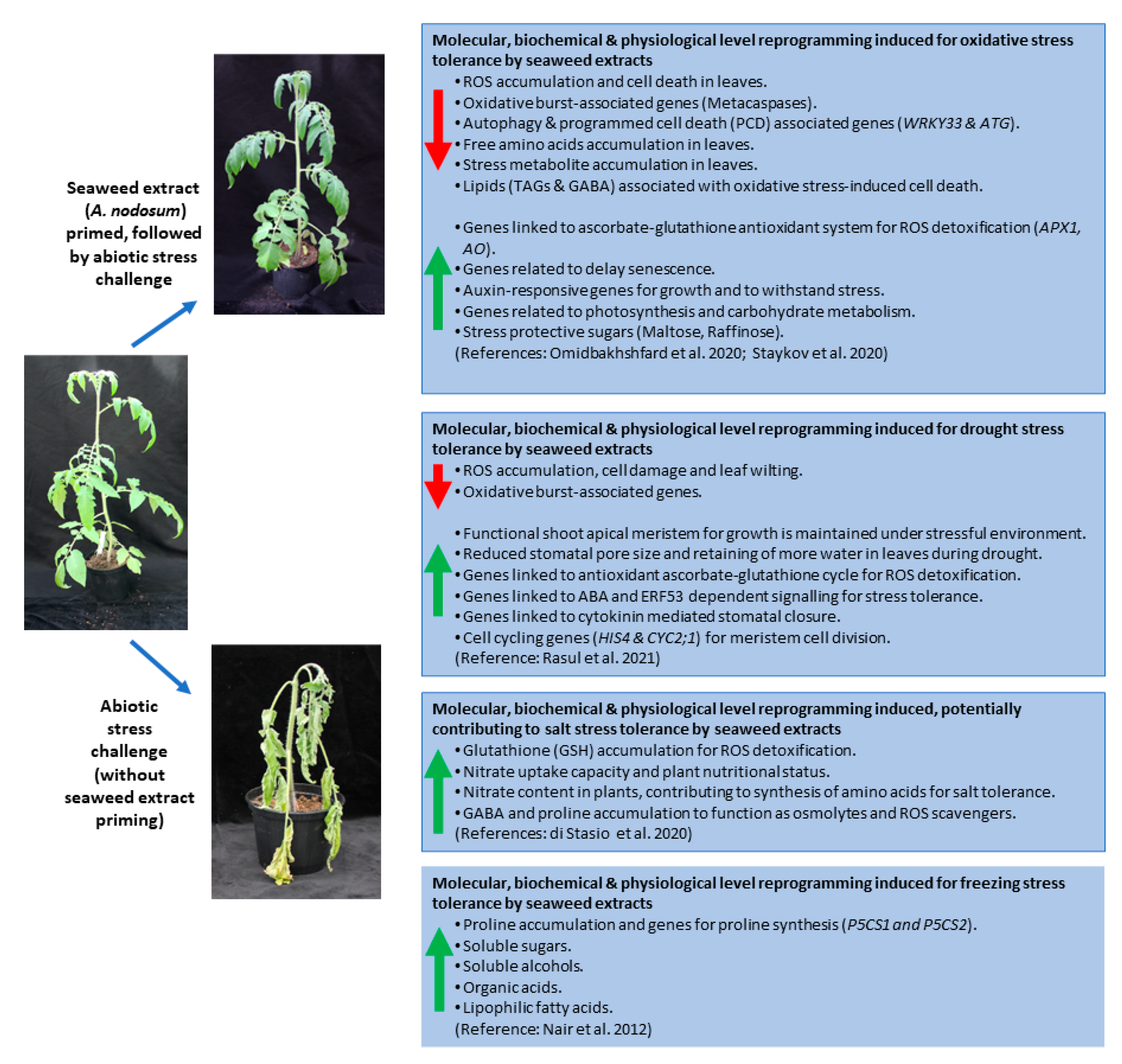
5. Seaweed Extract Induced Stress Mitigation
5.1. Seaweed Extracts Induce Plant Priming
5.2. Seaweed Extracts-Based Abiotic/Oxidative Stress Reduction
5.3. Seaweed Extract-Based Plant Priming and Drought Stress Mitigation
5.4. Seaweed Extract-Based Plant Priming for Salt and Freezing Induced Damage
6. Improvements in Nutrient Use Efficiency, Productivity and Quality Traits Induced by Seaweed Extracts
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, A.P., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 3–32. Available online: www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_SPM.pdf (accessed on 25 April 2022).
- Intergovernmental Panel on Climate Change (IPCC). Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. 2019. Available online: www.ipcc.ch/srccl/ (accessed on 25 April 2022).
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Zhang, H.; Li, Y.; Zhu, J.-K. Developing Naturally Stress-Resistant Crops for a Sustainable Agriculture. Nat. Plants 2018, 4, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed]
- Guyomard, H.; Bureau, J.C.; Chatellier, V.; Détang-Dessendre, C.; Dupraz, P.; Jacquet, F.; Reboud, X.; Réquillart, V.; Soler, L.G.; Tysebaert, M. The Green Deal and the CAP: Policy Implications to Adapt Farming Practices and to Preserve the EU’s Natural Resources. European Parliament, Policy Department for Structural and Cohesion Policies, Brussels. Available online: http://www.europarl.europa.eu/RegData/etudes/STUD/2020/629214/IPOL_STU(2020)629214_EN.pdf (accessed on 25 April 2022).
- Therond, O.; Duru, M.; Roger-Estrade, J.; Richard, G. A New Analytical Framework of Farming System and Agriculture Model Diversities. A Review Agron. Sustain. Dev. 2017, 37, 21. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Mattii, G.B. Effect of Agronomic Techniques on Aroma Composition of White Grapevines: A Review. Agronomy 2021, 11, 2027. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses. Plants 2022, 11, 162. [Google Scholar] [CrossRef]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant Biostimulants: A Categorical Review, Their Implications for Row Crop Production, and Relation to Soil Health Indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Kerchev, P.; van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.V.; van Breusegem, F.; Gechev, T. Molecular Priming as an Approach to Induce Tolerance against Abiotic and Oxidative Stresses in Crop Plants. Biotechnol. Adv. 2020, 40, 107503. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for Plant Growth and Mitigation of Abiotic Stresses: A Metabolomics Perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 20 January 2022).
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General Principles to Justify Plant Biostimulant Claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Critchley, A.T.; Critchley, J.S.C.; Norrie, J.; Gupta, S.; van Staden, J. Perspectives on the Global Biostimulant Market: Applications, Volumes, and Values, 2016 Data and Projections to 2022. In Biostimulants for Crops from Seed Germination to Plant Development; Elsevier: Amsterdam, The Netherlands, 2021; pp. 289–296. [Google Scholar]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Bio4Safe project. Identification of the seaweed biostimulant market (Phase2). In The North Sea Farm Foundation: AD Den Haag, The Netherlands; De Stichting: Rotterdam, The Netherlands, 2018; Available online: www.northseafarmers.org/public/documents/Bio4safe_WP1_D112_Seaweed-biostimulant-market_North-Sea-Farm-Foundation_December-2018.pdf (accessed on 21 May 2022).
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal Chemodiversity and Bioactivity: Sources of Natural Variability and Implications for Commercial Application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Abdala-Díaz, R.T.; Cabello-Pasini, A.; Pérez-Rodríguez, E.; Álvarez, R.M.; Figueroa, F.L. Daily and Seasonal Variations of Optimum Quantum Yield and Phenolic Compounds in Cystoseira Tamariscifolia (Phaeophyta). Mar. Biol. 2006, 148, 459–465. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Ross, A.B.; Anastasakis, K.; Hodgson, E.M.; Gallagher, J.A.; Jones, J.M.; Donnison, I.S. Seasonal Variation in the Chemical Composition of the Bioenergy Feedstock Laminaria digitata for Thermochemical Conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef]
- Aguilera, J.; Bischof, K.; Karsten, U.; Hanelt, D.; Wiencke, C. Seasonal Variation in Ecophysiological Patterns in Macroalgae from an Arctic Fjord II. Pigment Accumulation and Biochemical Defence Systems against High Light Stress. Mar. Biol. 2002, 140, 1087–1095. [Google Scholar]
- Garcia-Vaquero, M.; Rajauria, G.; Miranda, M.; Sweeney, T.; Lopez-Alonso, M.; O’Doherty, J. Seasonal Variation of the Proximate Composition, Mineral Content, Fatty Acid Profiles and Other Phytochemical Constituents of Selected Brown Macroalgae. Mar. Drugs 2021, 19, 204. [Google Scholar] [CrossRef] [PubMed]
- Guihéneuf, F.; Gietl, A.; Stengel, D.B. Temporal and Spatial Variability of Mycosporine-like Amino Acids and Pigments in Three Edible Red Seaweeds from Western Ireland. J. Appl. Phycol. 2018, 30, 2573–2586. [Google Scholar] [CrossRef]
- Kamiya, M.; Nishio, T.; Yokoyama, A.; Yatsuya, K.; Nishigaki, T.; Yoshikawa, S.; Ohki, K. Seasonal Variation of Phlorotannin in Sargassacean Species from the Coast of the Sea of Japan. Phycol. Res. 2010, 58, 53–61. [Google Scholar] [CrossRef]
- Kirke, D.A.; Rai, D.K.; Smyth, T.J.; Stengel, D.B. An Assessment of Temporal Variation in the Low Molecular Weight Phlorotannin Profiles in Four Intertidal Brown Macroalgae. Algal Res. 2019, 41, 101550. [Google Scholar] [CrossRef]
- Martínez, B.; Rico, J.M. Seasonal Variation of p Content and Major n Pools in Palmaria Palmata (Rhodophyta) 1. J. Phycol. 2002, 38, 1082–1089. [Google Scholar] [CrossRef]
- Parys, S.; Kehraus, S.; Pete, R.; Küpper, F.C.; Glombitza, K.-W.; König, G.M. Seasonal Variation of Polyphenolics in Ascophyllum nodosum (Phaeophyceae). Eur. J. Phycol. 2009, 44, 331–338. [Google Scholar] [CrossRef]
- Plouguerné, E.; le Lann, K.; Connan, S.; Jechoux, G.; Deslandes, E.; Stiger-Pouvreau, V. Spatial and Seasonal Variation in Density, Reproductive Status, Length and Phenolic Content of the Invasive Brown Macroalga Sargassum Muticum (Yendo) Fensholt along the Coast of Western Brittany (France). Aquat. Bot. 2006, 85, 337–344. [Google Scholar] [CrossRef]
- Ragan, M.A.; Jensen, A. Quantitative Studies on Brown Algal Phenols. II. Seasonal Variation in Polyphenol Content of Ascophyllum nodosum (L.) Le Jol. and Fucus Vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1978, 34, 245–258. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L. Chapter 7 Seaweed Carbohydrates. In Seaweed Sustainability; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; pp. 141–192. [Google Scholar] [CrossRef]
- Sampath-Wiley, P.; Neefus, C.D.; Jahnke, L.S. Seasonal Effects of Sun Exposure and Emersion on Intertidal Seaweed Physiology: Fluctuations in Antioxidant Contents, Photosynthetic Pigments and Photosynthetic Efficiency in the Red Alga Porphyra Umbilicalis Kützing (Rhodophyta, Bangiales). J. Exp. Mar. Biol. Ecol. 2008, 361, 83–91. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The Seasonal Variation in the Chemical Composition of the Kelp Species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Fatty Acid Contents and Profiles of 16 Macroalgae Collected from the Irish Coast at Two Seasons. J. Appl. Phycol. 2014, 26, 451–463. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Zvyagintseva, T.N.; Imbs, T.I. Monthly Changes in the Content and Monosaccharide Composition of Fucoidan from Undaria pinnatifida (Laminariales, Phaeophyta). J. Appl. Phycol. 2010, 22, 79–86. [Google Scholar] [CrossRef]
- Stengel, D.B.; Dring, M.J. Seasonal Variation in the Pigment Content and Photosynthesis of Different Thallus Regions of Ascophyllum nodosum (Fucales, Phaeophyta) in Relation to Position in the Canopy. Phycologia 1998, 37, 259–268. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Seasonal Variation of Chemical Composition and Biomethane Production from the Brown Seaweed Ascophyllum nodosum. Bioresour. Technol. 2016, 216, 219–226. [Google Scholar] [CrossRef]
- Guinan, K.J.; Sujeeth, N.; Copeland, R.B.; Jones, P.W.; O’brien, N.M.; Sharma, H.S.S.; Prouteau, P.F.J.; O’sullivan, J.T. Discrete Roles for Extracts of Ascophyllum nodosum in Enhancing Plant Growth and Tolerance to Abiotic and Biotic Stresses. In Proceedings of the I World Congress on the Use of Biostimulants in Agriculture 1009, Strasbourg, France, 26–29 November 2012; pp. 127–135. [Google Scholar]
- Goni, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative Transcriptome Analysis of Two Ascophyllum nodosum Extract Biostimulants: Same Seaweed but Different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aversana, E.; Cirillo, V.; van Oosten, M.J.; di Stasio, E.; Saiano, K.; Woodrow, P.; Ciarmiello, L.F.; Maggio, A.; Carillo, P. Ascophyllum nodosum Based Extracts Counteract Salinity Stress in Tomato by Remodeling Leaf Nitrogen Metabolism. Plants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- FAO. Fishery and Aquaculture Statistics. Global Capture Production 1950–2019 (FishstatJ). In FAO Fisheries Division; FAO: Rome, Italy, 2021; Available online: www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 27 January 2022).
- Crowe, T.; Brooks, P.; Scally, L. Kelp Harvesting in Bantry Bay: Monitoring to Meet Licensing Requirements Part 1: Pre-Harvest Survey, September 2016. BioAtlantis Aquamarine Ltd., Bantry Bay, Baseline Survey. Reference Number: FS006061 2017. Available online: https://www.gov.ie/en/foreshore-notice/e62b9-bioatlantis-ltd-bantry-bay/ (accessed on 10 June 2022).
- FAO. The Global Status of Seaweed Production, Trade and Utilization. Globefish Res. Programme 2018, 124, 120. Available online: https://www.fao.org/3/CA1121EN/ca1121en.pdf (accessed on 21 January 2022).
- Borges, D.; Araujo, R.; Azevedo, I.; Pinto, I.S. Sustainable Management of Economically Valuable Seaweed Stocks at the Limits of Their Range of Distribution: Ascophyllum nodosum (Phaeophyceae) and Its Southernmost Population in Europe. J. Appl. Phycol. 2020, 32, 1365–1375. [Google Scholar] [CrossRef]
- Aberg, P. Size-Based Demography of the Seaweed Ascophyllum nodosum in Stochastic Environments. Ecology 1992, 73, 1488–1501. [Google Scholar] [CrossRef]
- Aberg, P. A Demographic Study of Two Populations of the Seaweed Ascophyllum nodosum. Ecology 1992, 73, 1473–1487. [Google Scholar] [CrossRef]
- Werner, A.; Kraan, S. Review of the Potential Mechanisation of Kelp Harvesting in Ireland; Marine Environment and Health Series No. 17; Marine Institute, 2004; Available online: https://oar.marine.ie/handle/10793/261 (accessed on 10 June 2022).
- Bak, U.G.; Gregersen, Ó.; Infante, J. Technical Challenges for Offshore Cultivation of Kelp Species: Lessons Learned and Future Directions. Bot. Mar. 2020, 63, 341–353. [Google Scholar] [CrossRef]
- Kapetsky, J.M.; Aguilar-Manjarrez, J.; Jenness, J. A Global Assessment of Offshore Mariculture Potential from a Spatial Perspective; FAO Fisheries and Aquaculture Technical Paper No. 549; FAO: Rome, Italy, 2013; 181p. [Google Scholar]
- Bekkby, T.; Smit, C.; Gundersen, H.; Rinde, E.; Steen, H.; Tveiten, L.; Gitmark, J.K.; Fredriksen, S.; Albretsen, J.; Christie, H. The Abundance of Kelp Is Modified by the Combined Impact of Depth, Waves and Currents. Front. Mar. Sci. 2019, 6, 475. [Google Scholar] [CrossRef]
- Gorman, D.; Bajjouk, T.; Populus, J.; Vasquez, M.; Ehrhold, A. Modeling Kelp Forest Distribution and Biomass along Temperate Rocky Coastlines. Mar. Biol. 2013, 160, 309–325. [Google Scholar] [CrossRef]
- Smale, D.; Burrows, M.; Evans, A.; King, N.; Sayer, M.; Yunnie, A.; Moore, P. Linking Environmental Variables with Regional- Scale Variability in Ecological Structure and Standing Stock of Carbon within UK Kelp Forests. Mar. Ecol. Prog. Ser. 2016, 542, 79–95. [Google Scholar] [CrossRef]
- Harb, T.B.; Chow, F. An Overview of Beach-Cast Seaweeds: Potential and Opportunities for the Valorization of Underused Waste Biomass. Algal Res. 2022, 62, 102643. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Jamieson, J.; Oliver, D.M. Seaweeds and Plastic Debris Can Influence the Survival of Faecal Indicator Organisms in Beach Environments. Mar. Pollut. Bull. 2014, 84, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Swinscoe, I.; Oliver, D.M.; Gilburn, A.S.; Quilliam, R.S. The Seaweed Fly (Coelopidae) Can Facilitate Environmental Survival and Transmission of E. Coli O157 at Sandy Beaches. J. Environ. Manag. 2018, 223, 275–285. [Google Scholar] [CrossRef]
- Kelly, L.; Collier, L.; Costello, M.J.; Diver, M.; McGarvey, S.; Kraan, S.; Morrissey, J.; Giury, M.D. Impact Assessment of Hand and Mechanical Harvesting of Ascophyllum nodosum on Regeneration and Biodiversity. 2001. Available online: https://oar.marine.ie/handle/10793/207 (accessed on 16 April 2013).
- Kelly, E. (Ed.) The role of kelp in the marine environment. In Irish Wildlife Manuals, No. 17; National Parks and Wildlife Service, Department of Environment, Heritage and Local Government: Dublin, Ireland, 2005. [Google Scholar]
- NPWS, DAHG. Marine Natura Impact Statements in Irish Special Areas of Conservation. A Working Document; National Parks and Wildlife Service (NPWS), Department of Arts, Heritage and the Gaeltacht: Dublin, Ireland, 2012; Available online: https://www.npws.ie/sites/default/files/general/Marine%20Assessment%20Working%20Document.pdf (accessed on 10 June 2022).
- Nelson, B.; Cummins, S.; Fay, L.; Jeffrey, R.; Kelly, S.; Kingston, N.; Lockhart, N.; Marnell, F.; Tierney, D.; Wyse Jackson, M. Checklists of Protected and Threatened Species in Ireland. Irish Wildlife Manuals, No. 116. National Parks and Wildlife Service, Department of Culture, Heritage and the Gaeltacht, Ireland. 2019. Available online: https://www.npws.ie/sites/default/files/publications/pdf/IWM%20116%20Checklists%20Protected%20and%20Threatened%20Species%202019.pdf (accessed on 10 June 2022).
- NPWS. The Status of EU Protected Habitats and Species in Ireland. In Summary Overview; Lynn, D., O’Neill, F., Eds.; National Parks and Wildlife Service (NPWS) Report, Ireland; 2019; Volume 1, Available online: https://www.npws.ie/sites/default/files/publications/pdf/NPWS_2019_Vol1_Summary_Article17.pdf (accessed on 10 June 2022).
- Mesnildrey, L.; Jacob, C.; Frangoudes, K.; Reunavot, M.; Lesueur, M. Seaweed industry in France; Report Interreg program NETALGAE: 2012; Volume 42. Available online: https://hal-agrocampus-ouest.archives-ouvertes.fr/hal-00840572/document (accessed on 12 April 2022).
- Davoult, D.; Engel, C.R.; Arzel, P.; Knoch, D.; Laurans, M. Environmental Factors and Commercial Harvesting: Exploring Possible Links behind the Decline of the Kelp Laminaria digitata in Brittany France. Cah. De Biol. Mar. 2011, 52, 429–434. [Google Scholar]
- Leclerc, J.-C.; Riera, P.; Laurans, M.; Leroux, C.; Lévêque, L.; Davoult, D. Community, Trophic Structure and Functioning in Two Contrasting Laminaria hyperborea Forests. Estuar. Coast. Shelf Sci. 2015, 152, 11–22. [Google Scholar] [CrossRef][Green Version]
- Meland, M.; Rebours, C. Introduction to the Management and Regulation of the Norwegian Seaweed Industry. Bioforsk 2011, 7, 278–279. [Google Scholar]
- Gómez, T.C.; Lähteenmäki-Uutela, A. European and National Regulations on Seaweed Cultivation and Harvesting. “Growing algae Sustainably in the Baltic Sea”(GRASS) and “Sustainable Cultivation of Seaweed”(SUSCULT) Project Report 2021. Available online: https://www.submariner-network.eu/images/grass/FINAL-GRASS_GoA_3.2._SYKE_regulation_report.pdf (accessed on 21 June 2022).
- Steen, H.; Moy, F.E.; Bodvin, T.; Husa, V. Regrowth after Kelp Harvesting in Nord-Trøndelag, Norway. ICES J. Mar. Sci. J. Du Cons. 2016, 73, 2708–2720. [Google Scholar] [CrossRef]
- Maack, A. Ways to Encourage Sustainable Exploitation and Improve the Regulatory Framework on Wild Seaweed in Iceland. Master Thesis, Lund University. 2019. Available online: https://www.lunduniversity.lu.se/lup/publication/8997131 (accessed on 21 June 2022).
- BioAtlantis Aquamarine Ltd. Foreshore Licence Application for Mechanical Harvesting of Seaweed in Bantry Bay, County Cork, Ireland. Refer-Ence Number: FS006061. 2014. Available online: https://www.gov.ie/en/foreshore-notice/e62b9-bioatlantis-ltd-bantry-bay/ (accessed on 12 April 2022).
- Westermeier, R.; Murúa, P.; Patiño, D.J.; Muñoz, L.; Atero, C.; Müller, D.G. Repopulation Techniques for Macrocystis integrifolia (Phaeophyceae: Laminariales) in Atacama, Chile. J. Appl. Phycol. 2014, 26, 511–518. [Google Scholar] [CrossRef]
- Westermeier, R.; Murúa, P.; Patiño, D.J.; Manoli, G.; Müller, D.G. Evaluation of Kelp Harvest Strategies: Recovery of Lessonia berteroana (Phaeophyceae, Laminariales) in Pan de Azucar, Atacama, Chile. J. Appl. Phycol. 2019, 31, 575–585. [Google Scholar] [CrossRef]
- Hay, C.H.; South, G.R. Experimental Ecology with Particular Reference to Proposed Commercial Harvesting of Durvillaea (Phaeophyta, Durvilleales) in New Zealand. Bot. Mar. 1979, 22, 431–436. [Google Scholar] [CrossRef]
- Westermeier, R.; Murúa, P.; Patiño, D.J.; Müller, D.G. Population Biology and Long-Term Mariculture Studies in the Brown Alga Lessonia trabeculata in Atacama, Chile. J. Appl. Phycol. 2017, 29, 2267–2275. [Google Scholar] [CrossRef]
- Stagnol, D.; Michel, R.; Davoult, D. Unravelling the Impact of Harvesting Pressure on Canopy-Forming Macroalgae. Mar. Freshw. Res. 2016, 67, 153. [Google Scholar] [CrossRef]
- Stagnol, D.; Michel, R.; Davoult, D. Population Dynamics of the Brown Alga Himanthalia elongata under Harvesting Pressure. Estuar. Coast. Shelf Sci. 2016, 174, 65–70. [Google Scholar] [CrossRef]
- Wilding, C.; Tillin, H.M.; Corrigan, S.E.; Stuart, E.; Ashton, I.A.; Felstead, P.; Lubelski, A.; Burrows, M.; Smale, D.A. Seaweed Aquaculture and Mechanical Harvesting: An Evidence Review to Support Sustainable Management. Natural England Report NECR378. Natural England Commissioned Reports: 2021; pp. 117. Available online: http://plymsea.ac.uk/id/eprint/9590/ (accessed on 12 April 2022).
- Kitching, J.A. Studies in sublittoral ecology. Biol. Bull. 1941, 80, 324–337. [Google Scholar] [CrossRef]
- Kain, J.M.; Jones, N.S. Algal Recolonization of Some Cleared Subtidal Areas. J. Ecol. 1975, 63, 739. [Google Scholar] [CrossRef]
- Christie, H.; Fredriksen, S.; Rinde, E. Regrowth of Kelp and Colonization of Epiphyte and Fauna Community after Kelp Trawling at the Coast of Norway. Hydrobiologia 1998, 132, 49–58. [Google Scholar] [CrossRef]
- Sjøtun, K.; Christie, H.; Helge Fosså, J. The Combined Effect of Canopy Shading and Sea Urchin Grazing on Recruitment in Kelp Forest (Laminaria hyperborea). Mar. Biol. Res. 2006, 2, 24–32. [Google Scholar] [CrossRef]
- Hawkins, S.J.; Harkin, E. Preliminary Canopy Removal Experiments in Algal Dominated Communities Low on the Shore and in the Shallow Subtidal on the Isle of Man. Bot. Mar. 1985, 28, 223–230. [Google Scholar] [CrossRef]
- Engelen, A.H.; Leveque, L.; Destombe, C.; Valero, M. Spatial and Temporal Patterns of Recovery of Low Intertidal Laminaria digitata after Experimental Spring and Au-Tumn Removal. CBM-Cah. De Biol. Mar. 2011, 52, 441–453. [Google Scholar]
- Baardseth, E. Regrowth of Ascophyllum nodosum after Harvesting; Institute for Industrial Research and Standards: Dublin, Ireland, 1955. [Google Scholar]
- Baardseth, E. Regrowth of Ascophyllum nodosum after Harvesting. Report to Institute for Industrial Research and Standards, Dublin. Typewritten Manuscr. NUI Galway 1949. [Google Scholar]
- Guiry, M.D.; Morrison, L. The Sustainable Harvesting of Ascophyllum nodosum (Fucaceae, Phaeophyceae) in Ireland, with Notes on the Collection and Use of Some Other Brown Algae. J. Appl. Phycol. 2013, 25, 1823–1830. [Google Scholar] [CrossRef]
- Sharp, G.J.; Tremblay, D.M. An Assessment of Ascophyllum Nodosum Resources in Scotia Fundy; Canadian Atlantic Fisheries Scientific Advisory Committee: 1989. Available online: https://waves-vagues.dfo-mpo.gc.ca/Library/110806.pdf (accessed on 10 June 2022).
- Lauzon-Guay, J.S.; Ugarte, R.A.; Morse, B.L.; Robertson, C.A. Biomass and Height of Ascophyllum nodosum after Two Decades of Continuous Commercial Harvesting in Eastern Canada. J. Appl. Phycol. 2021, 33, 1695–1708. [Google Scholar] [CrossRef]
- Ugarte, R.A.; Sharp, G.; Moore, B. Changes in the Brown Seaweed Ascophyllum nodosum (L.) Le Jol. Plant Morphology and Biomass Produced by Cutter Rake Harvests in Southern New Brunswick, Canada. J. Appl. Phycol. 2006, 18, 351–359. [Google Scholar] [CrossRef]
- Lotze, H.K.; Milewski, I.; Fast, J.; Kay, L.; Worm, B. Ecosystem-Based Management of Seaweed Harvesting. Bot. Mar. 2019, 62, 395–409. [Google Scholar] [CrossRef]
- Gerring, P.K. Assessment of Pterocladia Lucida at Waihau Bay, New Zealand. N. Z. Minist. Fish. Assess. Rep. 2001. Available online: https://docs.niwa.co.nz/library/public/FAR2001_72.pdf (accessed on 10 June 2022).
- Higgins, J. Analysis of Ocle (Gelidium Corneum) Extraction along the Asturian Coast and Its Influence on the Sustainability of the Resource. Master Thesis, Master of Science in Marine Conservations, University of Oviedo, Spain. 2021. Available online: https://digibuo.uniovi.es/dspace/bitstream/handle/10651/60231/TFM_JaclynHiggins.pdf?sequence=4 (accessed on 10 June 2022).
- Marín, S.L.; Westermeier, R.; Melipillán, J. Simulation of Alternative Management Strategies for Red Algae, Luga Roja, (Gigartina Skottsbergii Setchell and Gardner) in Southern Chile. Ecol. Model. 2002, 154, 121–133. [Google Scholar] [CrossRef]
- Santelices, B.; Camus, P.; Hoffmann, A.J. Ecological Studies for Harvesting and Culturing Gymnogongrus furcellatus (Rhodophyta, Gigartinales) in Central Chile. J. Appl. Phycol. 1989, 1, 171. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Duarte, C.M.; Gattuso, J.; Hancke, K.; Gundersen, H.; Filbee-Dexter, K.; Pedersen, M.F.; Middelburg, J.J.; Burrows, M.T.; Krumhansl, K.A.; Wernberg, T.; et al. Global Estimates of the Extent and Production of Macroalgal Forests. Glob. Ecol. Biogeogr. 2022, 31, 1422–1439. [Google Scholar] [CrossRef]
- Cott, G.; Beca-Carretero, P.; Stengel, D. Blue Carbon and Marine Carbon Sequestration in Irish Waters and Coastal Habitats. Marine Institute, Ireland. 2021. Available online: https://oar.marine.ie/handle/10793/1685 (accessed on 10 June 2022).
- Bruton, T.; Lyons, H.; Lerat, Y.; Stanley, M.; Rasmussen, M.B. A Review of the Potential of Marine Algae as a Source of Biofuel in Ireland. Sustain. Energy Irel. 2009, 1–88. [Google Scholar]
- Krause-Jensen, D.; Duarte, C.M. Substantial Role of Macroalgae in Marine Carbon Sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Gallagher, J.B.; Shelamoff, V.; Layton, C. Seaweed Ecosystems May Not Mitigate CO2 Emissions. ICES J. Mar. Sci. 2022, 79, 585–592. [Google Scholar] [CrossRef]
- Jackson, G.A.; Winant, C.D. Effect of a Kelp Forest on Coastal Currents. Cont. Shelf Res. 1983, 2, 75–80. [Google Scholar] [CrossRef]
- Jackson, G.A. Internal Wave Attenuation by Coastal Kelp Stands. J. Phys. Oceanogr. 1984, 14, 1300–1306. [Google Scholar] [CrossRef]
- Gaylord, B.; Rosman, J.H.; Reed, D.C.; Koseff, J.R.; Fram, J.; MacIntyre, S.; Arkema, K.; McDonald, C.; Brzezinski, M.A.; Largier, J.L.; et al. Spatial Patterns of Flow and Their Modification within and around a Giant Kelp Forest. Limnol. Oceanogr. 2007, 52, 1838–1852. [Google Scholar] [CrossRef]
- Rosman, J.H.; Koseff, J.R.; Monismith, S.G.; Grover, J. A Field Investigation into the Effects of a Kelp Forest ( Macrocystis Pyrifera ) on Coastal Hydrodynamics and Transport. J. Geophys. Res. 2007, 112, C02016. [Google Scholar] [CrossRef]
- Andersen, K.H.; Mork, M.; Nilsen, J.E.Ø. Measurement of the Velocity-Profile in and above a Forest of Laminaria hyperborea. Sarsia 1996, 81, 193–196. [Google Scholar] [CrossRef]
- Mork, M. The Effect of Kelp in Wave Damping. Sarsia 1996, 80, 323–327. [Google Scholar] [CrossRef]
- Elwany, M.H.S.; Flick, R.E. Relationship between Kelp Beds and Beach Width in Southern California. J. Waterw. Port Coast. Ocean Eng. 1996, 122, 34–37. [Google Scholar] [CrossRef]
- Elwany, M.H.S.; O’Reilly, W.C.; Guza, R.T.; Flick, R.E. Effects of Southern California Kelp Beds on Waves. J. Waterw. Port Coast. Ocean Eng. 1995, 121, 143–150. [Google Scholar] [CrossRef]
- Morris, R.L.; Graham, T.D.J.; Kelvin, J.; Ghisalberti, M.; Swearer, S.E. Kelp Beds as Coastal Protection: Wave Attenuation of Ecklonia Radiata in a Shallow Coastal Bay. Ann. Bot. 2020, 125, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Smale, D.A.; Vance, T. Climate-Driven Shifts in Species’ Distributions May Exacerbate the Impacts of Storm Disturbances on North-East Atlantic Kelp Forests. Mar. Freshw. Res. 2016, 67, 65. [Google Scholar] [CrossRef]
- Pedersen, M.F.; Filbee-Dexter, K.; Norderhaug, K.M.; Fredriksen, S.; Frisk, N.L.; Fagerli, C.W.; Wernberg, T. Detrital Carbon Production and Export in High Latitude Kelp Forests. Oecologia 2020, 192, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Orland, C.; Queirós, A.M.; Spicer, J.I.; McNeill, C.L.; Higgins, S.; Goldworthy, S.; Zananiri, T.; Archer, L.; Widdicombe, S. Application of Computer-Aided Tomography Techniques to Visualize Kelp Holdfast Structure Reveals the Importance of Habitat Complexity for Supporting Marine Biodiversity. J. Exp. Mar. Biol. Ecol. 2016, 477, 47–56. [Google Scholar] [CrossRef]
- Leclerc, J.-C.; Riera, P.; Lévêque, L.; Davoult, D. Contrasting Temporal Variation in Habitat Complexity and Species Abundance Distributions in Four Kelp Forest Strata. Hydrobiologia 2016, 777, 33–54. [Google Scholar] [CrossRef]
- Gundersen, H.; Rinde, E.; Bekkby, T.; Hancke, K.; Gitmark, J.K.; Christie, H. Variation in Population Structure and Standing Stocks of Kelp Along Multiple Environmental Gradients and Implications for Ecosystem Services. Front. Mar. Sci. 2021, 8, 360. [Google Scholar] [CrossRef]
- Schoenrock, K.M.; O’Callaghan, R.; O’Callaghan, T.; O’Connor, A.; Stengel, D.B. An Ecological Baseline for Laminaria hyperborea Forests in Western Ireland. Limnol. Oceanogr. 2021, 66, 3439–3454. [Google Scholar] [CrossRef]
- Ryder, E.; Nelson, S.; Glenn, E.; Nagler, P.; Napolean, S.; Fitzsimmons, K. Production of Gracilaria Parvispora in Two-Phase Polyculture Systems in Relation to Nutrient Requirements and Uptake. Bull.-Fish. Res. Agency Jpn. 2004, Supplement No. 1, 71–76. [Google Scholar]
- Norambuena, R. Recent Trends of Seaweed Production in Chile. Hydrobiologia 1996, 116, 371–379. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Moreira, W.S.C.; Carneiro, M.A.A. Some Aspects of the Growth of Gracilaria birdiae (Gracilariales, Rhodophyta) in an Estuary in Northeast Brazil. Aquac. Int. 2006, 14, 327–336. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Hernandez-Gonzalez, M.D.C.; Varela, D. Seaweed Future Cultivation in Chile: Perspectives and Challenges. Int. J. Environ. Pollut. 2008, 33, 432. [Google Scholar] [CrossRef]
- Marinho-Soriano, E. Historical Context of Commercial Exploitation of Seaweeds in Brazil. J. Appl. Phycol. 2017, 29, 665–671. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Prescott, S.; Potin, P.; Faugeron, S.; Vásquez, J.A.; Camus, C.; Infante, J.; Hernández-González, M.C.; Gutíerrez, A.; Varela, D.A. The Status of Kelp Exploitation and Marine Agronomy, with Emphasis on Macrocystis pyrifera, in Chile. In Sea Plants, Advances in botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, Chapter 6; pp. 161–188. [Google Scholar]
- Ebeling, A.W.; Laur, D.R. The Influence of Plant Cover on Surfperch Abundance at an Offshore Temperate Reef. Environ. Biol. Fishes 1985, 12, 169–179. [Google Scholar] [CrossRef]
- Bodkin, J.L. Effects of Kelp Forest Removal on Associated Fish Assemblages in Central California. J. Exp. Mar. Biol. Ecol. 1988, 117, 227–238. [Google Scholar] [CrossRef]
- Vanella, F.A.; Fernández, D.A.; Carolina Romero, M.; Calvo, J. Changes in the Fish Fauna Associated with a Sub-Antarctic Macrocystis Pyrifera Kelp Forest in Response to Canopy Removal. Polar Biol. 2007, 30, 449–457. [Google Scholar] [CrossRef]
- Moreno, C.A.; Jara, F. Ecological Studies on Fish Fauna Associated with Macrocystis pyrifera Belts in the South of Fueguian Islands, Chile. Mar. Ecol. Prog. Ser. Oldendorf 1984, 15, 99–107. [Google Scholar] [CrossRef]
- Bularz, B.; Fernández, M.; Subida, M.D.; Wieters, E.A.; Pérez-Matus, A. Effects of Harvesting on Subtidal Kelp Forests ( Lessonia Trabeculata ) in Central Chile. Ecosphere 2022, 13, e3958. [Google Scholar] [CrossRef]
- Ugarte, R.A. An Evaluation of the Mortality of the Brown Seaweed Ascophyllum nodosum (L.) Le Jol. Produced by Cutter Rake Harvests in Southern New Brunswick, Canada. J. Appl. Phycol. 2011, 23, 401–407. [Google Scholar] [CrossRef]
- Vandermeulen, H. Information to Support Assessment of Stock Status of Commercially Harvested Species of Marine Plants in Nova Scotia: Irish Moss, Rockweed and Kelp; Canadian Science Advisory Secretariat. 2013. Available online: https://waves-vagues.dfo-mpo.gc.ca/Library/349705.pdf (accessed on 1 July 2014).
- Lorentsen, S.-H.; Sjøtun, K.; Grémillet, D. Multi-Trophic Consequences of Kelp Harvest. Biol. Conserv. 2010, 143, 2054–2062. [Google Scholar] [CrossRef]
- Christensen-Dalsgaard, S.; Mattisson, J.; Norderhaug, K.M.; Lorentsen, S.-H. Sharing the Neighbourhood: Assessing the Impact of Kelp Harvest on Foraging Behaviour of the European Shag. Mar. Biol. 2020, 167, 136. [Google Scholar] [CrossRef]
- Norderhaug, K.; Filbee-Dexter, K.; Freitas, C.; Birkely, S.; Christensen, L.; Mellerud, I.; Thormar, J.; van Son, T.; Moy, F.; Vázquez Alonso, M.; et al. Ecosystem-Level Effects of Large-Scale Disturbance in Kelp Forests. Mar. Ecol. Prog. Ser. 2020, 656, 163–180. [Google Scholar] [CrossRef]
- Seitz, R.D.; Wennhage, H.; Bergström, U.; Lipcius, R.N.; Ysebaert, T. Ecological Value of Coastal Habitats for Commercially and Ecologically Important Species. ICES J. Mar. Sci. 2014, 71, 648–665. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R1107 (accessed on 6 May 2021).
- Anon. Report of the Comptroller and Auditor General of India, for the Year Ended March 2017. Union Government (Department of Revenue—Customs. Compliance Audit No.41 of 2017). 2017. Available online: https://cag.gov.in/uploads/download_audit_report/2017/Report_No.41_of_2017_-_Compliance_Audit_on_Department_of_Revenue_%E2%80%93_Customs_Union_Government.pdf (accessed on 28 January 2022).
- Anon. Caltec Ag, Inc. v. Department of Pesticide Regulation et al. Court of Appeal of the State of California, Fifth Appellate District. Court of Appeal Case No.: F074334; Super. Ct. No.: 2016497. 2019. Available online: https://www.courts.ca.gov/opinions/archive/F074334.PDF (accessed on 27 January 2022).
- Khan, W.; Hiltz, D.; Critchley, A.T.; Prithiviraj, B. Bioassay to Detect Ascophyllum nodosum Extract-Induced Cytokinin-like Activity in Arabidopsis Thaliana. J. Appl. Phycol. 2011, 23, 409–414. [Google Scholar] [CrossRef]
- Wally, O.S.D.; Critchley, A.T.; Hiltz, D.; Craigie, J.S.; Han, X.; Zaharia, L.I.; Abrams, S.R.; Prithiviraj, B. Regulation of Phytohormone Biosynthesis and Accumulation in Arabidopsis Following Treatment with Commercial Extract from the Marine Macroalga Ascophyllum nodosum. J. Plant Growth Regul. 2013, 32, 324–339. [Google Scholar] [CrossRef]
- Rayorath, P.; Khan, W.; Palanisamy, R.; MacKinnon, S.L.; Stefanova, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Extracts of the Brown Seaweed Ascophyllum nodosum Induce Gibberellic Acid (GA3)-Independent Amylase Activity in Barley. J. Plant Growth Regul. 2008, 27, 370–379. [Google Scholar] [CrossRef]
- Akazawa, T.; Hara-Nishimura, I. Topographic Aspects of Biosynthesis, Extracellular Secretion, and Intracellular Storage of Proteins in Plant Cells. Annu. Rev. Plant Physiol. 1985, 36, 441–472. [Google Scholar] [CrossRef]
- Beck, E.; Ziegler, P. Biosynthesis and Degradation of Starch in Higher Plants. Annu. Rev. Plant Biol. 1989, 40, 95–117. [Google Scholar] [CrossRef]
- Sun, T.; Gubler, F. Molecular Mechansim of Gibberellin Signaling in Plants. Annu. Rev. Plant Biol. 2004, 55, 197–223. [Google Scholar] [CrossRef]
- Woodger, F.; Jacobsen, J.V.; Gubler, F. Gibberellin Action in Germinated Cereal Grains. In Plant Hormones; Springer: Dordrecht, The Netherlands, 2010; pp. 221–240. [Google Scholar]
- Ghaderiardakani, F.; Collas, E.; Damiano, D.K.; Tagg, K.; Graham, N.S.; Coates, J.C. Effects of Green Seaweed Extract on Arabidopsis Early Development Suggest Roles for Hormone Signalling in Plant Responses to Algal Fertilisers. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Rasul, F.; Gupta, S.; Olas, J.J.; Gechev, T.; Sujeeth, N.; Mueller-Roeber, B. Priming with a Seaweed Extract Strongly Improves Drought Tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1469. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.; Cotas, J.; Rocha, C.P.; Araújo, G.S.; Figueirinha, A.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweeds’ Carbohydrate Polymers as Plant Growth Promoters. Carbohydr. Polym. Technol. Appl. 2021, 2, 100097. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates From Seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–274. [Google Scholar]
- Hien, N.Q.; Nagasawa, N.; Tham, L.X.; Yoshii, F.; Dang, V.H.; Mitomo, H.; Makuuchi, K.; Kume, T. Growth-Promotion of Plants with Depolymerized Alginates by Irradiation. Radiat. Phys. Chem. 2000, 59, 97–101. [Google Scholar] [CrossRef]
- Iwasaki, K.; Matsubara, Y. Purification of Alginate Oligosaccharides with Root Growth-Promoting Activity toward Lettuce. Biosci. Biotechnol. Biochem. 2000, 64, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Iwamoto, Y.; Kitamura, Y.; Oda, T.; Muramatsu, T. Root Growth-Promoting Activity of Unsaturated Oligomeric Uronates from Alginate on Carrot and Rice Plants. Biosci. Biotechnol. Biochem. 2003, 67, 2022–2025. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed Oligosaccharides Stimulate Plant Growth by Enhancing Carbon and Nitrogen Assimilation, Basal Metabolism, and Cell Division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- De Saeger, J.; van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the Molecular Understanding of the Action Mechanism of Ascophyllum nodosum Extracts on Plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A Commercial Extract of Brown Macroalga (Ascophyllum nodosum) Affects Yield and the Nutritional Quality of Spinach in Vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Brassica Napus Growth Is Promoted by Ascophyllum nodosum (L.) Le Jol. Seaweed Extract: Microarray Analysis and Physiological Characterization of N, C, and S Metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Gechev, T.; Petrov, V. Reactive Oxygen Species and Abiotic Stress in Plants. Int. J. Mol. Sci. 2020, 21, 7433. [Google Scholar] [CrossRef]
- Omidbakhshfard, M.A.; Sujeeth, N.; Gupta, S.; Omranian, N.; Guinan, K.J.; Brotman, Y.; Nikoloski, Z.; Fernie, A.R.; Mueller-Roeber, B.; Gechev, T.S. A Biostimulant Obtained from the Seaweed Ascophyllum nodosum Protects Arabidopsis thaliana from Severe Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 474. [Google Scholar] [CrossRef] [PubMed]
- Huijser, P.; Schmid, M. The Control of Developmental Phase Transitions in Plants. Development 2011, 138, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Beckett, R.P.; van Staden, J. The Effect of Seaweed Concentrate on the Growth and Yield of Potassium Stressed Wheat. Plant Soil 1989, 116, 29–36. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Cytokinin-Containing Seaweed and Humic Acid Extracts Associated with Creeping Bentgrass Leaf Cytokinins and Drought Resistance. Crop Sci. 2004, 44, 1737–1745. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Impact of Seaweed Extract-Based Cytokinins and Zeatin Riboside on Creeping Bentgrass Heat Tolerance. Crop Sci. 2008, 48, 364–370. [Google Scholar] [CrossRef]
- Sako, K.; Nguyen, H.M.; Seki, M. Advances in Chemical Priming to Enhance Abiotic Stress Tolerance in Plants. Plant Cell Physiol. 2021, 61, 1995–2003. [Google Scholar] [CrossRef]
- Llorens, E.; González-Hernández, A.I.; Scalschi, L.; Fernández-Crespo, E.; Camañes, G.; Vicedo, B.; García-Agustín, P. Priming Mediated Stress and Cross-Stress Tolerance in Plants: Concepts and Opportunities. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–20. [Google Scholar]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting Ready for Battle. Mol. Plant-Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and Chromatin-Based Mechanisms in Environmental Stress Adaptation and Stress Memory in Plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming Crops for the Future: Rewiring Stress Memory. Trends Plant Sci. 2021, 27, 699–716. [Google Scholar] [CrossRef]
- Staykov, N.S.; Angelov, M.; Petrov, V.; Minkov, P.; Kanojia, A.; Guinan, K.J.; Alseekh, S.; Fernie, A.R.; Sujeeth, N.; Gechev, T.S. An Ascophyllum nodosum-Derived Biostimulant Protects Model and Crop Plants from Oxidative Stress. Metabolites 2020, 11, 24. [Google Scholar] [CrossRef]
- Di Stasio, E.; Cirillo, V.; Raimondi, G.; Giordano, M.; Esposito, M.; Maggio, A. Osmo-Priming with Seaweed Extracts Enhances Yield of Salt-Stressed Tomato Plants. Agronomy 2020, 10, 1559. [Google Scholar] [CrossRef]
- Nair, P.; Kandasamy, S.; Zhang, J.; Ji, X.; Kirby, C.; Benkel, B.; Hodges, M.D.; Critchley, A.T.; Hiltz, D.; Prithiviraj, B. Transcriptional and Metabolomic Analysis of Ascophyllum nodosum Mediated Freezing Tolerance in Arabidopsis Thaliana. BMC Genom. 2012, 13, 643. [Google Scholar] [CrossRef] [PubMed]
- Santaniello, A.; Scartazza, A.; Gresta, F.; Loreti, E.; Biasone, A.; di Tommaso, D.; Piaggesi, A.; Perata, P. Ascophyllum nodosum Seaweed Extract Alleviates Drought Stress in Arabidopsis by Affecting Photosynthetic Performance and Related Gene Expression. Front. Plant Sci. 2017, 8, 1362. [Google Scholar] [CrossRef] [PubMed]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Di Stasio, E.; van Oosten, M.J.; Silletti, S.; Raimondi, G.; Carillo, P.; Maggio, A. Ascophyllum nodosum-Based Algal Extracts Act as Enhancers of Growth, Fruit Quality, and Adaptation to Stress in Salinized Tomato Plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Carillo, P.; Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Dell’Aversana, E.; D’Amelia, L.; Colla, G.; Caruso, G.; de Pascale, S.; Rouphael, Y. Sensory and Functional Quality Characterization of Protected Designation of Origin ‘Piennolo Del Vesuvio’Cherry Tomato Landraces from Campania-Italy. Food Chem. 2019, 292, 166–175. [Google Scholar] [CrossRef]
- Goñi, O.; Łangowski, Ł.; Feeney, E.; Quille, P.; O’Connell, S. Reducing Nitrogen Input in Barley Crops While Maintaining Yields Using an Engineered Biostimulant Derived from Ascophyllum nodosum to Enhance Nitrogen Use Efficiency. Front. Plant Sci. 2021, 12, 789. [Google Scholar] [CrossRef]
- Durand, N.; Briand, X.; Meyer, C. The Effect of Marine Bioactive Substances (N PRO) and Exogenous Cytokinins on Nitrate Reductase Activity in Arabidopsis thaliana. Physiol. Plant. 2003, 119, 489–493. [Google Scholar] [CrossRef]
- Billard, V.; Etienne, P.; Jannin, L.; Garnica, M.; Cruz, F.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Two Biostimulants Derived from Algae or Humic Acid Induce Similar Responses in the Mineral Content and Gene Expression of Winter Oilseed Rape (Brassica napus L.). J. Plant Growth Regul. 2014, 33, 305–316. [Google Scholar] [CrossRef]

| No. | Country | Category | Tonnes (Live Weight) | |||||
|---|---|---|---|---|---|---|---|---|
| Brown Seaweed | Red Seaweed | Green Seaweed | Seaweed Nei | Aquatic Plants Nei | Total | |||
| 1 | Chile | † Bull kelp (Durvillaea antarctica), Lessonia trabeculata, Chilean kelp (Lessonia nigrescens), Giant kelps nei, (e.g., Macrocystis pyrifera). ‡ Chondracanthus chamissoi, Gymnogongrus furcellatus, Mazzaella laminarioides, Gelidium spp. (Gelidiaceae), Gracilaria spp. (Gracilariaceae), Leister (Sarcothalia crispate), Nori nei (Porphyra spp.), Skottsberg’s Gigartina (Gigartina skottsbergii). # Aquatic plants nei. | 288,486.00 | 115,973.00 | 467.00 | 404,926.00 | ||
| 2 | China | # Aquatic plants nei | 174,450.00 | 174,450.00 | ||||
| 3 | Norway | † Babberlocks (Alaria esculenta), Brown seaweeds (general), North Atlantic rockweed (Ascophyllum nodosum), North European kelp (Laminaria hyperborea). * Gut weed (Ulva intestinalis). | 162,824.00 | 128.00 | 162,952.00 | |||
| 4 | Japan | † Japanese kelp (Laminaria japonica). # Aquatic plants nei. | 46,500.00 | 20,300.00 | 66,800.00 | |||
| 5 | France | † North European kelp (Laminaria hyperborea), Tangle (Laminaria digitata), Sea thong (Himanthalia elongata). ‡ Dulse (Palmaria palmata), Giant gelidium (Gelidium corneum), Red seaweeds (general). # Other Seaweeds nei. | 51,141.92 | 158.12 | 0.01 | 51,300.05 | ||
| 6 | Indonesia | ‡ Red seaweeds (general). | 44,500.00 | 44,500.00 | ||||
| 7 | Peru | † Lessonia trabeculata, Chilean kelp (Lessonia nigrescens), Giant kelp (Macrocystis pyrifera). ‡ Chondracanthus chamissoi. | 34,836.78 | 1511.00 | 36,347.78 | |||
| 8 | Ireland | † North Atlantic rockweed (Ascophyllum nodosum), North European kelp (Laminaria hyperborea). ‡ Red seaweeds (general). | 29,400.00 | 100.00 | 29,500.00 | |||
| 9 | India | † Brown seaweeds (general) * Green seaweeds (general). ‡ Red seaweeds (general). | 3219.28 | 4136.78 | 11,043.93 | 18,399.99 | ||
| 10 | Iceland | † North Atlantic rockweed (Ascophyllum nodosum), North European kelp (Laminaria hyperborea), Tangle (Laminaria digitata). | 17,533.00 | 17,533.00 | ||||
| 11 | Morocco | ‡ Red seaweeds (general). | 17,317.71 | 17,317.71 | ||||
| 12 | Canada | † North Atlantic rockweed (Ascophyllum nodosum). | 12,655.00 | 12,655.00 | ||||
| 13 | South Africa | † Brown seaweeds (general). ‡ Gelidium spp. (Gelidiaceae). | 8265.00 | 735.00 | 9000.00 | |||
| 14 | Russia | † North European kelp (Laminaria hyperborea), Brown seaweeds (general). ‡ Red seaweeds (general). # Aquatic plants nei. | 8968.00 | 1.00 | 2.00 | 8971.00 | ||
| 15 | Rep. of Korea | † Japanese kelp, Wakame (Undaria pinnatifida), Brown seaweeds (general). ‡ Gracilaria spp. (Gracilariaceae), Laver (Nori, Porphyra tenera). * Fragile codium (Codium fragile), Green laver (Monostroma nitidum). # Other Aquatic plants nei. | 4290.00 | 75.00 | 1060.00 | 3285.00 | 8710.00 | |
| 16 | Mexico | † Brown seaweeds (general). ‡ Red seaweeds (general). | 5291.23 | 2034.41 | 7325.64 | |||
| 17 | Spain | † Wakame (Undaria pinnatifida), Brown seaweeds (general). ‡ Gelidium spp. (Gelidiaceae), Red seaweeds (general), Ribboned nori (Porphyra linearis). * Green seaweeds (general). # Seaweeds nei. | 314.90 | 242.29 | 0.01 | 2595.09 | 3152.29 | |
| 18 | USA | † Giant kelps nei, (e.g., Macrocystis pyrifera). * Green seaweeds (general). | 6.00 | 3125.00 | 3131.00 | |||
| 19 | Australia | † Brown seaweeds (general). | 1923.00 | 1923.00 | ||||
| 20 | Italy | ‡ Red seaweeds (general). * Green seaweeds (general). | 400.00 | 800.00 | 1200.00 | |||
| 21 | Portugal | ‡ Red seaweeds (general). | 1111.49 | 1111.49 | ||||
| 22 | Madagascar | ‡ Red seaweeds (general). | 800.00 | 800.00 | ||||
| 23 | New Zealand | ‡ Pterocladia lucida. * Sea lettuces nei. # Seaweeds nei. | 0.64 | 0.01 | 508.81 | 509.46 | ||
| 24 | Philippines | ‡ Red seaweeds (general) | 364.53 | 364.53 | ||||
| 25 | Taiwan | ‡ Gelidium spp. (Gelidiaceae), Laver (Nori; Porphyra tenera). * Lacy sea lettuce (Ulva pertusa). # Aquatic plants nei. | 116.71 | 73.13 | 112.64 | 302.48 | ||
| 26 | Estonia | ‡ Red seaweeds (general). | 60.00 | 60.00 | ||||
| Total | 675,654.11 | 189,637.68 | 16,230.08 | 3,103.91 | 198,616.64 | 1,083,242.42 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sujeeth, N.; Petrov, V.; Guinan, K.J.; Rasul, F.; O’Sullivan, J.T.; Gechev, T.S. Current Insights into the Molecular Mode of Action of Seaweed-Based Biostimulants and the Sustainability of Seaweeds as Raw Material Resources. Int. J. Mol. Sci. 2022, 23, 7654. https://doi.org/10.3390/ijms23147654
Sujeeth N, Petrov V, Guinan KJ, Rasul F, O’Sullivan JT, Gechev TS. Current Insights into the Molecular Mode of Action of Seaweed-Based Biostimulants and the Sustainability of Seaweeds as Raw Material Resources. International Journal of Molecular Sciences. 2022; 23(14):7654. https://doi.org/10.3390/ijms23147654
Chicago/Turabian StyleSujeeth, Neerakkal, Veselin Petrov, Kieran J. Guinan, Fiaz Rasul, John T. O’Sullivan, and Tsanko S. Gechev. 2022. "Current Insights into the Molecular Mode of Action of Seaweed-Based Biostimulants and the Sustainability of Seaweeds as Raw Material Resources" International Journal of Molecular Sciences 23, no. 14: 7654. https://doi.org/10.3390/ijms23147654
APA StyleSujeeth, N., Petrov, V., Guinan, K. J., Rasul, F., O’Sullivan, J. T., & Gechev, T. S. (2022). Current Insights into the Molecular Mode of Action of Seaweed-Based Biostimulants and the Sustainability of Seaweeds as Raw Material Resources. International Journal of Molecular Sciences, 23(14), 7654. https://doi.org/10.3390/ijms23147654






