Transcriptional Profiling of the Candida albicans Response to the DNA Damage Agent Methyl Methanesulfonate
Abstract
:1. Introduction
2. Results
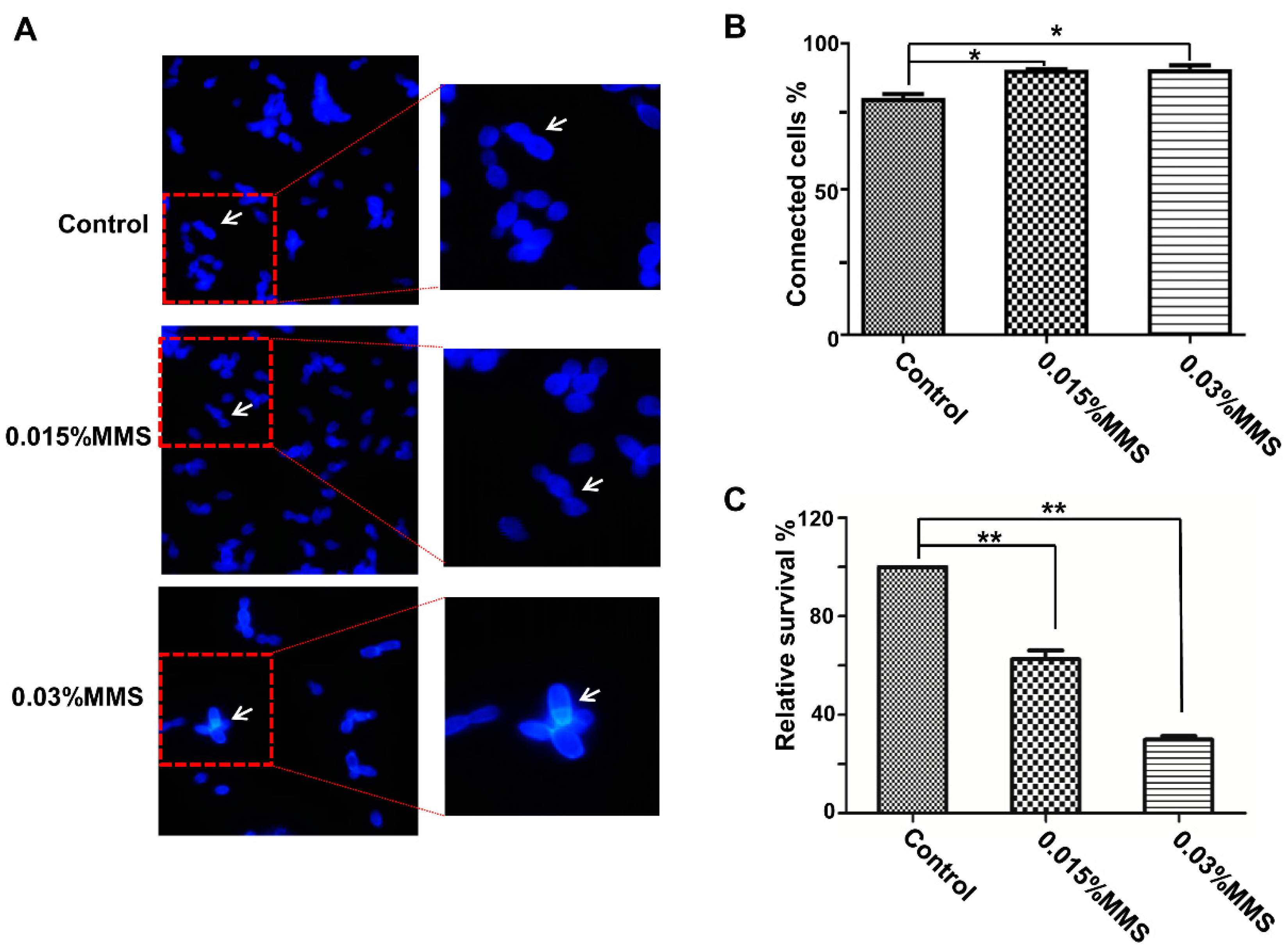
2.1. MMS Treatment Inhibits the Separation and Growth of C. albicans Cells
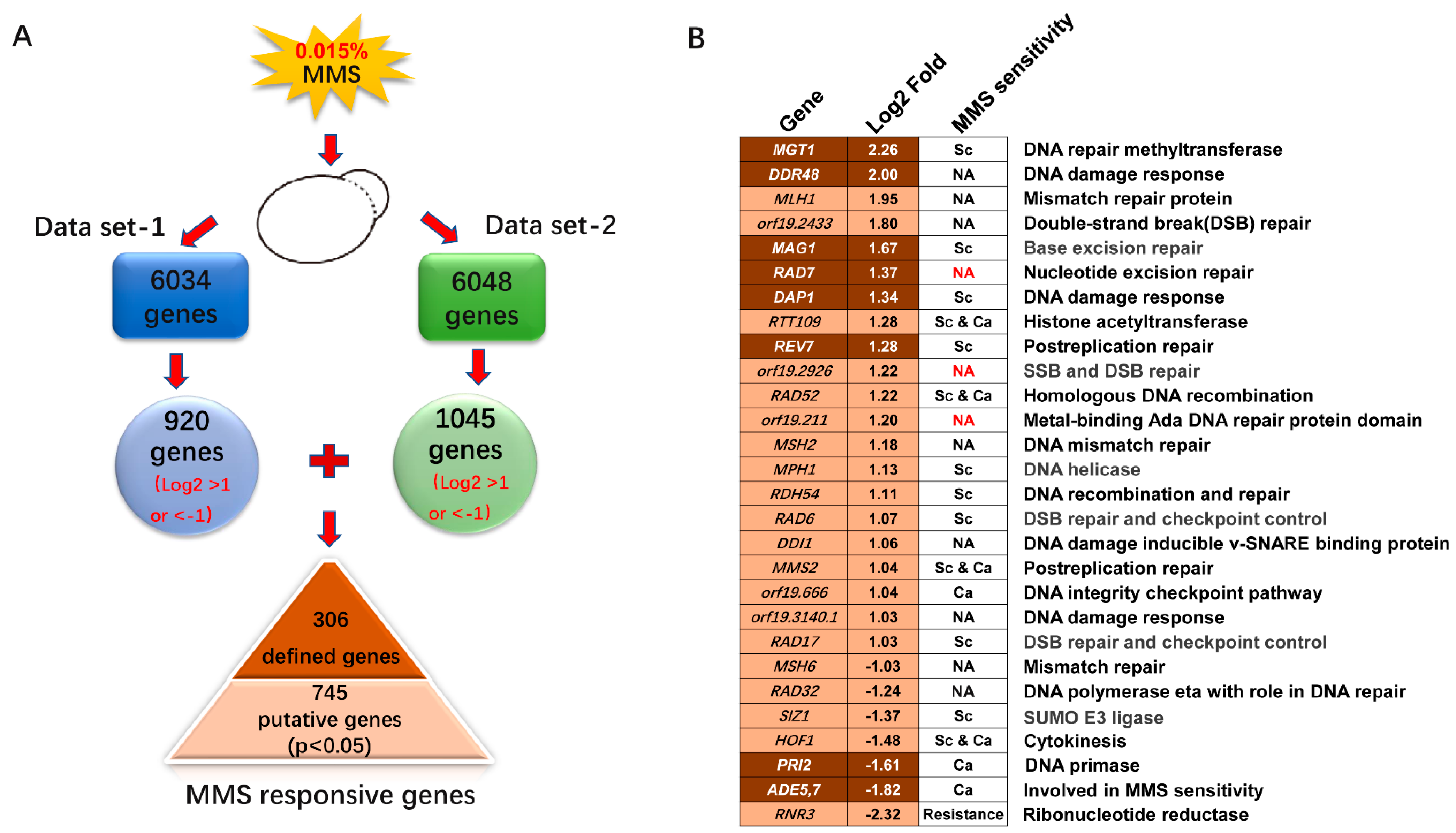
2.2. Transcriptional Profiling of the Response to MMS
2.3. DNA Damage Response Genes Induced by MMS in C. albicans
2.4. Evaluation of MMS-Responsive Genes
2.4.1. Oxidative Stress-Related Genes
2.4.2. ‘De Novo’ IMP Biosynthetic Process
2.4.3. Nucleosome Assembly
2.4.4. Glutathione Metabolism
2.4.5. Cell Wall Genes
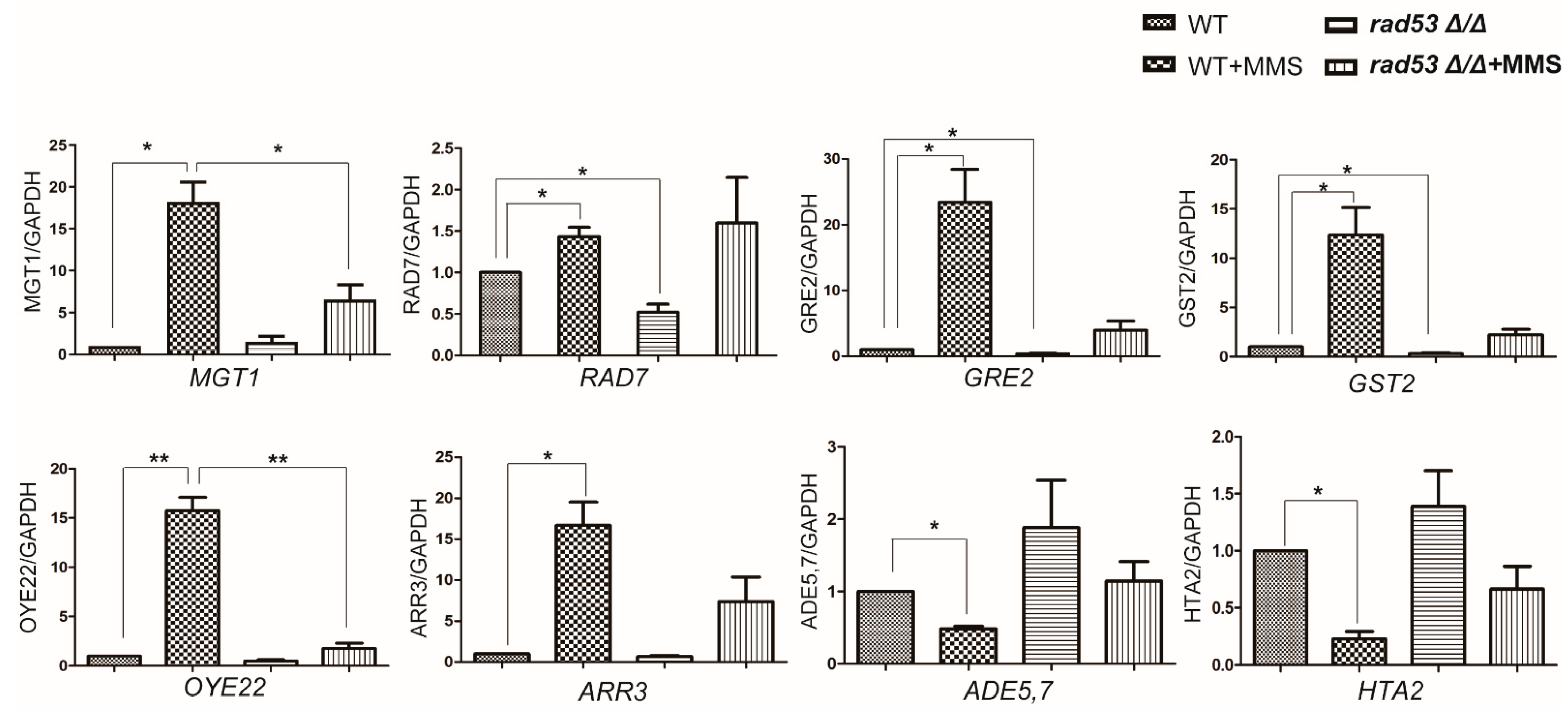
2.5. Rad53 Plays a Critical Role in Coordinating MMS-Induced Transcription
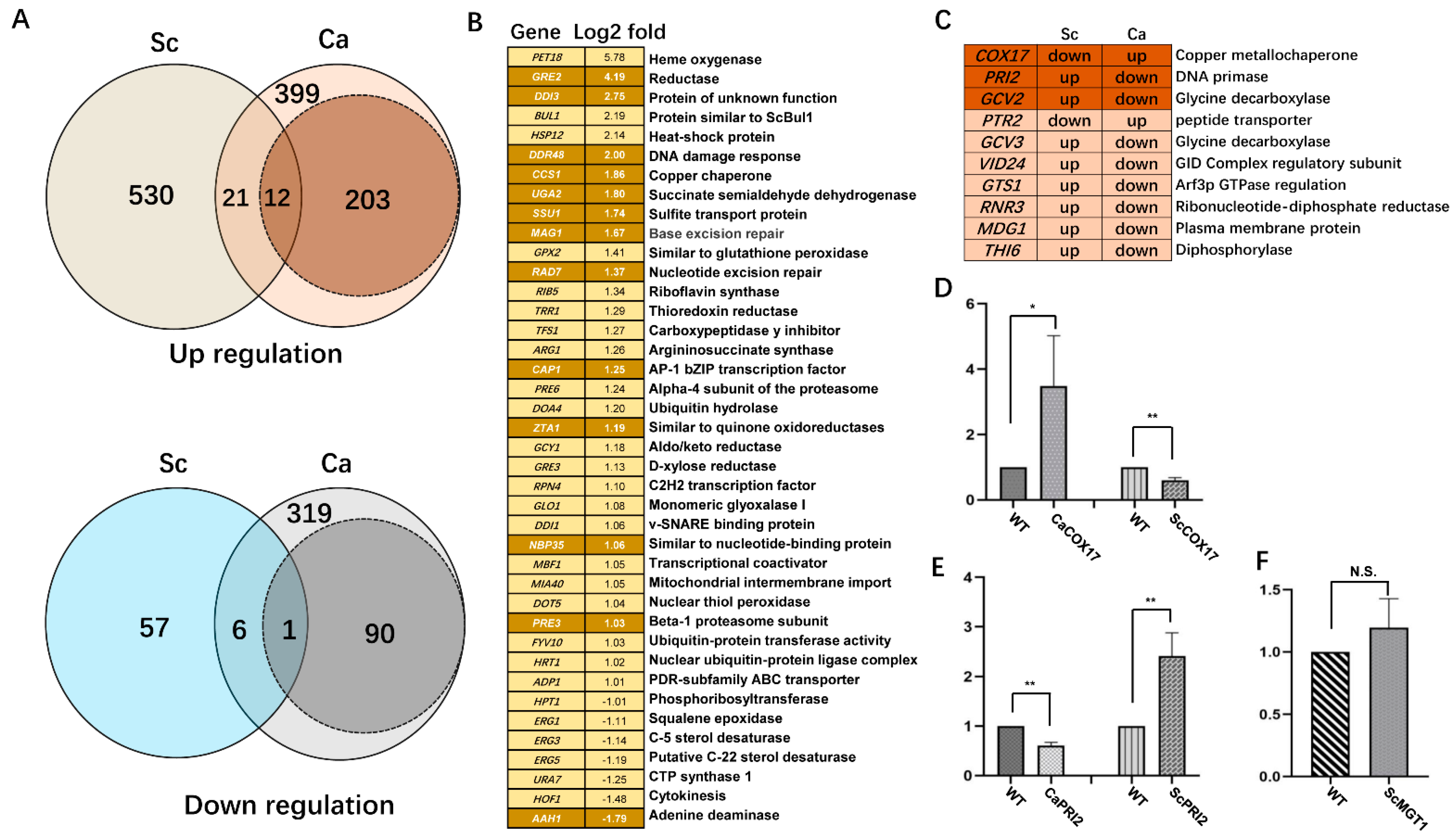
2.6. Differential Transcriptome to MMS in C. albicans and S. cerevisiae
3. Discussion
4. Materials and Methods
4.1. Strains, Media, and Reagents
4.2. Survival Assay of C. albicans Cells
4.3. Calcofluor White (CFW) Staining
4.4. RNA Preparation and RNA-seq Assay
4.5. Real Time PCR (qRT-PCR)
4.6. Construction of the CAP1 Deletion Strain
4.7. Overexpression of GST2 and GST3 Genes in C. albicans
4.8. SOD Activity and GST Activity Assay
4.9. Protein Extraction and Western Blot Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bukata, L.; Parker, S.L.; D’Angelo, M.A. Nuclear pore complexes in the maintenance of genome integrity. Curr. Opin. Cell Biol. 2013, 25, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Ames, B.N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic. Res. Commun. 1989, 7, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Feng, Y.; Zhang, Y.; Feng, J. DNA damage checkpoint and repair: From the budding yeast Saccharomyces cerevisiae to the pathogenic fungus Candida albicans. Comput. Struct. Biotechnol. J. 2021, 19, 6343–6354. [Google Scholar] [CrossRef] [PubMed]
- van Berlo, D.; Wessels, A.; Boots, A.W.; Wilhelmi, V.; Scherbart, A.M.; Gerloff, K.; van Schooten, F.J.; Albrecht, C.; Schins, R.P. Neutrophil-derived ROS contribute to oxidative DNA damage induction by quartz particles. Free Radic. Biol. Med. 2010, 49, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Al Aboud, N.M.; Basit, H.; Al-Jindan, F.A. Genetics, DNA Damage and Repair; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- de la Torre Ruiz, M.A.; Lowndes, N.F. DUN1 defines one branch downstream of RAD53 for transcription and DNA damage repair in Saccharomyces cerevisiae. FEBS Lett. 2000, 485, 205–206. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, E.J.; Chevalier, C.; Pinto, A.V.; Thiberge, J.M.; Ielpi, L.; Labigne, A.; Radicella, J.P. Pathogen DNA as target for host-generated oxidative stress: Role for repair of bacterial DNA damage in Helicobacter pylori colonization. Proc. Natl. Acad. Sci. USA 2003, 100, 2789–2794. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Feng, Y.; Islam, A.; Shrivastava, M.; Gu, H.; Lu, Y.; Sheng, J.; Whiteway, M.; Feng, J. Loss of Arp1, a putative actin-related protein, triggers filamentous and invasive growth and impairs pathogenicity in Candida albicans. Comput. Struct. Biotechnol. J. 2020, 18, 4002–4015. [Google Scholar] [CrossRef]
- Jelinsky, S.A.; Samson, L.D. Global response of Saccharomyces cerevisiae to an alkylating agent. Proc. Natl. Acad. Sci. USA 1999, 96, 1486–1491. [Google Scholar] [CrossRef] [Green Version]
- Gasch, A.P.; Huang, M.; Metzner, S.; Botstein, D.; Elledge, S.J.; Brown, P.O. Genomic expression responses to DNA-damaging agents and the regulatory role of the yeast ATR homolog Mec1p. Mol. Biol. Cell 2001, 12, 2987–3003. [Google Scholar] [CrossRef] [Green Version]
- van Attikum, H.; Fritsch, O.; Hohn, B.; Gasser, S.M. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 2004, 119, 777–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benton, M.G.; Somasundaram, S.; Glasner, J.D.; Palecek, S.P. Analyzing the dose-dependence of the Saccharomyces cerevisiae global transcriptional response to methyl methanesulfonate and ionizing radiation. BMC Genom. 2006, 7, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caba, E.; Dickinson, D.A.; Warnes, G.R.; Aubrecht, J. Differentiating mechanisms of toxicity using global gene expression analysis in Saccharomyces cerevisiae. Mutat. Res. 2005, 575, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Kim, B.J.; Choi, H.K.; Ryu, M.J.; Kim, S.B.; Kang, K.M.; Cho, E.J.; Youn, H.D.; Huh, W.K.; Kim, S.T. Global protein expression profiling of budding yeast in response to DNA damage. Yeast 2007, 24, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.H.; Jeong, S.J.; Seol, J.W.; Kim, H.J.; Han, J.W.; Lee, H.W.; Cho, E.J. Differential regulation of gene expression by RNA polymerase II in response to DNA damage. Biochem. Biophys. Res. Commun. 2004, 325, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Masuda, C.A.; Chang, W.H.; Komori, H.; Wang, D.; Hunter, T.; Joazeiro, C.A.; Kornberg, R.D. Ubiquitin ligase activity of TFIIH and the transcriptional response to DNA damage. Mol. Cell 2005, 18, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Balakirev, M.Y.; Mullally, J.E.; Favier, A.; Assard, N.; Sulpice, E.; Lindsey, D.F.; Rulina, A.V.; Gidrol, X.; Wilkinson, K.D. Wss1 metalloprotease partners with Cdc48/Doa1 in processing genotoxic SUMO conjugates. Elife 2015, 4, e06763. [Google Scholar] [CrossRef]
- Chernenkov, A.; Gracheva, L.M.; Evstiukhina, T.A.; Koval’tsova, S.V.; Peshekhonov, V.T.; Fedorova, I.V.; Korolev, V.G. Interaction of gene HSM3 with genes of the epistatic RAD6 group in yeast Saccharomyces cerevisiae. Genetika 2012, 48, 160–167. [Google Scholar] [CrossRef]
- Chernenkov, A.; Fedorov, D.V.; Gracheva, L.M.; Evstukhina, T.A.; Koval’tsova, S.V.; Peshekhonov, V.T.; Fedorova, I.; Korolev, V. Interaction of the HSM3 gene with genes initiating homologous recombination repair in yeast Saccharomyces cerevisiae. Genetika 2012, 48, 333–339. [Google Scholar] [CrossRef]
- Chang, M.; Bellaoui, M.; Boone, C.; Brown, G.W. A genome-wide screen for methyl methanesulfonate-sensitive mutants reveals genes required for S phase progression in the presence of DNA damage. Proc. Natl. Acad. Sci. USA 2002, 99, 16934–16939. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.A.; Sherlock, G.; Myers, C.L.; Burrows, N.M.; Deng, C.; Wu, H.I.; McCann, K.E.; Troyanskaya, O.G.; Brown, J.M. Global analysis of gene function in yeast by quantitative phenotypic profiling. Mol. Syst. Biol. 2006, 2, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birrell, G.W.; Brown, J.A.; Wu, H.I.; Giaever, G.; Chu, A.M.; Davis, R.W.; Brown, J.M. Transcriptional response of Saccharomyces cerevisiae to DNA-damaging agents does not identify the genes that protect against these agents. Proc. Natl. Acad. Sci. USA 2002, 99, 8778–8783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.I.; Giannakopoulos, N.V.; Virgin, H.W.; Zhang, D.E. Interferon-inducible ubiquitin E2, Ubc8, is a conjugating enzyme for protein ISGylation. Mol. Cell Biol. 2004, 24, 9592–9600. [Google Scholar] [CrossRef] [Green Version]
- Enenkel, C.; Lehmann, H.; Kipper, J.; Guckel, R.; Hilt, W.; Wolf, D.H. PRE3, highly homologous to the human major histocompatibility complex-linked LMP2 (RING12) gene, codes for a yeast proteasome subunit necessary for the peptidylglutamyl-peptide hydrolyzing activity. FEBS Lett. 1994, 341, 193–196. [Google Scholar] [CrossRef] [Green Version]
- Kapitzky, L.; Beltrao, P.; Berens, T.J.; Gassner, N.; Zhou, C.; Wuster, A.; Wu, J.; Babu, M.M.; Elledge, S.J.; Toczyski, D.; et al. Cross-species chemogenomic profiling reveals evolutionarily conserved drug mode of action. Mol. Syst. Biol. 2010, 6, 451. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Rosa, J.; Kaufman, P.D. Chromatin-mediated Candida albicans virulence. Biochim. Biophys. Acta 2013, 1819, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Bellido, A.; Hermosa, B.; Ciudad, T.; Larriba, G. Role of homologous recombination genes RAD51, RAD52, and RAD59 in the repair of lesions caused by gamma-radiation to cycling and G2/M-arrested cells of Candida albicans. Cell Microbiol. 2018, 20, e12950. [Google Scholar] [CrossRef]
- Feng, J.R.; Duan, Y.N.; Qin, Y.W.; Sun, W.; Zhuang, Z.; Zhu, D.D.; Jiang, L.H. The N-terminal pY33XL motif of CaPsy2 is critical for the function of protein phosphatase 4 in CaRad53 deactivation, DNA damage-induced filamentation and virulence in Candida albicans. Int. J. Med. Microbiol. 2017, 307, 471–480. [Google Scholar] [CrossRef]
- Yao, G.Y.; Wan, J.H.; Liu, Q.Z.; Mu, C.H.; Wang, Y.; Sang, J.L. Characterization of Pph3-mediated dephosphorylation of Rad53 during methyl methanesulfonate-induced DNA damage repair in Candida albicans. Biochem. J. 2017, 474, 1293–1306. [Google Scholar] [CrossRef]
- Shor, E.; Garcia-Rubio, R.; DeGregorio, L.; Perlin, D.S. A Noncanonical DNA Damage Checkpoint Response in a Major Fungal Pathogen. mBio 2020, 11, e03044-20. [Google Scholar] [CrossRef]
- Milo-Cochavi, S.; Pareek, M.; Delulio, G.; Almog, Y.; Anand, G.; Ma, L.J.; Covo, S. The response to the DNA damaging agent methyl methanesulfonate in a fungal plant pathogen. Fungal Biol. 2019, 123, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Islam, A.; Bean, B.; Feng, J.; Sparapani, S.; Shrivastava, M.; Goyal, A.; Omran, R.P.; Mallick, J.; Whiteway, M. Hof1 plays a checkpoint-related role in MMS-induced DNA damage response in Candida albicans. Mol. Biol. Cell 2020, 31, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Loser, D.A.; Shibata, A.; Shibata, A.K.; Woodbine, L.J.; Jeggo, P.A.; Chalmers, A.J. Sensitization to radiation and alkylating agents by inhibitors of poly(ADP-ribose) polymerase is enhanced in cells deficient in DNA double-strand break repair. Mol. Cancer Ther. 2010, 9, 1775–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessulat, M.; Alamgir, M.; Salsali, H.; Greenblatt, J.; Xu, J.; Golshani, A. Interacting proteins Rtt109 and Vps75 affect the efficiency of non-homologous end-joining in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 2008, 469, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Brosh, R.M., Jr. DNA helicases involved in DNA repair and their roles in cancer. Nat. Rev. Cancer 2013, 13, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Prieto, F.; Gomez-Raja, J.; Andaluz, E.; Calderone, R.; Larriba, G. Role of the homologous recombination genes RAD51 and RAD59 in the resistance of Candida albicans to UV light, radiomimetic and anti-tumor compounds and oxidizing agents. Fungal Genet. Biol. 2010, 47, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Legrand, M.; Chan, C.L.; Jauert, P.A.; Kirkpatrick, D.T. Role of DNA mismatch repair and double-strand break repair in genome stability and antifungal drug resistance in Candida albicans. Eukaryot Cell 2007, 6, 2194–2205. [Google Scholar] [CrossRef] [Green Version]
- Lopes da Rosa, J.; Boyartchuk, V.L.; Zhu, L.J.; Kaufman, P.D. Histone acetyltransferase Rtt109 is required for Candida albicans pathogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 1594–1599. [Google Scholar] [CrossRef] [Green Version]
- Chi, P.; Kwon, Y.; Seong, C.; Epshtein, A.; Lam, I.; Sung, P.; Klein, H.L. Yeast recombination factor Rdh54 functionally interacts with the Rad51 recombinase and catalyzes Rad51 removal from DNA. J. Biol. Chem. 2006, 281, 26268–26279. [Google Scholar] [CrossRef] [Green Version]
- Teo, S.H.; Jackson, S.P. Identification of Saccharomyces cerevisiae DNA ligase IV: Involvement in DNA double-strand break repair. EMBO J. 1997, 16, 4788–4795. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; St Onge, R.P.; Proctor, M.; Flaherty, P.; Jordan, M.I.; Arkin, A.P.; Davis, R.W.; Nislow, C.; Giaever, G. Genome-wide requirements for resistance to functionally distinct DNA-damaging agents. PLoS Genet. 2005, 1, e24. [Google Scholar] [CrossRef] [PubMed]
- Murakami-Sekimata, A.; Huang, D.; Piening, B.D.; Bangur, C.; Paulovich, A.G. The Saccharomyces cerevisiae RAD9, RAD17 and RAD24 genes are required for suppression of mutagenic post-replicative repair during chronic DNA damage. DNA Repair 2010, 9, 824–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.E.; Yu, S.L.; Prakash, S.; Prakash, L. A role for yeast and human translesion synthesis DNA polymerases in promoting replication through 3-methyl adenine. Mol. Cell Biol. 2007, 27, 7198–7205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heideker, J.; Lis, E.T.; Romesberg, F.E. Phosphatases, DNA damage checkpoints and checkpoint deactivation. Cell Cycle 2007, 6, 3058–3064. [Google Scholar] [CrossRef] [Green Version]
- Priebe, S.; Kreisel, C.; Horn, F.; Guthke, R.; Linde, J. FungiFun2: A comprehensive online resource for systematic analysis of gene lists from fungal species. Bioinformatics 2015, 31, 445–446. [Google Scholar] [CrossRef] [Green Version]
- Alarco, A.M.; Raymond, M. The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans. J. Bacteriol. 1999, 181, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Enjalbert, B.; Smith, D.A.; Cornell, M.J.; Alam, I.; Nicholls, S.; Brown, A.J.; Quinn, J. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol. Biol. Cell 2006, 17, 1018–1032. [Google Scholar] [CrossRef] [Green Version]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, S.C.; Shin, J.; Oh, K.B. GST2 is required for nitrogen starvation-induced filamentous growth in Candida albicans. J. Microbiol. Biotechnol. 2014, 24, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Elledge, S.J. Cell cycle checkpoints: Preventing an identity crisis. Science 1996, 274, 1664–1672. [Google Scholar] [CrossRef]
- Chakrabortee, S.; Byers, J.S.; Jones, S.; Garcia, D.M.; Bhullar, B.; Chang, A.; She, R.; Lee, L.; Fremin, B.; Lindquist, S.; et al. Intrinsically Disordered Proteins Drive Emergence and Inheritance of Biological Traits. Cell 2016, 167, 369–381.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Yao, S.; Dong, Y.; Hu, J.; Whiteway, M.; Feng, J. Nucleotide Excision Repair Protein Rad23 Regulates Cell Virulence Independent of Rad4 in Candida albicans. mSphere 2020, 5, e00062-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.Y.; Kim, M.A.; Kim, H.J.; Bae, Y.S.; Park, J.I.; Kwak, J.Y.; Chung, J.H.; Yun, J. Alkylating agent methyl methanesulfonate (MMS) induces a wave of global protein hyperacetylation: Implications in cancer cell death. Biochem. Biophys. Res. Commun. 2007, 360, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, K.; Glascott, P.A., Jr.; Farber, J.L. Roles for oxidative stress and poly(ADP-ribosyl)ation in the killing of cultured hepatocytes by methyl methanesulfonate. Biochem. Pharmacol. 1993, 46, 1811–1818. [Google Scholar] [CrossRef]
- Rai, R.; Tate, J.J.; Cooper, T.G. Ure2, a prion precursor with homology to glutathione S-transferase, protects Saccharomyces cerevisiae cells from heavy metal ion and oxidant toxicity. J. Biol. Chem. 2003, 278, 12826–12833. [Google Scholar] [CrossRef] [Green Version]
- Veal, E.A.; Toone, W.M.; Jones, N.; Morgan, B.A. Distinct roles for glutathione S-transferases in the oxidative stress response in Schizosaccharomyces pombe. J. Biol. Chem. 2002, 277, 35523–35531. [Google Scholar] [CrossRef] [Green Version]
- Fraser, J.A.; Davis, M.A.; Hynes, M.J. A gene from Aspergillus nidulans with similarity to URE2 of Saccharomyces cerevisiae encodes a glutathione S-transferase which contributes to heavy metal and xenobiotic resistance. Appl. Environ. Microbiol. 2002, 68, 2802–2808. [Google Scholar] [CrossRef] [Green Version]
- Gunjan, A.; Verreault, A. A Rad53 kinase-dependent surveillsnce mechanism 3that regulates histone protein levels6 in S. cerevisiae. Cell 2003, 115, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Dantas Ada, S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef] [Green Version]
- Travesa, A.; Kuo, D.; de Bruin, R.A.; Kalashnikova, T.I.; Guaderrama, M.; Thai, K.; Aslanian, A.; Smolka, M.B.; Yates, J.R., 3rd; Ideker, T.; et al. DNA replication stress differentially regulates G1/S genes via Rad53-dependent inactivation of Nrm1. EMBO J. 2012, 31, 1811–1822. [Google Scholar] [CrossRef] [Green Version]
- Treger, J.M.; McEntee, K. Structure of the DNA damage-inducible gene DDR48 and evidence for its role in mutagenesis in Saccharomyces cerevisiae. Mol. Cell Biol. 1990, 10, 3174–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, B.; Clancy, C.J.; Cheng, S.; Raman, S.B.; Iczkowski, K.A.; Nguyen, M.H. Candida albicans RFX2 encodes a DNA binding protein involved in DNA damage responses, morphogenesis, and virulence. Eukaryot. Cell 2009, 8, 627–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaehnig, E.J.; Kuo, D.; Hombauer, H.; Ideker, T.G.; Kolodner, R.D. Checkpoint kinases regulate a global network of transcription factors in response to DNA damage. Cell Rep. 2013, 4, 174–188. [Google Scholar] [CrossRef] [Green Version]
- MacPherson, S.; Akache, B.; Weber, S.; De Deken, X.; Raymond, M.; Turcotte, B. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob. Agents Chemother. 2005, 49, 1745–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tebung, W.A.; Omran, R.P.; Fulton, D.L.; Morschhauser, J.; Whiteway, M. Put3 Positively Regulates Proline Utilization in Candida albicans. mSphere 2017, 2, e00354-17. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, H.; Sellam, A.; Askew, C.; Nantel, A.; Whiteway, M. A toolbox for epitope-tagging and genome-wide location analysis in Candida albicans. BMC Genom. 2008, 9, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murad, A.M.; Lee, P.R.; Broadbent, I.D.; Barelle, C.J.; Brown, A.J. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 2000, 16, 325–327. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, Y.; Duan, Y.; Jiang, L. Genetic interactions between protein phosphatases CaPtc2p and CaPph3p in response to genotoxins and rapamycin in Candida albicans. FEMS Yeast Res. 2013, 13, 85–96. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Zhang, Y.; Li, J.; Omran, R.P.; Whiteway, M.; Feng, J. Transcriptional Profiling of the Candida albicans Response to the DNA Damage Agent Methyl Methanesulfonate. Int. J. Mol. Sci. 2022, 23, 7555. https://doi.org/10.3390/ijms23147555
Feng Y, Zhang Y, Li J, Omran RP, Whiteway M, Feng J. Transcriptional Profiling of the Candida albicans Response to the DNA Damage Agent Methyl Methanesulfonate. International Journal of Molecular Sciences. 2022; 23(14):7555. https://doi.org/10.3390/ijms23147555
Chicago/Turabian StyleFeng, Yuting, Yan Zhang, Jie Li, Raha Parvizi Omran, Malcolm Whiteway, and Jinrong Feng. 2022. "Transcriptional Profiling of the Candida albicans Response to the DNA Damage Agent Methyl Methanesulfonate" International Journal of Molecular Sciences 23, no. 14: 7555. https://doi.org/10.3390/ijms23147555
APA StyleFeng, Y., Zhang, Y., Li, J., Omran, R. P., Whiteway, M., & Feng, J. (2022). Transcriptional Profiling of the Candida albicans Response to the DNA Damage Agent Methyl Methanesulfonate. International Journal of Molecular Sciences, 23(14), 7555. https://doi.org/10.3390/ijms23147555




