Vesicular and Planar Membranes of Archaea Lipids: Unusual Physical Properties and Biomedical Applications
Abstract
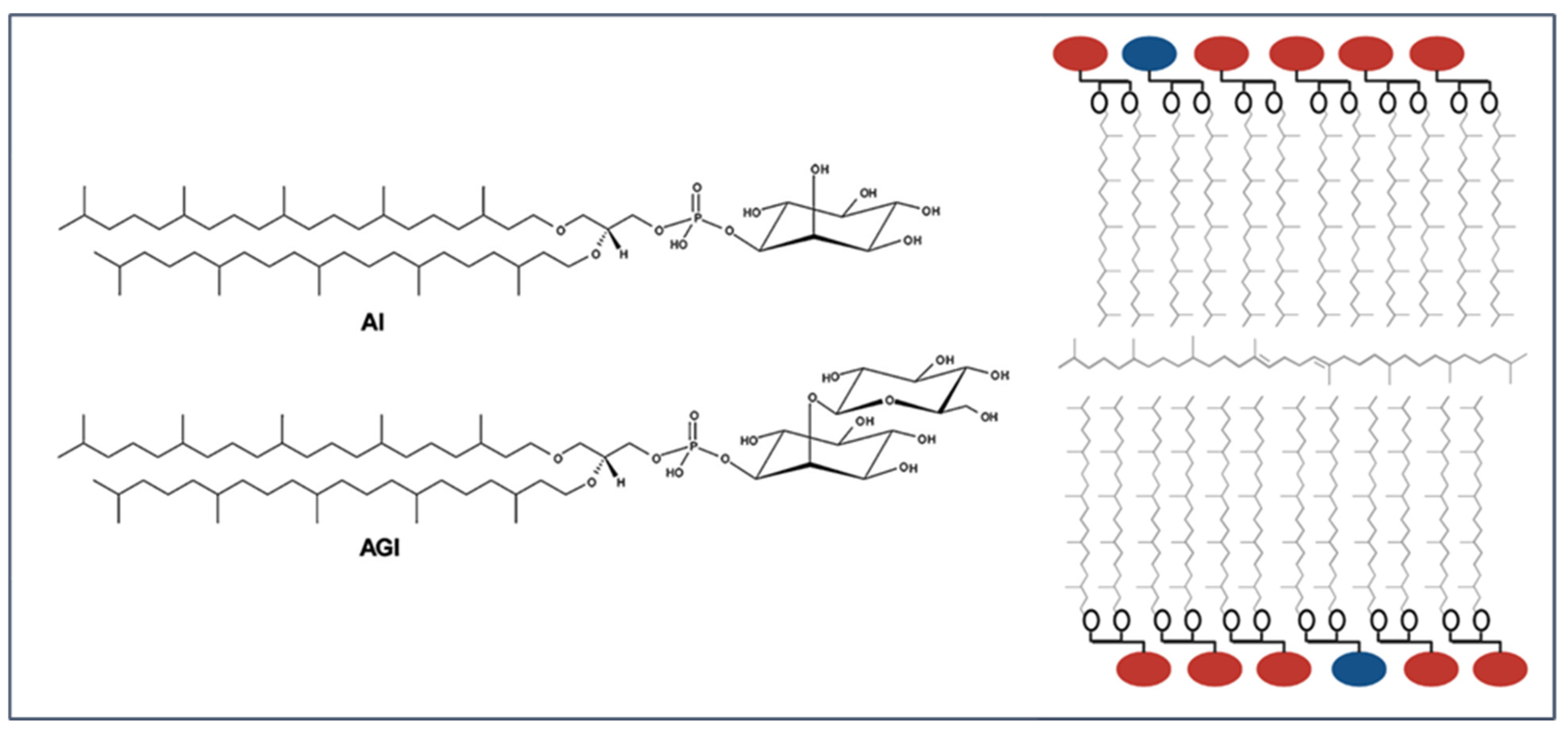
1. Vesicular Archaea Lipid Membranes (Archaeosomes)
1.1. Formation and Characterization of Archaeosomes
1.2. Stability and Cytotoxicity of Archaeosomes
1.2.1. In Vitro Stability of Tetraether Archaeosomes
1.2.2. In Vitro Stability of Diether Archaeosomes
1.2.3. Other Remarks about In Vitro Archaeosome Stability
1.2.4. Archaeosome Cytotoxicity
1.3. Archaeosomes as Nanocarriers of Therapeutics
1.3.1. Tetraether Archaeosomes for Oral Delivery
1.3.2. Tetraether Archaeosomes for Intravenous Delivery
1.4. Archaeosomes That Can Conduct Controlled Release and Target Delivery
1.4.1. Archaeosomal Photosensitizer
1.4.2. Thermosensitive Archaeosomes
1.5. Archaeosomes as Vaccine Adjuvants
1.5.1. Conventional Liposomal Vaccines
1.5.2. Archaeosomal Vaccines
2. Archaeal Planar Membranes
2.1. Free-Standing Archaea Lipid Planar Membranes
2.1.1. Stability
2.1.2. Possible Applications
2.2. Solid-Supported Planar Membranes
2.3. Archaea Lipid Planar Membranes as Novel Coating Materials on Biomedical Devices
3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGI | 2:3-di-O-sesterterpanyl-sn-glycerol-1-phospho-1’-(2’-O-α-D-glucosyl)-myo-inositol (C25,25-archaetidyl (glucosyl) inositol) |
| AI | 2,3-di-O-sesterterpanyl-sn-glycerol-1-phospho-myo-inositol (C25,25-archaetidylinositol) |
| APC | antigen presenting cells |
| BLM | black lipid membranes |
| CAM | chicken chorioallantoic membrane |
| CA4P | combretastatin A4 disodium phosphate |
| CPP | cell penetrating peptide |
| DoPhPC | 1,2-di-O-phytanyl-sn-glycero-3- phosphocholine |
| DoPhPE | 1,2-di-O-phytanyl-sn-glycero-3-phosphoethanolamine |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane |
| DOX | doxorubicin |
| DPPC | dipalmitoyl-sn-glycerol-3-phosphocholine |
| DSPC | distearoyl-sn-glycerol-3-phosphocholine |
| EPC | egg phosphatidylcholine |
| GCTE | glyceryl caldityl tetraether |
| GDGT | glycerol dialky glycerol tetraether (caldarchaeols) |
| GDNT | glycerol dialky calditol tetraether (calditolglycerocaldarchaeol) |
| GTGT | glycerol trialkyl glycerol tetraether |
| GUV | giant unilamellar vesicles |
| hGDNT | hydrolysed glycerol-dialkyl-nonitol tetraether |
| Laurdan | 6-dodecanoyl-2-dimethylaminonaphthalene |
| PDMS | polydimethylsiloxane |
| PLMF | the polar lipid methanol fraction |
| PLFE | the polar lipid fraction E |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| PS | phosphoserine |
| RES | the reticuloendothelial system |
| SLA | sulfated lactosylarchaeol |
| TPL | total polar lipids |
| TL | total lipids |
| TSL | thermosensitive liposomes |
References
- Gliozzi, A.; Relini, A.; Chong, P.L.-G. Structure and permeability properties of biomimetic membranes of bolaform archaeal tetraether lipids. J. Membr. Sci. 2002, 206, 131–147. [Google Scholar] [CrossRef]
- Jacquemet, A.; Barbeau, J.; Lemiegre, L.; Benvegnu, T. Archaeal tetraether bipolar lipids: Structures, functions and applications. Biochimie 2009, 91, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.-G. Archaebacterial bipolar tetraether lipids: Physico-chemical and membrane properties. Chem. Phys. Lipids 2010, 163, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Schouten, S.; Hopmans, E.C.; Sinninghe Damste, J.S. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review. Org. Geochem. 2013, 54, 19–61. [Google Scholar] [CrossRef]
- Caforio, A.; Driessen, A.J.M. Archaeal phospholipids: Structural properties and biosynthesis. Biochim. Biophys. Acta 2017, 1862, 1325–1339. [Google Scholar] [CrossRef]
- Thompson, D.H.; Wong, K.F.; Humphry-Baker, R.; Wheeler, J.; Kim, J.M.; Rananavare, S.B. Tetraether bolaform amphiphiles as models of archaebacterial membrane lipids: Raman spectroscopy, 31P NMR, X-ray scattering, and electron microscopy. J. Am. Chem. Soc. 1992, 114, 9035–9042. [Google Scholar] [CrossRef]
- Benvegnu, T.; Lemiègre, L.; Cammas-Marion, S. Archaeal lipids: Innovative materials for biotechnological applications. Eur. J. Org. Chem. 2008, 2008, 4725–4744. [Google Scholar] [CrossRef]
- Kriegler, S.P.; Paulisch, T.O.; Wegner, T.; Glorius, F.; Winter, R. Bipolar imidazolium-based lipid analogues for artificial archaeosomes. Langmuir 2021, 37, 11996–12006. [Google Scholar] [CrossRef]
- Elferink, M.G.; de Wit, J.G.; Demel, R.; Driessen, A.J.; Konings, W.N. Functional reconstitution of membrane proteins in monolayer liposomes from bipolar lipids of Sulfolobus acidocaldarius. J. Biol. Chem. 1992, 267, 1375–1381. [Google Scholar] [CrossRef]
- Galimzyanov, T.R.; Kuzmin, P.I.; Pohl, P.; Akimov, S.A. Elastic deformations of bolalipid membranes. Soft Matter 2016, 12, 2357–2364. [Google Scholar] [CrossRef][Green Version]
- Hanford, J.H.; Peeples, T.L. Archaeal tetraether lipids, unique structures and applications. Appl. Biochem. Biotechnol. 2002, 97, 45–62. [Google Scholar] [CrossRef]
- Krishnan, L.; Sprott, G.D. Archaeosome adjuvants: Immunological capabilities and mechanism(s) of action. Vaccine 2008, 26, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.L.; Chang, E.L. Purification and characterization of a liposomal-forming tetraether lipid fraction. Biochem. Biophys. Res. Commun. 1990, 167, 238–243. [Google Scholar] [CrossRef]
- Morii, H.; Yagi, H.; Akutsu, H.; Nomura, N.; Sako, Y.; Koga, Y. A novel phosphoglycolipid archaetidyl(glucosyl)inositol with two sesterterpanyl chains from the aerobic hyperthermophilic archaeon Aeropyrum pernix K1. Biochim. Biophys. Acta 1999, 1436, 426–436. [Google Scholar] [CrossRef]
- Chang, E.L. Unusual thermal stability of liposomes made from bipolar tetraether lipids. Biochem. Biophys. Res. Commun. 1994, 202, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Ayesa, U.; Chong, P.L.-G. Polar lipid fraction E from Sulfolobus acidocaldarius and dipalmitoylphosphatidylcholine can form stable yet thermo-sensitive tetraether/diester hybrid archaeosomes with controlled release capability. Int. J. Mol. Sci. 2020, 21, 8388. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Helm, F.; Hofhaus, G.; Brings, S.; Kaufman, C.; Leotta, K.; Urban, S.; Haberkorn, U.; Mier, W.; Fricker, G. A liposomal formulation for the oral application of the investigational hepatitis B drug Myrcludex B. Eur. J. Pharm. Biopharm. 2016, 103, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, J.; Thewes, B.; Gropp, F.; Fricker, G. Oral peptide delivery by tetraether lipid liposomes. Int. J. Pharm. 2011, 415, 150–157. [Google Scholar] [CrossRef]
- Parmentier, J.; Becker, M.M.M.; Heintz, U.; Fricker, G. Stability of liposomes containing bio-enhancers and tetraether lipids in simulated gastro-intestinal fluids. Int. J. Pharm. 2011, 405, 210–217. [Google Scholar] [CrossRef]
- Brown, D.A.; Venegas, B.; Cooke, P.H.; English, V.; Chong, P.L.-G. Bipolar tetraether archaeosomes exhibit unusual stability against autoclaving as studied by dynamic light scattering and electron microscopy. Chem. Phys. Lipids 2009, 159, 95–103. [Google Scholar] [CrossRef]
- Cario, A.; Grossi, V.; Schaeffer, P.; Oger, P.M. Membrane homeoviscous adaptation in the piezo-hyperthermophilic archaeon Thermococcus barophilus. Front. Microbiol. 2015, 6, 1152. [Google Scholar] [CrossRef] [PubMed]
- LoRicco, J.G.; Salvador-Castell, M.; Deme, B.; Peters, J.; Oger, P.M. Apolar polyisoprenoids located in the midplane of the bilayer regulate the response of an archaeal-like membrane to high temperature and pressure. Front. Chem. 2020, 8, 594039. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Castell, M.; Demé, B.; Oger, P.; Peters, J. Structural characterization of an archaeal lipid bilayer as a function of hydration and temperature. Int. J. Mol. Sci. 2020, 21, 1816. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Castell, M.; Tourte, M.; Oger, P.M. In search for the membrane regulators of archaea. Int. J. Mol. Sci. 2019, 20, 4434. [Google Scholar] [CrossRef]
- Gmajner, D.; Ota, A.; Sentjurc, M.; Ulrih, N.P. Stability of diether C25,25 liposomes from the hyperthermophilic archaeon Aeropyrum pernix K1. Chem. Phys. Lipids 2011, 164, 236–245. [Google Scholar] [CrossRef]
- Plenagl, N.; Duse, L.; Seitz, B.S.; Goergen, N.; Pinnapireddy, S.R.; Jedelska, J.; Brubler, J.; Bakowsky, U. Photodynamic therapy—Hypericin tetraether liposome conjugates and their antitumor and antiangiogenic activity. Drug Deliv. 2019, 26, 23–33. [Google Scholar] [CrossRef]
- Komatsu, H.; Chong, P.L.-G. Low permeability of liposomal membranes composed of bipolar tetraether lipids from thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biochemistry 1998, 37, 107–115. [Google Scholar] [CrossRef]
- Bagatolli, L.; Gratton, E.; Khan, T.K.; Chong, P.L.-G. Two-photon fluorescence microscopy studies of bipolar tetraether giant liposomes from thermoacidophilic archaebacteria Sulfolobus acidocaldarius. Biophys. J. 2000, 79, 416–425. [Google Scholar] [CrossRef]
- Rezelj, S.K.; Kozorog, M.; Švigelj, T.; Ulrih, N.P.; Žnidaršič, N.; Podobnik, M.; Anderluh, G. Cholesterol enriched archaeosomes as a molecular system for studying interactions of cholesterol-dependent cytolysins with membranes. J. Membr. Biol. 2018, 251, 491–505. [Google Scholar] [CrossRef]
- Kanichay, R.; Boni, L.T.; Cooke, P.H.; Khan, T.K.; Chong, P.L.-G. Calcium-induced aggregation of archaeal bipolar tetraether liposomes derived from thermoacidophilic archaeon Sulfolobus acidocaldarius. Archaea 2003, 1, 175–183. [Google Scholar] [CrossRef]
- Dobro, M.J.; Samson, R.Y.; Yu, Z.; McCullough, J.; Ding, H.J.; Chong, P.L.-G.; Bell, S.D.; Jensen, G.J. Electron cryotomography of ESCRT assemblies and dividing Sulfolobus cells suggests that spiraling filaments are involved in membrane scission. Mol. Biol. Cell 2013, 24, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.-G.; Zein, M.; Khan, T.K.; Winter, R. Structure and conformation of bipolar tetraether lipid membranes derived from thermoacidophilic archaeon Sulfolobus acidocaldarius as revealed by small-angle X-ray scattering and high pressure FT-IR spectroscopy. J. Phys. Chem. 2003, 107, 8694–8700. [Google Scholar] [CrossRef]
- Salvador-Castell, M.; Golub, M.; Erwin, N.; Demé, B.; Brooks, N.J.; Winter, R.; Peters, J.; Oger, P.M. Characterisation of a synthetic Archeal membrane reveals a possible new adaptation route to extreme conditions. Commun. Biol. 2021, 4, 653. [Google Scholar] [CrossRef] [PubMed]
- Rethore, G.; Montier, T.; Le Gall, T.; Delepine, P.; Cammas-Marion, S.; Lemiegre, L.; Lehn, P.; Benvegnu, T. Archaeosomes based on synthetic tetraether-like lipids as novel versatile gene delivery systems. Chem. Commun. 2007, 20, 2054–2056. [Google Scholar] [CrossRef]
- Chong, P.L.-G.; Bonanno, A.; Ayesa, U. Dynamics and organization of archaeal tetraether lipid membranes. In Membrane Organization and Dynamics; Springer Series in Biophysics; Chattopadhyay, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 11–30. [Google Scholar]
- Daswani, V.P.; Ayesa, U.; Venegas, B.; Chong, P.L.-G. Concentration-induced J-aggregate formation causes a biphasic change in the release of trans-combretastatin A4 disodium phosphate from archaeosomes and the subsequent cytotoxicity on mammary cancer cells. Mol. Pharm. 2015, 12, 3724–3734. [Google Scholar] [CrossRef]
- Daswani, V.P.; Ayesa, U.; Chong, P.L.-G. The polar lipid fraction E from Sulfolobus acidocaldarius can be used as liposomal drug stabilizing agents to reduce the leakage of the antivascular drug combretastatin A4 disodium phosphate from tetraether/diester hybrid archaeosomes. Biophysica 2021, 1, 474–486. [Google Scholar] [CrossRef]
- Mahmoud, G.J.; Jedelská, J.; Oma, S.M.; Strehlow, B.; Schneider, M.; Bakowsky, U. Stabilized tetraether lipids based particles guided prophyrins photodynamic therapy. Drug Deliv. 2018, 25, 1526–1536. [Google Scholar] [CrossRef]
- Wang, X.; Lv, B.; Cai, G.; Fu, L.; Wu, Y.; Ren, B.; Ma, H. A proton shelter inspired by the sugar coating of acidophilic archaea. Sci. Rep. 2012, 2, 892. [Google Scholar] [CrossRef]
- Chong, P.L.-G.; Sulc, M.; Winter, R. Compressibilities and volume fluctuations of archaeal tetraether liposomes. Biophys. J. 2010, 99, 3319–3326. [Google Scholar] [CrossRef]
- Kaur, G.; Garg, T.; Rath, G.; Goyal, A.K. Archaeosomes: An excellent carrier for drug and cell delivery. Drug Delivery 2016, 23, 2497–2512. [Google Scholar] [CrossRef]
- Chugunov, A.O.; Volynsky, P.E.; Krylov, N.A.; Boldyrev, I.A.; Efremov, R.G. Liquid but durable: Molecular dynamics simulations explain the unique properties of archaeal-like membranes. Sci. Rep. 2014, 4, 7462. [Google Scholar] [CrossRef] [PubMed]
- Batishchev, O.V.; Alekseeva, A.S.; Tretyakova, D.S.; Galimzyanov, T.R.; Onishchenko, N.R.; Volynsky, P.E.; Boldyrev, I.A. Cyclopentane rings in hydrophobic chains of a phospholipid enhance the bilayer stability to electric breakdown. Soft Matter 2019, 16, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Elferink, M.G.; de Wit, J.G.; Driessen, A.J.; Konings, W.N. Stability and proton-permeability of liposomes composed of archaeal tetraether lipids. Biochim. Biophys. Acta 1994, 1193, 247–254. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Chong, P.L.-G. Molecular modeling of archaebacterial bipolar tetraether lipid membranes. Chem. Phys. Lipids 2000, 105, 193–200. [Google Scholar] [CrossRef]
- Choquet, C.G.; Patel, G.B.; Sprott, G.D. Heat sterilization of archaeal liposomes. Can. J. Microbiol. 1996, 42, 183–186. [Google Scholar] [CrossRef]
- Falck, E.; Patra, M.; Karttunen, M.; Hyvonen, M.T.; Vattulainen, I. Impact of cholesterol on voids in phospholipid membranes. J. Chem. Phys. 2004, 121, 12676–12689. [Google Scholar] [CrossRef]
- Relini, A.; Cassinadri, D.; Mirghani, Z.; Brandt, O.; Gambacorta, A.; Trincone, A.; De Rosa, M.; Gliozzi, A. Calcium-induced interaction and fusion of archaeobacterial lipid vesicles: A fluorescence study. Biochim. Biophys. Acta 1994, 1194, 17–24. [Google Scholar] [CrossRef]
- Relini, A.; Cassinadri, D.; Fan, Q.; Gulik, A.; Mirghani, Z.; De Rosa, M.; Gliozzi, A. Effect of physical constraints on the mechanisms of membrane fusion: Bolaform lipid vesicles as model systems. Biophys. J. 1996, 71, 1789–1795. [Google Scholar] [CrossRef][Green Version]
- Sako, Y.; Nomura, N.; Uchida, A.; Ishida, Y.; Morii, H.; Koga, Y.; Hoaki, T.; Maruyama, T. Aeropyrum pernix gen. nov., sp. nov., a novel aerobic hyperthermophilic archaeon growing at temperatures up to 10 °C. Int. J. Syst. Bacteriol. 1996, 46, 1070–1077. [Google Scholar] [CrossRef]
- Hafenbradl, D.; Keller, M.; Stetter, K.O. Lipid analysis of Methanopyrus kandleri. FEMS Microbiol. 1996, 136, 199–202. [Google Scholar] [CrossRef]
- Polak, A.; Tarek, M.; Tomsic, M.; Valant, J.; Ulrih, N.P.; Jamnik, A.; Kramar, P.; Miklavcic, D. Structural properties of archaeal lipid bilayers: Small-angle X-ray scattering and molecular dynamics simulation study. Langmuir 2014, 30, 8308–8315. [Google Scholar] [CrossRef]
- Gmajner, D.; Grabnar, P.A.; Žnidarič, M.T.; Štrus, J.; Šentjurc, M.; Ulrih, N.P. Structural characterization of liposomes made of diether archaeal lipids and dipalmitoyl-L-α-phosphatidylcholine. Biophys. Chem. 2011, 158, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.B.; Agnew, B.J.; Deschatelets, L.A.; Fleming, L.P.; Sprott, G.D. In vitro assessment of archaeosome stability for developing oral delivery systems. Int. J. Pharm. 2000, 194, 39–49. [Google Scholar] [CrossRef]
- Barbeau, J.; Cammas-Marion, S.; Auvray, P.; Benvegnu, T. Preparation and characterization of stealth archaeosomes based on a synthetic PEGylated archaeal tetraether lipid. J. Drug Deliv. 2011, 2011, 396068. [Google Scholar] [CrossRef] [PubMed]
- Vitkova, V.M.; Mitkova, D.; Yordanova, V.; Pohl, P.; Bakowsky, U.; Staneva, G.; Batishchev, O. Elasticity and phase behaviour of biomimetic membrane systems containing tetraether archaeal lipids. Eng. Asp. 2020, 601, 124974. [Google Scholar] [CrossRef]
- Venegas, B.; Zhu, W.; Haloupek, N.B.; Lee, J.; Zellhart, E.; Sugar, I.P.; Kiani, M.; Chong, P.L.-G. Cholesterol supelattice modulates combretastatin A4 disodium phosphate (CA4P) release from liposomes and CA4P cytotoxicity on mammary cancer cells. Biophys. J. 2012, 102, 2086–2094. [Google Scholar] [CrossRef]
- Huang, J.; Buboltz, J.T.; Feigenson, G.W. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim. Biophys. Acta 1999, 1417, 89–100. [Google Scholar] [CrossRef]
- Chong, P.L.-G. Evidence for regular distribution of sterols in liquid crystalline phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA 1994, 91, 10069–10073. [Google Scholar] [CrossRef]
- Napotnik, T.B.; Valant, J.; Gmajner, D.; Passamonti, S.; Miklavcic, D.; Ulrih, N.P. Cytotoxicity and uptake of archaeosomes prepared from Aeropyrum pernix lipids. Hum. Exp. Toxicol. 2013, 32, 950–959. [Google Scholar] [CrossRef]
- Patel, G.B.; Omri, A.; Deschatelets, L.; Sprott, G.D. Safety of archaeosome adjuvants evaluated in a mouse model. J. Liposome Res. 2002, 12, 353–372. [Google Scholar] [CrossRef]
- Patel, G.B.; Sprott, G.D. Archaeobacterial ether lipid liposomes (archaeosomes) as novel vaccine and drug delivery systems. Crit. Rev. Biotechnol. 1999, 19, 317–357. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.B.; Ponce, A.; Zhou, H.; Chen, W. Safety of intranasally administered archaeal lipid mucosal vaccine adjuvant and delivery (AMVAD) vaccine in mice. Int. J. Toxicol. 2008, 27, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Omri, A.; Agnew, B.J.; Patel, G.B. Short-term repeated-dose toxicity profile of archaeosomes administrated to mice via intravenous and oral routes. Int. J. Toxicol. 2003, 22, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.C.; Jensen, S.M.; Fricker, G.; Brandl, M.; Treusch, A.H. Archaeal lipids in oral delivery of therapeutic peptides. Eur. J. Pharm. Sci. 2017, 108, 101–110. [Google Scholar] [CrossRef]
- Jensen, S.M.; Christensen, C.J.; Petersen, J.M.; Treusch, A.H.; Brandl, M. Liposomes containing lipids from Sulfolobus islandicus withstand intestinal bile salts: An approach for oral drug delivery? Int. J. Pharm. 2015, 493, 63–69. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Sun, W.; Xu, Y. Investigation of archaeosomes as carriers for oral delivery of peptides. Biochem. Biophys. Res. Commun. 2010, 394, 412–417. [Google Scholar] [CrossRef]
- Uhl, P.; Sauter, M.; Hertlein, T.; Witzigmann, D.; Laffleur, F.; Hofhaus, G.; Fidelj, V.; Tursch, A.; Özbek, S.; Hopke, E.; et al. Overcoming the mucosal barrier: Tetraether lipid-stabilized liposomal nanocarriers decorated with cell-penetrating peptides enable oral delivery of vancomycin. Adv. Ther. 2021, 4, 2000247. [Google Scholar] [CrossRef]
- Mahmoud, G.J.; Jedelská, J.; Strehlow, B.; Omar, S.; Schneider, M.; Bakowsky, U. Photo-responsive tetraether lipids based vesicles for prophyrin mediated vascular targeting and direct phototherapy. Colloids Surf. B Biointerfaces 2017, 159, 720–728. [Google Scholar] [CrossRef]
- Duse, L.; Pinnapireddy, S.R.; Strehlow, B.; Jedelská, J.; Bakowsky, U. Low level LED photodynamic therapy using curcumin loaded tetraether liposomes. Eur. J. Pharm. Biopharm. 2018, 126, 233–241. [Google Scholar] [CrossRef]
- Uhl, P.; Pantze, S.; Storck, P.; Parmentier, J.; Witzigmann, D.; Hofhaus, G.; Huwyler, J.; Mier, W.; Fricker, G. Oral delivery of vancomycin by tetraether lipid liposomes. Eur. J. Pharm. Sci. 2017, 108, 111–118. [Google Scholar] [CrossRef]
- Hernández-Caselles, T.; Villalaín, J.; Gómez-Fernández, J.C. Influence of liposome charge and composition on their interaction with human blood serum proteins. Mol. Cell Biochem. 1993, 120, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Zhaorigetu, S.; Rodriguez-Aguayo, C.; Sood, A.K.; Lopez-Berestein, G.; Walton, B.L. Delivery of negatively charged liposomes into the atherosclerotic plague of apolipoprotein E-deficient mouse aortic tissue. J. Liposome Res. 2014, 24, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Papahadjopoulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc. Natl. Acad. Sci. USA 1988, 85, 6949–6953. [Google Scholar] [CrossRef]
- Maruyama, K.; Unezaki, S.; Takahashi, N.; Iwatsuru, M. Enhanced delivery of doxorubicin to tumor by long-circulating thermosensitive liposomes and local hyperthermia. Biochim. Biophys. Acta—Biomembr. 1993, 1149, 209–216. [Google Scholar] [CrossRef]
- Papini, E.; Tavano, R.; Mancin, F. Opsonins and dysopsonins of nanoparticles: Facts, concepts, and methodological guidelines. Front. Immunol 2020, 11, 567365. [Google Scholar] [CrossRef]
- Mahmoud, G.; Jedelska, J.; Strehlow, B.; Bakowsky, U. Bipolar tetraether lipids derived from thermoacidophilic archaeon Sulfolobus acidocaldarius for membrane stabilization of chlorin e6 based liposomes for photodynamic therapy. Eur. J. Pharm. Biopharm. 2015, 95, 88–98. [Google Scholar] [CrossRef]
- Li, L.; ten Hagen, T.L.M.; Hossann, M.; Suess, R.; van Rhoon, G.C.; Eggermont, A.M.M.; Haemmerich, D.; Koning, G.A. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J. Control. Release 2013, 168, 142–150. [Google Scholar] [CrossRef]
- Ta, T.; Porter, T.M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef]
- Gray, M.D.; Lyon, P.C.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Focused ultrasound hyperthermia for targeted drug release from thermosensitive liposomes: Results from a phase I trial. Radiology 2019, 291, 232–238. [Google Scholar] [CrossRef]
- Ross, J.S.; Stagliano, N.E.; Donovan, M.J.; Breitbart, R.E.; Ginsburg, G.S. Atherosclerosis and cancer: Common molecular pathways of disease development and progression. Ann. N. Y. Acad. Sci. 2001, 947, 271–292. [Google Scholar] [CrossRef]
- Gaber, M.H.; Hong, K.; Huang, S.K.; Papahadjopoulos, D. Thermosensitive sterically stabilized liposomes: Formulation and in vitro studies on mechanism of doxorubicin release by bovine serum and human plasma. Pharm. Res. 1995, 12, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.G.; Mathieu, D.; Loudet, C.; Buchoux, S.; Dufourc, E.J. Plant sterols in “rafts”: A better way to regulate membrane thermal shocks. FASEB J. 2007, 21, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Cattel, L.; Ceruti, M.; Dosio, F. From conventional to stealth liposomes: A new frontier in cancer chemotherapy. Tumori 2003, 89, 237–249. [Google Scholar] [CrossRef]
- Sandstrom, M.C.; Ickenstein, L.M.; Mayer, L.D.; Edwards, K. Effects of lipid segregation and lysolipid dissociation on drug release from thermosensitive liposomes. J. Control. Release 2005, 107, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Banno, B.; Ickenstein, L.M.; Chiu, G.N.; Bally, M.B.; Thewalt, J.; Brief, E.; Wasan, E.K. The functional roles of poly(ethylene glycol)-lipid and lysolipid in the drug retention and release from lysolipid-containing thermosensitive liposomes in vitro and in vivo. J. Pharm. Sci. 2010, 99, 2295–2308. [Google Scholar] [CrossRef] [PubMed]
- May, J.P.; Li, S.D. Thermosensitive liposomes in cancer therapy. Recent Pat. Biomed. Eng. 2012, 5, 148–158. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamashita, K.; Itoh, Y.; Yoshino, K.; Nozawa, S.; Kasukawa, H. Comparative studies of polyethylene glycol-modified liposomes prepared using different PEG-modification methods. Biochim. Biophys. Acta 2012, 1818, 2801–2807. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Wang, L.; Yang, Q.; Tang, W.; She, Z.; Deng, Y. A frustrating problem: Accelerated blood clearance of PEGylated solid lipid nanoparticles following subcutaneous injection in rats. Eur. J. Pharm. Biopharm. 2012, 81, 506–513. [Google Scholar] [CrossRef]
- Mulligan, K.; Brownholland, D.; Carnini, A.; Thompson, D.H.; Johnston, L.J. AFM investigations of phase separation in supported membranes of binary mixtures of POPC and an eicosanyl-based bisphosphocholine bolalipid. Langmuir 2010, 26, 8525–8533. [Google Scholar] [CrossRef]
- Benvegnu, T.; Rethore, G.; Brard, M.; Richter, W.; Plusquellec, D. Archaeosomes based on novel synthetic tetraether-type lipids for the development of oral delivery systems. Chem. Commun. 2005, 44, 5536–5538. [Google Scholar] [CrossRef]
- Ren, H.; He, Y.; Liang, J.; Cheng, Z.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.; Wang, J. Role of liposome size, surface charge, and PEGylation on rheumatoid arthritis targeting therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef] [PubMed]
- Copland, M.J.; Rades, T.; Davies, N.M.; Baird, M.A. Lipid based particulate formulations for the delivery of antigen. Immunol. Cell Biol. 2005, 83, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.T.; Foged, C.; Korsholm, K.S.; Rades, T.; Christensen, D. Liposome-based adjuvants for subunit vaccines: Formulation strategies for subunit atigens and immunostimulators. Pharmaceutics 2016, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Corthésy, B.; Bioley, G. Lipid-based particles: Versatile delivery systems for mucosal vaccination against infection. Front. Immunol. 2018, 9, 431. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Allison, A.C.; Gregoriadis, G. Liposomes as immunological adjuvants. Nature 1974, 252, 252. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupeze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garcon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Mazumdar, T.; Anam, K.; Ali, N. Influence of phospholipid composition on the adjuvanticity and protective efficacy of liposomes-encapsulated Leishmania donovani antigens. J. Parasitol. 2005, 91, 269–274. [Google Scholar] [CrossRef]
- Kaur, R.; Bramwell, V.W.; Kirby, D.J.; Perrie, Y. Pegylation of dda:Tdb liposomal adjuvants reduces the vaccine depot effect and alters the th1/th2 immune responses. J. Control. Release 2012, 158, 72–77. [Google Scholar] [CrossRef]
- Higa, L.H.; Arnal, L.; Vermeulen, M.; Perez, A.P.; Schilrreff, P.; Mundiña-Weilenmann, C.; Yantorno, O.; Vela, M.E.; Morilla, M.J.; Romero, E.L. Ultradeformable archaeosomes for needle free nanovaccination with Leishmania braziliensis antigens. PLoS ONE 2016, 11, e0150185. [Google Scholar] [CrossRef]
- Akache, B.; Renner, T.M.; Tran, A.; Deschatelets, L.; Dudani, R.; Harrison, B.A.; Duque, D.; Haukenfrers, J.; Rossotti, M.A.; Gaudreault, F.; et al. Immunogenic and efficacious SARS-CoV-2 vaccine based on resistin-trimerized spike antigen SmT1 and SLA archaeosome adjuvant. Sci. Rep. 2021, 11, 21849. [Google Scholar] [CrossRef] [PubMed]
- Tolson, D.L.; Latta, R.K.; Patel, G.B.; Sprott, G.D. Uptake of archaeobacterial liposomes and conventional liposomes by phagocytic cells. J. Liposome Res. 1996, 64, 755–776. [Google Scholar] [CrossRef]
- Agbayani, G.; Jia, Y.; Akache, B.; Chandan, V.; Iqbal, U.; Stark, F.C.; Deschatelets, L.; Lam, E.; Hemraz, U.D.; Régnier, S.; et al. Mechanistic insight into the induction of cellular immune responses by encapsulated and admixed archaeosome-based vaccine formulations. Hum. Vaccines Immunother. 2020, 16, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; Stark, F.C.; Iqbal, U.; Chen, W.; Jia, Y.; Krishnan, L.; McCluskie, M.J. Safety and biodistribution of sulfated archaeal glycolipid archaeosomes as vaccine adjuvants. Hum. Vaccin. Immunother. 2018, 14, 1746–1759. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Deschatelets, L.; Krishnan, L. Sulfated archaeal glycolipid archaeosomes as a safe and effective vaccine adjuvant for induction of cell-mediated immunity. Hum. Vaccines Immunother. 2017, 13, 2772–2779. [Google Scholar] [CrossRef]
- Stark, F.C.; Akache, B.; Ponce, A.; Dudani, R.; Deschatelets, L.; Jia, Y.; Sauvageau, J.; Williams, D.; Jamshidi, M.P.; Agbayani, G.; et al. Archaeal glycolipid adjuvanted vaccines induce strong influenza-specific immune responses through direct immunization in young and aged mice or through passive maternal immunization. Vaccine 2019, 37, 7108–7116. [Google Scholar] [CrossRef]
- Jia, Y.; Akachea, B.; Deschatelets, L.; Qian, H.; Dudani, R.; Harrison, B.A.; Stark, F.C.; Chandan, V.; Jamshidi, M.P.; Krishnan, L.; et al. A comparison of the immune responses induced by antigens in three different archaeosome-based vaccine formulations. Int. J. Pharm. 2019, 561, 187–196. [Google Scholar] [CrossRef]
- Karimi, H.; Soleimanjahi, H.; Abdoli, A.; Banijamali, R.S. Combination therapy using human papillomavirus L1/E6/E7 genes and archaeosome: A nanovaccine confer immuneadjuvanting effects to fight cervical cancer. Sci. Rep. 2020, 10, 5787. [Google Scholar] [CrossRef]
- Van den Hurk, R.; Evoy, S. A review of membrane-based biosensors for pathogen detection. Sensors 2015, 15, 14045–14078. [Google Scholar] [CrossRef]
- Khan, M.S.; Dosoky, N.S.; Williams, J.D. Engineering lipid bilayer membranes for protein studies. Int. J. Mol. Sci. 2013, 14, 21561–21597. [Google Scholar] [CrossRef]
- Freisleben, H.J. The main (glyco) phospholipid (MPL) of Thermoplasma acidophilum. Int. J. Mol. Sci. 2019, 20, 5217. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, A.; Chong, P.L.-G. Certain, but not all, tetraether lipids from the thermoacidophilic archaeon Sulfolobus acidocaldarius can form black Lipid membranes with remarkable stability and exhibiting Mthk channel activity with unusually high Ca2+ sensitivity. Int. J. Mol. Sci. 2021, 22, 12941. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, A.; Rolandi, R.; De Rosa, M.; Gambacorta, A. Artificial black membranes from bipolar lipids of thermophilic archaebacteria. Biophys. J. 1982, 37, 563–566. [Google Scholar] [CrossRef][Green Version]
- Stern, J.; Freisleben, H.J.; Janku, S.; Ring, K. Black lipid membranes of tetraether lipids from Thermoplasma acidophilum. Biochim. Biophys. Acta 1992, 1128, 227–236. [Google Scholar] [CrossRef]
- Melikyan, G.B.; Matinyan, N.S.; Kocharov, S.L.; Arakelian, V.B.; Prangishvili, D.A.; Nadareishvili, K.G. Electromechanical stability of planar lipid membranes from bipolar lipids of the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biochim. Biophys. Acta 1991, 1068, 245–248. [Google Scholar] [CrossRef]
- Ren, X.; Liu, K.; Zhang, Q.; Noh, H.M.; Kumbur, E.C.; Yuan, W.W.; Zhou, J.G.; Chong, P.L.-G. Design, fabrication and characterization of archaeal tetraether free-standing planar membranes in a PDMS- and PCB-based fluidic platform. ACS Appl. Mater. Interfaces 2014, 6, 12618–12628. [Google Scholar] [CrossRef]
- Ren, X.; Kumber, E.C.; Zhou, J.G.; Noh, M.; Chong, P.L.-G. Stability of free-standing tetraether planar membranes in microchips. J. Membr. Sci. 2017, 540, 27–34. [Google Scholar] [CrossRef]
- Pau, V.P.T.; Abarca-Heidemann, K.; Rothberg, B.S. Allosteric mechanism of Ca2+ activation and H+-inhibited gating of the MthK K+ channel. J. Gen. Physiol. 2010, 135, 509–526. [Google Scholar] [CrossRef]
- Norimatsu, Y.; Hasegawa, K.; Shimizu, N.; Toyoshima, C. Protein–phospholipid interplay revealed with crystals of a calcium pump. Nature 2017, 545, 193–197. [Google Scholar] [CrossRef]
- Iida, K.; Kiriyama, H.; Fukai, A.; Konings, W.N.; Nango, M. Two-dimensional self-organization of the light-harvesting polypeptides/BChl a complex into a thermostable liposomal membrane. Langmuir 2001, 17, 2821–2827. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Crane, B.M.; Rothberg, B.S. Modulation of MthK potassium channel activity at the intracellular entrance to the pore. J. Biol. Chem. 2006, 281, 21131–21138. [Google Scholar] [CrossRef] [PubMed]
- Castellana, E.T.; Cremer, P.S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, A.; Terme, N.; Benvegnu, T.; Vie, V.; Lemiegre, L. Collapsed bipolar glycolipids at the air/water interface: Effect of the stereochemistry on the stretched/bent conformations. J. Colloid Interface Sci. 2013, 412, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Terme, N.; Jacquemet, A.; Benvegnu, T.; Vie, V.; Lemiegre, L. Modification of bipolar lipid conformation at the air/water interface by a single stereochemical variation. Chem. Phys. Lipids 2014, 183, 9–17. [Google Scholar] [CrossRef]
- Bakowsky, U.; Rothe, U.; Antonopoulos, E.; Martini, T.; Henkel, L.; Freisleben, H.J. Monomolecular organization of the main tetraether lipid from Thermoplasma acidophilum at the water-air interface. Chem. Phys. Lipids 2000, 105, 31–42. [Google Scholar] [CrossRef]
- Vilalta, I.; Gliozzi, A.; Prats, M. Interfacial air/water proton conduction from long distances by Sulfolobus solfataricus archaeal bolaform lipids. Eur. J. Biochem./FEBS 1996, 240, 181–185. [Google Scholar] [CrossRef]
- Vidawati, S.; Sitterberg, J.; Bakowsky, U.; Rothe, U. AFM and ellipsometric studies on LB films of natural asymmetric and symmetric bolaamphiphilic archaebacterial tetraether lipids on silicon wafers. Colloids Surf. B Biointerfaces 2010, 78, 303–309. [Google Scholar] [CrossRef]
- Schuster, B.; Pum, D.; Sleytr, U.B. Voltage clamp studies on S-layer-supported tetraether lipid membranes. Biochim. Biophys. Acta 1998, 1369, 51–60. [Google Scholar] [CrossRef]
- Gufler, P.C.; Pum, D.; Sleytr, U.B.; Schuster, B. Highly robust lipid membranes on crystalline S-layer supports investigated by electrochemical impedance spectroscopy. Biochim. Biophys. Acta 2004, 1661, 154–165. [Google Scholar] [CrossRef]
- Gilmore, S.F.; Yao, A.I.; Tietel, Z.; Kind, T.; Facciotti, M.T.; Parikh, A.N. Role of squalene in the organization of monolayers derived from lipid extracts of Halobacterium salinarum. Langmuir 2013, 29, 7922–7930. [Google Scholar] [CrossRef]
- Dayyoub, E.; Sitterberg, J.; Bakowsky, U. New antibacterial, antiadhesive films based on self assembles of modified tetraether lipids. Adv. Sci. Technol. 2008, 57, 188–194. [Google Scholar]
- Liefeith, K.; Frant, M.; Müller, U.; Stenstad, P.; Johnsen, H.; Schmid, R. Archaeal tetraether lipid coatings—A strategy for the development of membrane analog spacer systems for the site-specific functionalization of medical surfaces. Biointerphases 2018, 13, 011004. [Google Scholar] [CrossRef]
- Swain, M.; Brisson, J.R.; Sprott, G.D.; Cooper, F.P.; Patel, G.B. Identification of beta-L-gulose as the sugar moiety of the main polar lipids of Thermoplasma acidophilum. Biochim. Biophys. Acta 1997, 1345, 56–64. [Google Scholar] [CrossRef]
- Bucher, C.; Grosse, X.; Rothe, H.; Fiethen, A.; Kuhn, H. Biomimetic surface modification with bolaamphiphilic archaeal tetraether lipids via liposome spreading. Biointerphases 2014, 9, 011002. [Google Scholar] [CrossRef]



| Formulation (Molar Ratio) | Prepared by | Therapeutics Entrapped | Cultured Cells | In Vivo Model | Controlled Release by | Major Findings | References |
|---|---|---|---|---|---|---|---|
| PLFE/DPPC (1:9) | sonication | hypericin (photosensitizer) | human ovarian carcinoma cells (SKOV-3) | CAM | light irradiation | hypericin archaeosomes are suited for antivascular targeting | [26] |
| PLFE/DPPC (9:91), (29:71), and (62:38) | sonication and extrusion | protoporphyrin IX (photosensitizer) | SKOV-3 and mouse fibroblast L929 cells; CAM | CAM | light irradiation | PLFE-stabilized PDT liposomes suppressed angiogenesis and removed thrombosis in the chick | [38,69] |
| PLFE/DSPC (10:90) | sonication and extrusion | curcumin (photosensitizer) | SKOV-3 and primary human coronary artery endothelial cells (PCS-100-020™ cells) | CAM | light irradiation | Archaeosomes are hemocompatble, coagulation time < 50 s | [70] |
| PLFE | sonication | insulin | Caco-2 cells | mice (oral) | N/A | Archaeosomal insulin leads to lower blood glucose than conventional liposomal insulin | [67] |
| PLFE/DPPC (3:7) | extrusion | doxorubicin (Anticancer drug) | human breast cancer MCF-7 cells | N/A | hyperthermia-like treatment | an abrupt DOX release and a dramatic change in particle surface charge | [16] |
| GCTE/ cholesterol/EPC (5:10:85), with and without CPP | speed mixing with glass beads | vancomycin (antibiotic) | Caco-2 cells | mice (oral) | N/A | GCTE and CPP work synergistically in enhancing oral bioavailability and anti-infection efficacy | [68,71] |
| GCTE/DPPC (3:1), GCTE/EPC (4:1), other molar ratios | extrusion | octreotide (peptide inhibitor of exocrine and endocrine secretion) | N/A | rats (oral) | N/A | a 4.1-fold increase in oral bioavailability | [18] |
| GCTE/cholesterol/EPC (5:10:85) | speed mixing with glass beads | myrcludex B (Hepatitis B entry inhibitor) | N/A | rats (oral) | N/A | 7% of the initial dose detected in the liver (a 3.5-fold increase vs. control) | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, P.L.-G.; Chang, A.; Yu, A.; Mammedova, A. Vesicular and Planar Membranes of Archaea Lipids: Unusual Physical Properties and Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 7616. https://doi.org/10.3390/ijms23147616
Chong PL-G, Chang A, Yu A, Mammedova A. Vesicular and Planar Membranes of Archaea Lipids: Unusual Physical Properties and Biomedical Applications. International Journal of Molecular Sciences. 2022; 23(14):7616. https://doi.org/10.3390/ijms23147616
Chicago/Turabian StyleChong, Parkson Lee-Gau, Abby Chang, Allyson Yu, and Ayna Mammedova. 2022. "Vesicular and Planar Membranes of Archaea Lipids: Unusual Physical Properties and Biomedical Applications" International Journal of Molecular Sciences 23, no. 14: 7616. https://doi.org/10.3390/ijms23147616
APA StyleChong, P. L.-G., Chang, A., Yu, A., & Mammedova, A. (2022). Vesicular and Planar Membranes of Archaea Lipids: Unusual Physical Properties and Biomedical Applications. International Journal of Molecular Sciences, 23(14), 7616. https://doi.org/10.3390/ijms23147616







