Promising Strategies for Preserving Adult Endothelium Health and Reversing Its Dysfunction: From Liquid Biopsy to New Omics Technologies and Noninvasive Circulating Biomarkers
Abstract
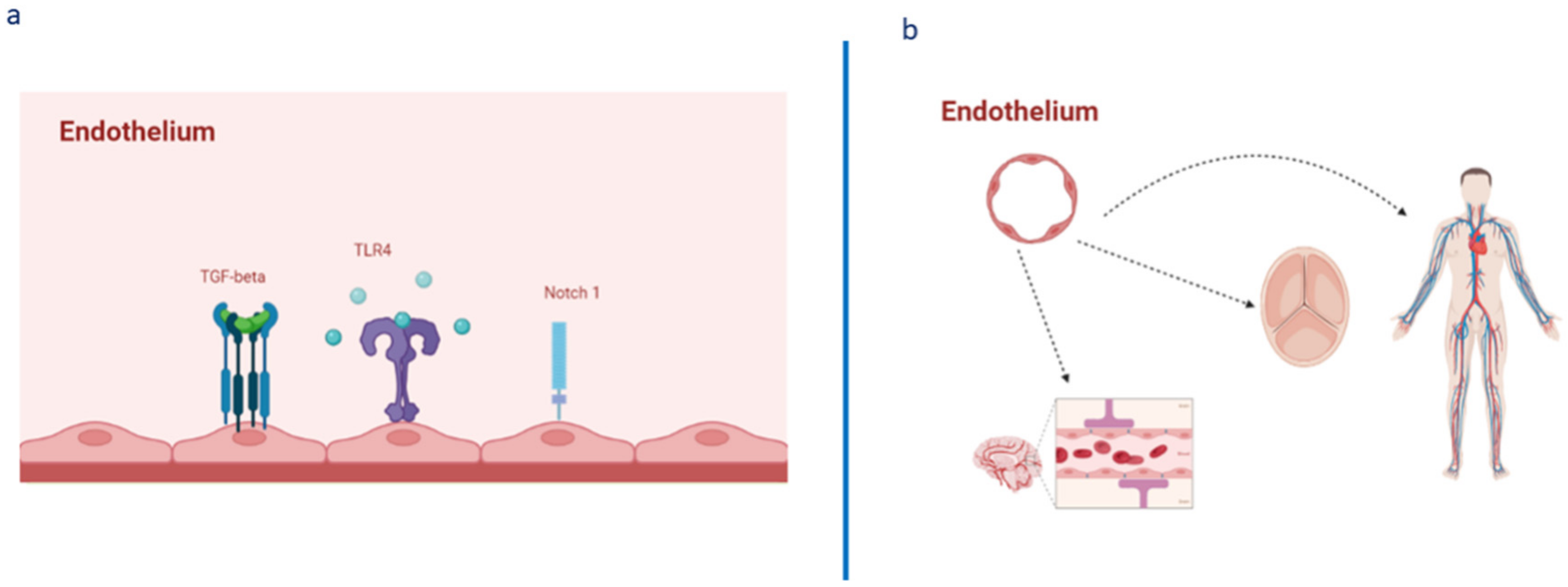
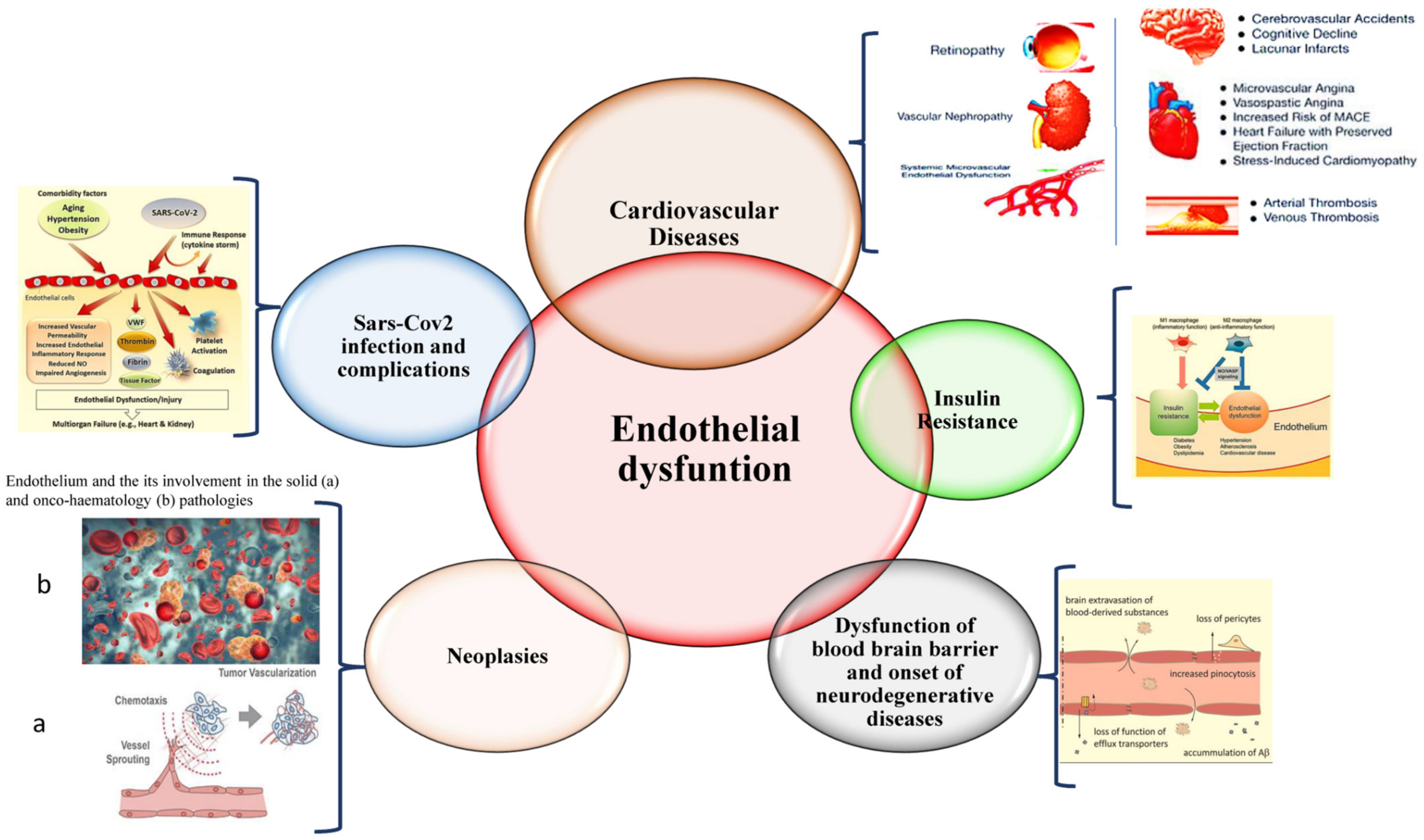
1. Introduction
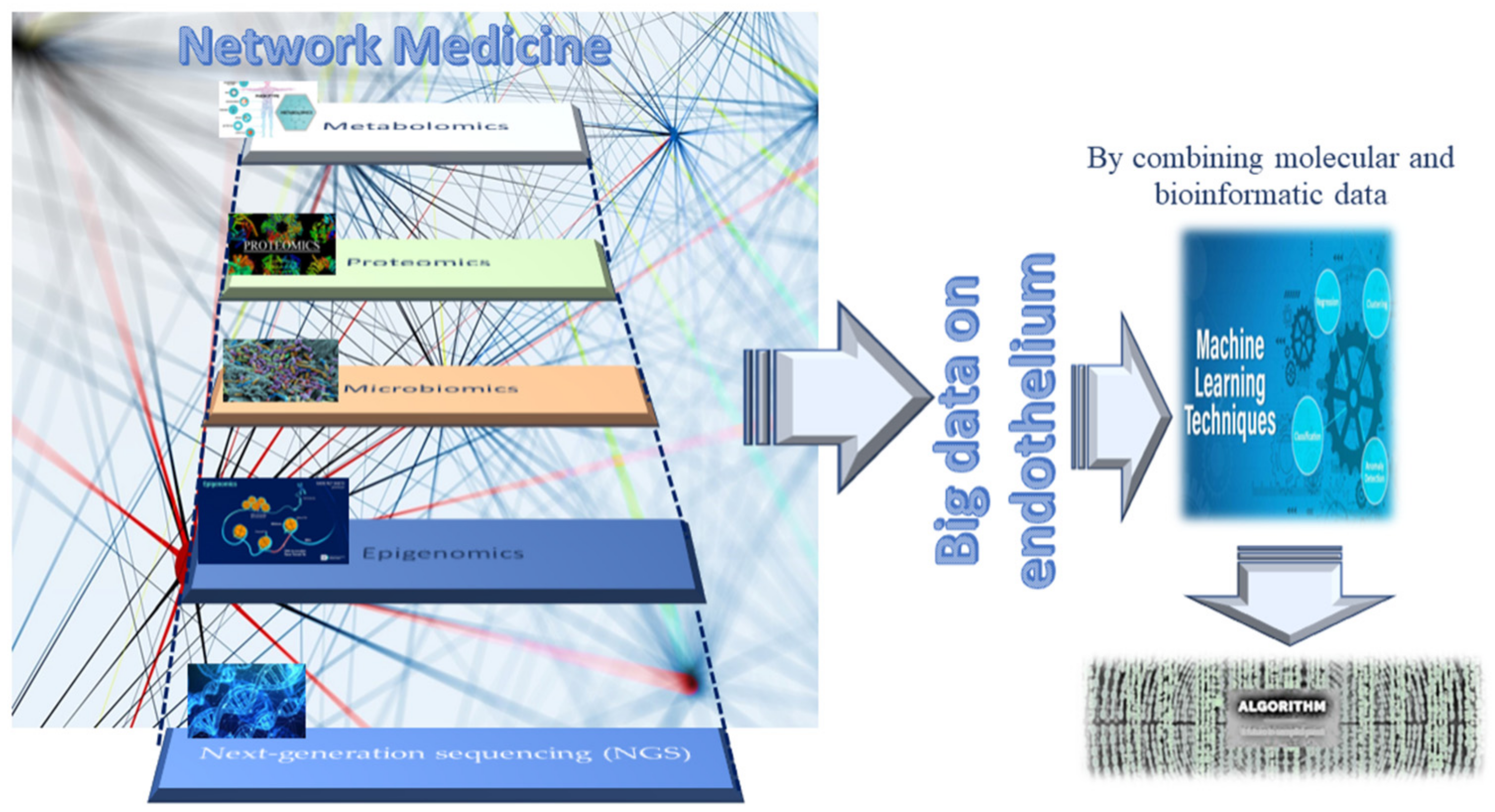
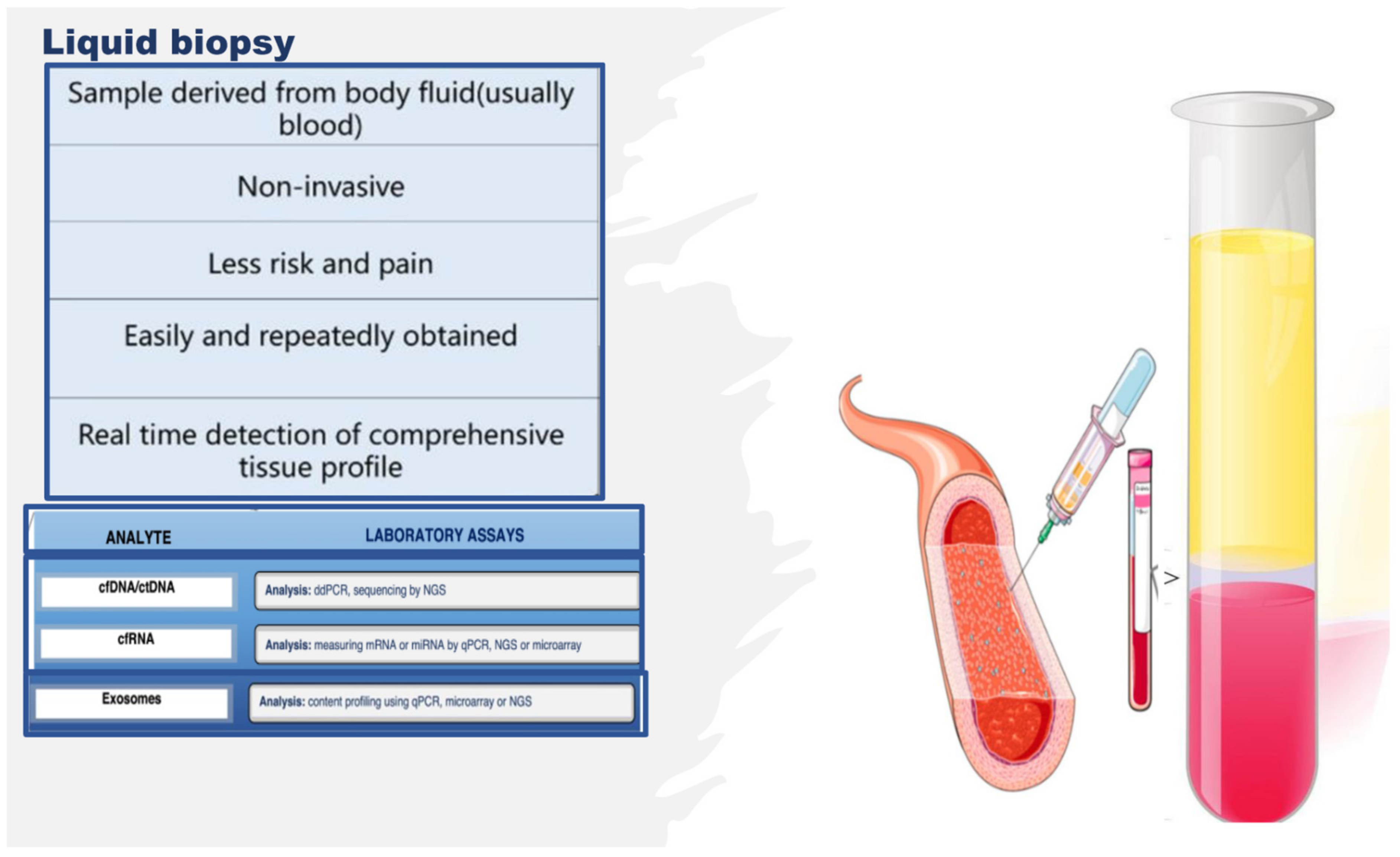
2. Omics Technologies and Liquid Biopsy as Emerging Tools for Identifying Endothelial Biomarkers and Therapeutic Targets
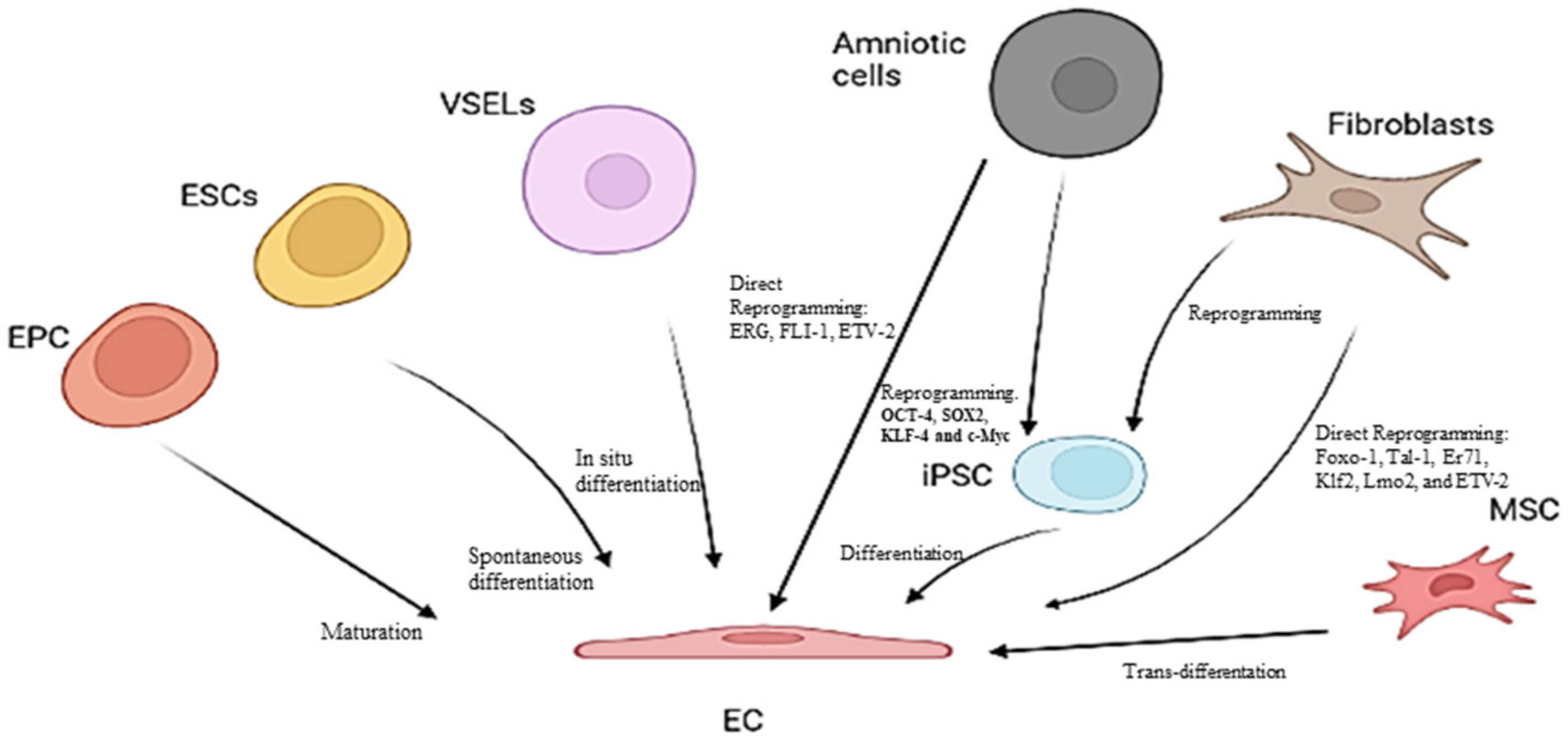
3. Endothelial Progenitor Cells (EPCs) as Potential Biomarkers of Endothelium Dysfunction and Therapeutic Agents
Other Candidates for Therapeutic Agents of Endothelium Dysfunction
4. CircRNAs and Their Potential as Biomarkers and Therapeutic Targets
4.1. CircRNAs and the Endothelium: Recent Literature Evidence
4.2. Limitations and Future Perspectives
5. ADAR Protein Family as Biomarkers and Therapeutic Targets
5.1. ADAR Enzymes in the Endothelium Literature
5.2. Limitations and Future Perspectives
6. Vascular Endothelial Glycocalyx and Its Components as Emerging Endothelial Biomarkers and Therapeutic Targets of Endothelium Dysfunction
7. Circulating Metabolites
8. Suggestions and Recommendations for Further Investigations Needed to Develop Biomarker Endothelial Profiles
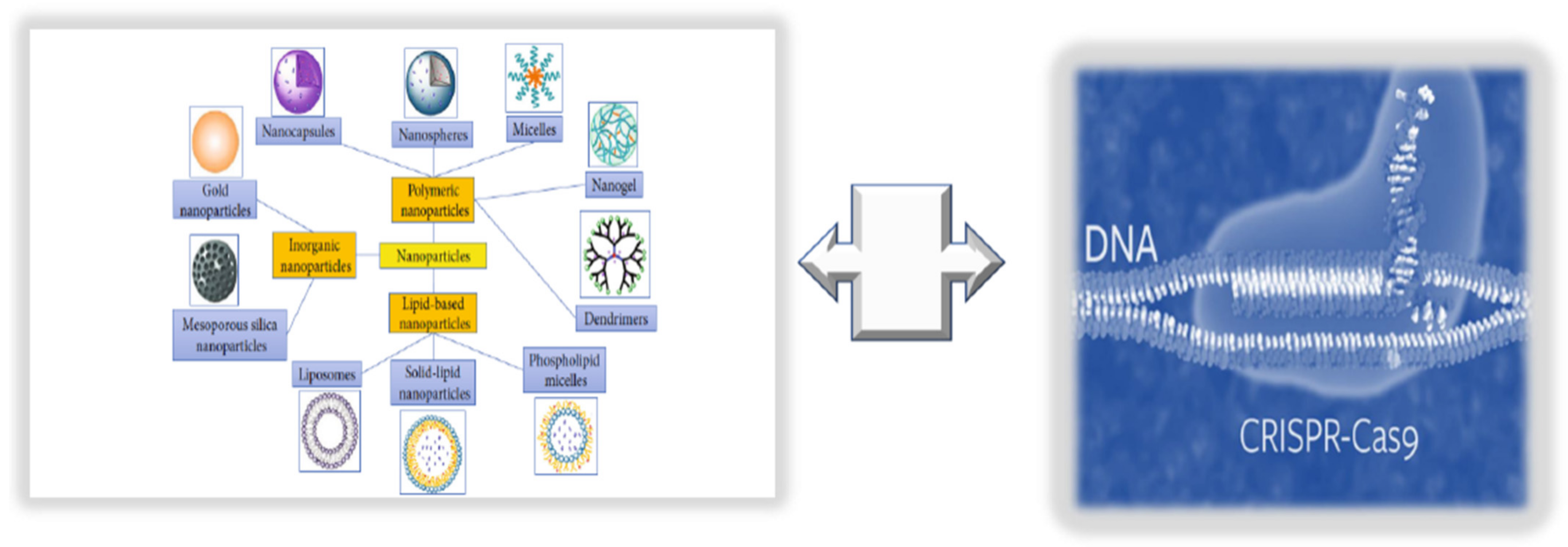
9. Innovative Technologies for Targeted Endothelium Treatments: Nanomedicine and Its Benefits and Limitations
9.1. Consideration of NPs as New Endothelium Treatment Strategy
9.2. Integrating NPs with CRISPR/Cas9 Editing and Base Primers
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inai, K. Chapter 17: Atrioventricular Valve Abnormalities: From Molecular Mechanisms Underlying Morphogenesis to Clinical Perspective. In Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology [Internet]; Nakanishi, T., Markwald, R.R., Baldwin, H.S., Keller, B.B., Srivastava, D., Yamagishi, H., Eds.; Springer: Tokyo, Japan, 2016. [Google Scholar]
- Vita, J.A. Endothelial Function. Circulation 2011, 124, e906–e912. [Google Scholar] [CrossRef] [PubMed]
- Sabe, S.A.; Feng, J.; Sellke, F.W.; Abid, M.R. Mechanisms and clinical implications of endothelium-dependent vasomotor dysfunction in coronary microvasculature. Am. J. Physiol. Circ. Physiol. 2022, 322, H819–H841. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M. Endothelium. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e26–e31. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Madonna, R.; Melino, G.; Caruso, C. The emerging role of Notch pathway in ageing: Focus on the related mechanisms in age-related diseases. Ageing Res. Rev. 2016, 29, 50–65. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Garagnani, P.; Madonna, R.; Vaiserman, A.; Melino, G. Developmental programming of adult haematopoiesis system. Ageing Res. Rev. 2019, 54, 100918. [Google Scholar] [CrossRef]
- Allegra, A.; Giarratana, R.M.; Scola, L.; Balistreri, C.R. The close link between the fetal programming imprinting and neurodegeneration in adulthood: The key role of “hemogenic endothelium” programming. Mech. Ageing Dev. 2021, 195, 111461. [Google Scholar] [CrossRef]
- Schirò, G.; Balistreri, C.R. The close link between brain vascular pathological conditions and neurodegenerative diseases: Focus on some examples and potential treatments. Vasc. Pharmacol. 2021, 142, 106951. [Google Scholar] [CrossRef]
- Pober, J.S.; Min, W.; Bradley, J.R. Mechanisms of Endothelial Dysfunction, Injury, and Death. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 71–95. [Google Scholar] [CrossRef]
- Regina, C.; Panatta, E.; Candi, E.; Melino, G.; Amelio, I.; Balistreri, C.R.; Annicchiarico-Petruzzelli, M.; Di Daniele, N.; Ruvolo, G. Vascular ageing and endothelial cell senescence: Molecular mechanisms of physiology and diseases. Mech. Ageing Dev. 2016, 159, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Novo, G.; Balistreri, C.R. Cellular and molecular basis of the imbalance between vascular damage and repair in ageing and age-related diseases: As biomarkers and targets for new treatments. Mech. Ageing Dev. 2016, 159, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Hsu, Y.-J.; Wu, H.-F.; Lee, G.-L.; Yang, Y.-S.; Wu, J.-Y.; Yet, S.-F.; Wu, K.K.; Kuo, C.-C. Endothelium-Derived 5-Methoxytryptophan Is a Circulating Anti-Inflammatory Molecule That Blocks Systemic Inflammation. Circ. Res. 2016, 119, 222–236. [Google Scholar] [CrossRef]
- Miura, S.; Morimoto, Y.; Furihata, T.; Takeuchi, S. Functional analysis of human brain endothelium using a microfluidic device integrating a cell culture insert. APL Bioeng. 2022, 6, 016103. [Google Scholar] [CrossRef]
- Jalnapurkar, S.; Landes, S.; Wei, J.; Mehta, P.K.; Shufelt, C.; Minissian, M.; Pepine, C.J.; Handberg, E.; Cook-Wiens, G.; Sopko, G.; et al. Coronary endothelial dysfunction appears to be a manifestation of a systemic process: A report from the Women’s Ischemia Syndrome Evaluation—Coronary Vascular Dysfunction (WISE-CVD) study. PLoS ONE 2021, 16, e0257184. [Google Scholar] [CrossRef]
- Meilhac, A.; Cautela, J.; Thuny, F. Cancer Therapies and Vascular Toxicities. Curr. Treat. Options Oncol. 2022, 23, 333–347. [Google Scholar] [CrossRef]
- Camilli, M.; La Vecchia, G.; Lillo, R.; Iannaccone, G.; Lamendola, P.; Montone, R.A.; Hohaus, S.; Aspromonte, N.; Massetti, M.; Lanza, G.A.; et al. Cardiovascular involvement in patients affected by multiple myeloma: A comprehensive review of recent advances. Expert Rev. Hematol. 2021, 14, 1115–1128. [Google Scholar] [CrossRef]
- Love, K.M.; Barrett, E.J.; Malin, S.K.; Reusch, J.E.B.; Regensteiner, J.G.; Liu, Z. Diabetes pathogenesis and management: The endothelium comes of age. J. Mol. Cell Biol. 2021, 13, 500–512. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2021, 19, 173–185. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Grillari, J.; Balistreri, C.R. Cellular Senescence and Inflammaging in Age-Related Diseases. Mediat. Inflamm. 2018, 2018, 9076485. [Google Scholar] [CrossRef]
- Peng, H.; Wang, X.; Du, J.; Cui, Q.; Huang, Y.; Jin, H. Metabolic Reprogramming of Vascular Endothelial Cells: Basic Research and Clinical Applications. Front. Cell Dev. Biol. 2021, 9, 626047. [Google Scholar] [CrossRef]
- Jaul, E.; Barron, J. Characterizing the Heterogeneity of Aging: A Vision for a Staging System for Aging. Front. Public Health 2021, 9, 513557. [Google Scholar] [CrossRef]
- Pisano, C.; Polisano, D.; Balistreri, C.; Altieri, C.; Nardi, P.; Bertoldo, F.; Trombetti, D.; Asta, L.; Ferrante, M.; Buioni, D.; et al. Role of Cachexia and Fragility in the Patient Candidate for Cardiac Surgery. Nutrients 2021, 13, 517. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.J.; Kruse, L.D.; Sanchez-Rivera, L.; Enge, L.; Dusart, P.; Hong, M.-G.; Uhlén, M.; Renné, T.; Schwenk, J.M.; Bergstrom, G.; et al. Identification of Endothelial Proteins in Plasma Associated with Cardiovascular Risk Factors. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2990–3004. [Google Scholar] [CrossRef] [PubMed]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef] [PubMed]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef]
- Zhang, J.; Tecson, K.M.; McCullough, P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020, 21, 315–319. [Google Scholar] [CrossRef]
- Lio, D.; Scola, L.; Giarratana, R.M.; Candore, G.; Colonna-Romano, G.; Caruso, C.; Balistreri, C.R. SARS CoV2 infection _The longevity study perspectives. Ageing Res. Rev. 2021, 67, 101299. [Google Scholar] [CrossRef]
- Wingo, M.; Rafii, S. Endothelial reprogramming for vascular regeneration: Past milestones and future directions. Semin. Cell Dev. Biol. 2021, 122, 50–55. [Google Scholar] [CrossRef]
- León, P.; Wang, J.; Huertas-Vazquez, A. Relevance of Multi-Omics Studies in Cardiovascular Diseases. Front. Cardiovasc. Med. 2019, 6, 91. [Google Scholar] [CrossRef]
- Scola, L.; Giarratana, R.M.; Torre, S.; Argano, V.; Lio, D.; Balistreri, C.R. On the Road to Accurate Biomarkers for Cardiometabolic Diseases by Integrating Precision and Gender Medicine Approaches. Int. J. Mol. Sci. 2019, 20, 6015. [Google Scholar] [CrossRef]
- Greene, J.A.; Loscalzo, J. Putting the Patient Back Together—Social Medicine, Network Medicine, and the Limits of Reductionism. N. Engl. J. Med. 2017, 377, 2493–2499. [Google Scholar] [CrossRef]
- Cheng, F.; Lu, W.; Liu, C.; Fang, J.; Hou, Y.; Handy, D.E.; Wang, R.; Zhao, Y.; Yang, Y.; Huang, J.; et al. A genome-wide positioning systems network algorithm for in silico drug repurposing. Nat. Commun. 2019, 10, 3476. [Google Scholar] [CrossRef]
- Lee, L.Y.-H.; Loscalzo, J. Network Medicine in Pathobiology. Am. J. Pathol. 2019, 189, 1311–1326. [Google Scholar] [CrossRef]
- Abasht, B.; Papah, M.B.; Qiu, J. Evidence of vascular endothelial dysfunction in Wooden Breast disorder in chickens: Insights through gene expression analysis, ultra-structural evaluation and supervised machine learning methods. PLoS ONE 2021, 16, e0243983. [Google Scholar] [CrossRef]
- Treder, M.; Lauermann, J.L.; Alnawaiseh, M.; Eter, N. Using Deep Learning in Automated Detection of Graft Detachment in Descemet Membrane Endothelial Keratoplasty: A Pilot Study. Cornea 2018, 38, 157–161. [Google Scholar] [CrossRef]
- Sun, X.; Feinberg, M.W. Vascular Endothelial Senescence: Pathobiological Insights, Emerging Long Noncoding RNA Targets, Challenges and Therapeutic Opportunities. Front. Physiol. 2021, 12, 693067. [Google Scholar] [CrossRef]
- Kumar, S.; Andueza, A.; Kim, J.; Kang, D.-W.; Jo, H. Isolation of Endothelial Cells from the Lumen of Mouse Carotid Arteries for Single-cell Multi-omics Experiments. J. Vis. Exp. 2021, 176, e63128. [Google Scholar] [CrossRef]
- Andueza, A.; Kumar, S.; Kim, J.; Kang, D.-W.; Mumme, H.L.; Perez, J.I.; Villa-Roel, N.; Jo, H. Endothelial Reprogramming by Disturbed Flow Revealed by Single-Cell RNA and Chromatin Accessibility Study. Cell Rep. 2020, 33, 108491. [Google Scholar] [CrossRef]
- He, M.; Huang, T.-S.; Li, S.; Hong, H.-C.; Chen, Z.; Martin, M.; Zhou, X.; Huang, H.-Y.; Su, S.-H.; Zhang, J.; et al. Atheroprotective Flow Upregulates ITPR3 (Inositol 1,4,5-Trisphosphate Receptor 3) in Vascular Endothelium via KLF4 (Krüppel-Like Factor 4)-Mediated Histone Modifications. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 902–914. [Google Scholar] [CrossRef]
- Zhang, M.; Urabe, G.; Ozer, H.G.; Xie, X.; Webb, A.; Shirasu, T.; Li, J.; Han, R.; Kent, K.C.; Wang, B.; et al. Angioplasty induces epigenomic remodeling in injured arteries. Life Sci. Alliance 2022, 5, e202101114. [Google Scholar] [CrossRef]
- Pepin, M.E.; Schiano, C.; Miceli, M.; Benincasa, G.; Mansueto, G.; Grimaldi, V.; Soricelli, A.; Wende, A.R.; Napoli, C. The human aortic endothelium undergoes dose-dependent DNA methylation in response to transient hyperglycemia. Exp. Cell Res. 2021, 400, 112485. [Google Scholar] [CrossRef]
- Matsuoka, R.L.; Buck, L.D.; Vajrala, K.P.; Quick, R.E.; Card, O.A. Historical and current perspectives on blood endothelial cell heterogeneity in the brain. Experientia 2022, 79, 372. [Google Scholar] [CrossRef]
- Benincasa, G.; Mansueto, G.; Napoli, C. Fluid-based assays and precision medicine of cardiovascular diseases: The ‘hope’ for Pandora’s box? J. Clin. Pathol. 2019, 72, 785–799. [Google Scholar] [CrossRef]
- Mattox, A.K.; Bettegowda, C.; Zhou, S.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B. Applications of liquid biopsies for cancer. Sci. Transl. Med. 2019, 11, eaay1984. [Google Scholar] [CrossRef]
- Rubis, G.; Krishnan, S.; Bebawy, M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol. Sci. 2019, 40, 172–186. [Google Scholar] [CrossRef]
- Perakis, S.; Speicher, M.R. Emerging concepts in liquid biopsies. BMC Med. 2017, 15, 75. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Buffa, S.; Pisano, C.; Lio, D.; Ruvolo, G.; Mazzesi, G. Are Endothelial Progenitor Cells the Real Solution for Cardiovascular Diseases? Focus on Controversies and Perspectives. BioMed Res. Int. 2015, 2015, 835934. [Google Scholar] [CrossRef]
- Balistrieri, C. Endothelial Progenitor Cells: A New Real Hope? Springer International Publishing: Dordrecht, The Netherlands, 2017; pp. 1–80. [Google Scholar]
- Olivieri, F.; Pompilio, G.; Balistreri, C.R. Endothelial progenitor cells in ageing. Mech. Ageing Dev. 2016, 159, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Altabas, V.; Altabas, K.; Kirigin, L. Endothelial progenitor cells (EPCs) in ageing and age-related diseases: How currently available treatment modalities affect EPC biology, atherosclerosis, and cardiovascular outcomes. Mech. Ageing Dev. 2016, 159, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Crapanzano, F.; Schirone, L.; Allegra, A.; Pisano, C.; Ruvolo, G.; Forte, M.; Greco, E.; Cavarretta, E.; Marullo, A.G.M.; et al. Deregulation of Notch1 pathway and circulating endothelial progenitor cell (EPC) number in patients with bicuspid aortic valve with and without ascending aorta aneurysm. Sci. Rep. 2018, 8, 13834. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Pisano, C.; Bertoldo, F.; Massoud, R.; Dolci, S.; Ruvolo, G. Red Blood Cell Distribution Width, Vascular Aging Biomarkers, and Endothelial Progenitor Cells for Predicting Vascular Aging and Diagnosing/Prognosing Age-Related Degenerative Arterial Diseases. Rejuvenation Res. 2019, 22, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Guggino, G.; Di Liberto, D.; Ciccia, F.; Cipriani, P.; Balistreri, C.R.; Sireci, G.; Giacomelli, R.; Triolo, G. Endothelial progenitor cells: Are they displaying a function in autoimmune disorders? Mech. Ageing Dev. 2016, 159, 44–48. [Google Scholar] [CrossRef][Green Version]
- Loiola, R.A.; García-Gabilondo, M.; Grayston, A.; Bugno, P.; Kowalska, A.; Duban-Deweer, S.; Rizzi, E.; Hachani, J.; Sano, Y.; Shimizu, F.; et al. Secretome of endothelial progenitor cells from stroke patients promotes endothelial barrier tightness and protects against hypoxia-induced vascular leakage. Stem Cell Res. Ther. 2021, 12, 552. [Google Scholar] [CrossRef]
- Morrone, D.; Picoi, M.E.L.; Felice, F.; De Martino, A.; Scatena, C.; Spontoni, P.; Naccarato, A.G.; Di Stefano, R.; Bortolotti, U.; Monte, M.D.; et al. Endothelial Progenitor Cells: An Appraisal of Relevant Data from Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 12874. [Google Scholar] [CrossRef]
- Ota, Y.; Kuwana, M. Endothelial cells and endothelial progenitor cells in the pathogenesis of systemic sclerosis. Eur. J. Rheumatol. 2020, 7, 139–146. [Google Scholar] [CrossRef]
- Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Atienza-Mateo, B.; Mora-Cuesta, V.; Iturbe-Fernández, D.; Lera-Gómez, L.; Pérez-Fernández, R.; Prieto-Peña, D.; Portilla, V.; et al. Endothelial Progenitor Cells: Relevant Players in the Vasculopathy and Lung Fibrosis Associated with the Presence of Interstitial Lung Disease in Systemic Sclerosis Patients. Biomedicines 2021, 9, 847. [Google Scholar] [CrossRef]
- Endothelial progenitor cells and coronary artery disease: Current concepts and future research directions. World J. Clin. Cases 2021, 9, 8953–8966. [CrossRef]
- Buffa, S.; Borzì, D.; Chiarelli, R.; Crapanzano, F.; Lena, A.M.; Nania, M.; Candi, E.; Triolo, F.; Ruvolo, G.; Melino, G.; et al. Biomarkers for vascular ageing in aorta tissues and blood samples. Exp. Gerontol. 2019, 128, 110741. [Google Scholar] [CrossRef]
- Poz, D.; De Falco, E.; Pisano, C.; Madonna, R.; Ferdinandy, P.; Balistreri, C.R. Diagnostic and Prognostic Relevance of Red Blood Cell Distribution Width for Vascular Aging and Cardiovascular Diseases. Rejuvenation Res. 2019, 22, 146–162. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Naruse, K.; Hamada, Y.; Nakashima, E.; Kato, K.; Mizubayashi, R.; Kamiya, H.; Yuzawa, Y.; Matsuo, S.; Murohara, T.; Matsubara, T.; et al. Therapeutic Neovascularization Using Cord Blood–Derived Endothelial Progenitor Cells for Diabetic Neuropathy. Diabetes 2005, 54, 1823–1828, Erratum in Diabetes 2006, 55, 1534. [Google Scholar] [CrossRef]
- Shyu, W.-C.; Lin, S.-Z.; Chiang, M.-F.; Su, C.-Y.; Li, H. Intracerebral Peripheral Blood Stem Cell (CD34+) Implantation Induces Neuroplasticity by Enhancing beta1 Integrin-Mediated Angiogenesis in Chronic Stroke Rats. J. Neurosci. 2006, 26, 3444–3453. [Google Scholar] [CrossRef]
- Gómez-Navarro, J.; Contreras, J.L.; Arafat, W.; Jiang, X.L.; Krisky, D.; Oligino, T.; Marconi, P.; Hubbard, B.; Glorioso, J.C.; Curiel, D.T.; et al. Genetically modified CD34+ cells as cellular vehicles for gene delivery into areas of angiogenesis in a rhesus model. Gene Ther. 2000, 7, 43–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohtsuka, M.; Takano, H.; Zou, Y.; Toko, H.; Akazawa, H.; Qin, Y.; Suzuki, M.; Hasegawa, H.; Nakaya, H.; Komuro, I. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. FASEB J. 2004, 18, 851–853. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kondo, T.; Suzuki, S.; Izawa, H.; Kobayashi, M.; Emi, N.; Komori, K.; Naoe, T.; Takamatsu, J.; Murohara, T. Molecular Evaluation of Endothelial Progenitor Cells in Patients with Ischemic Limbs. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e192–e196. [Google Scholar] [CrossRef]
- Lenk, K.; Adams, V.; Lurz, P.; Erbs, S.; Linke, A.; Gielen, S.; Schmidt, A.; Scheinert, D.; Biamino, G.; Emmrich, F.; et al. Therapeutical potential of blood-derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischaemia. Eur. Heart J. 2005, 26, 1903–1909. [Google Scholar] [CrossRef]
- Erbs, S.; Linke, A.; Adams, V.; Lenk, K.; Thiele, H.; Diederich, K.-W.; Emmrich, F.; Kluge, R.; Kendziorra, K.; Sabri, O.; et al. Transplantation of Blood-Derived Progenitor Cells After Recanalization of Chronic Coronary Artery Occlusion. Circ. Res. 2005, 97, 756–762. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Liang, H.; Cai, Y.; Li, X.; Yan, L.; Zhou, L.; Shan, L.; Wang, H. Human umbilical cord-derived mesenchymal stem cells not only ameliorate blood glucose but also protect vascular endothelium from diabetic damage through a paracrine mechanism mediated by MAPK/ERK signaling. Stem Cell Res. Ther. 2022, 13, 258. [Google Scholar] [CrossRef]
- Chen, L.-L. The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 2016, 17, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Chen, L.-L. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell 2018, 71, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules ex-isting as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-T.; Coca-Prados, M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature 1979, 280, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Bao, Y.; Yee, M.-C.; Barrett, S.P.; Hogan, G.J.; Olsen, M.N.; Dinneny, J.R.; Brown, P.O.; Salzman, J. Circular RNA Is Expressed across the Eukaryotic Tree of Life. PLoS ONE 2014, 9, e90859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xie, X.; Li, M.; Shi, J.; Zhou, J.J.; Knox, K.S.; Wang, T.; Chen, Q.; Gu, W. Rat BodyMap transcriptomes reveal unique circular RNA features across tissue types and developmental stages. RNA 2018, 24, 1443–1456. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Huang, A.; Zheng, H.; Wu, Z.; Chen, M.; Huang, Y. Circular RNA-protein interactions: Functions, mechanisms, and identification. Theranostics 2020, 10, 3503–3517. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Tan, X.; Deng, J.; Li, Y.; Piao, Y.; Li, C.; Yang, W.; Mo, W.; Sun, J.; et al. Microarray expression profile of circular RNAs and mRNAs in children with systemic lupus erythematosus. Clin. Rheumatol. 2019, 38, 1339–1350. [Google Scholar] [CrossRef]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37.e9. [Google Scholar] [CrossRef]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of CircRNAs. Mol. Cell 2017, 66, 9–21.e7. [Google Scholar] [CrossRef]
- Shang, Q.; Yang, Z.; Jia, R.; Ge, S. The novel roles of circRNAs in human cancer. Mol. Cancer 2019, 18, 6. [Google Scholar] [CrossRef]
- Vo, J.N.; Cieslik, M.; Zhang, Y.; Shukla, S.; Xiao, L.; Zhang, Y.; Wu, Y.-M.; Dhanasekaran, S.M.; Engelke, C.G.; Cao, X.; et al. The Landscape of Circular RNA in Cancer. Cell 2019, 176, 869–881.e13. [Google Scholar] [CrossRef]
- Aufiero, S.; Reckman, Y.J.; Pinto, Y.M.; Creemers, E.E. Circular RNAs open a new chapter in cardiovascular biology. Nat. Rev. Cardiol. 2019, 16, 503–514. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Huang, S.; Zhao, L. Roles of circular RNAs in immune regulation and autoimmune diseases. Cell Death Dis. 2019, 10, 503. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Xiao, J. Circular RNAs: Promising Biomarkers for Human Diseases. eBioMedicine 2018, 34, 267–274. [Google Scholar] [CrossRef]
- Wu, W.-P.; Zhou, M.-Y.; Liu, D.-L.; Min, X.; Shao, T.; Xu, Z.-Y.; Jing, X.; Cai, M.-Y.; Xu, S.; Liang, X.; et al. circGNAQ, a circular RNA enriched in vascular endothelium, inhibits endothelial cell senescence and atherosclerosis progression. Mol. Ther. Nucleic Acids 2021, 26, 374–387. [Google Scholar] [CrossRef]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Sun, N.; Wang, T.; Zhu, J.; Yang, S.; Song, X.; Wang, R.; Wang, X.; Zhao, Y.; et al. Differentially Expressed Circular Non-coding RNAs in Atherosclerotic Aortic Vessels and Their Potential Functions in Endothelial Injury. Front. Cardiovasc. Med. 2021, 8, 657544. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Liu, Z.; Ma, X.; Li, M.; Lu, Q.; Li, Y.; Lu, Z.; Niu, L.; Fan, Z.; et al. Circular RNA circ_0124644 exacerbates the ox-LDL-induced endothelial injury in human vascular endothelial cells through regulating PAPP-A by acting as a sponge of miR-149-5p. Mol. Cell. Biochem. 2020, 471, 51–61. [Google Scholar] [CrossRef]
- Yu, H.; Pan, Y.; Dai, M.; Xu, H.; Li, J. Circ_0003423 Alleviates ox-LDL-Induced Human Brain Microvascular Endothelial Cell Injury via the miR-589-5p/TET2 Network. Neurochem. Res. 2021, 46, 2885–2896. [Google Scholar] [CrossRef]
- Zhu, Q.-Q.; Pu, X.-B.; Chen, T.-C.; Qiu, C.-Y.; Wu, Z.-H.; Tian, L.; He, Y.-Y.; Wang, X.-H.; Shang, T.; Wang, X.; et al. Hsa_circ_0008360 sponges miR-186-5p to target CCND2 to modulate high glucose-induced vascular endothelial dysfunction. Cell Cycle 2021, 20, 1389–1401. [Google Scholar] [CrossRef]
- Huang, H.-S.; Huang, X.-Y.; Yu, H.-Z.; Xue, Y.; Zhu, P.-L. Circular RNA circ-RELL1 regulates inflammatory response by miR-6873-3p/MyD88/NF-κB axis in endothelial cells. Biochem. Biophys. Res. Commun. 2020, 525, 512–519, Erratum in Biochem. Biophys. Res. Commun. 2020, 532, 682. [Google Scholar] [CrossRef] [PubMed]
- Byron, S.; Van Keuren-Jensen, K.R.; Engelthaler, D.M.; Carpten, J.D.; Craig, D.W. Translating RNA sequencing into clinical diagnostics: Opportunities and challenges. Nat. Rev. Genet. 2016, 17, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Salzman, J. Detecting circular RNAs: Bioinformatic and experimental challenges. Nat. Rev. Genet. 2016, 17, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.F.; Bindereif, A.; Bozzoni, I.; Hanan, M.; Hansen, T.B.; Irimia, M.; Kadener, S.; Kristensen, L.S.; Legnini, I.; Morlando, M.; et al. Best practice standards for circular RNA research. Nat. Methods 2022, 1–13. [Google Scholar] [CrossRef]
- Mannion, N.; Arieti, F.; Gallo, A.; Keegan, L.P.; O’Connell, M.A. New Insights into the Biological Role of Mammalian ADARs; the RNA Editing Proteins. Biomolecules 2015, 5, 2338–2362. [Google Scholar] [CrossRef]
- Hogg, M.; Paro, S.; Keegan, L.P.; O’Connell, M.A. RNA Editing by Mammalian ADARs. Adv. Genet. 2011, 73, 87–120. [Google Scholar] [CrossRef]
- Song, C.; Sakurai, M.; Shiromoto, Y.; Nishikura, K. Functions of the RNA Editing Enzyme ADAR1 and Their Relevance to Human Diseases. Genes 2016, 7, 129. [Google Scholar] [CrossRef]
- Cayir, A. RNA A-to-I editing, environmental exposure, and human diseases. Crit. Rev. Toxicol. 2021, 51, 456–466. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, H.; Zhou, S.; Zhuang, Y. ADAR1 is targeted by miR-143 to regulate IL-1β-induced endothelial activation through the NFκB pathway. Int. J. Biochem. Cell Biol. 2017, 89, 25–33. [Google Scholar] [CrossRef]
- Van der Kwast, R.V.; Parma, L.; van der Bent, M.L.; van Ingen, E.; Baganha, F.; Peters, H.A.; Goossens, E.A.; Simons, K.H.; Palmen, M.; de Vries, M.R.; et al. Adenosine-to-Inosine Editing of Vasoactive MicroRNAs Alters Their Targetome and Function in Ischemia. Mol. Ther. Nucleic Acids 2020, 21, 932–953. [Google Scholar] [CrossRef]
- Vlachogiannis, N.I.; Sachse, M.; Georgiopoulos, G.; Zormpas, E.; Bampatsias, D.; Delialis, D.; Bonini, F.; Galyfos, G.; Sigala, F.; Stamatelopoulos, K.; et al. Adenosine-to-inosine Alu RNA editing controls the stability of the pro-inflammatory long noncoding RNA NEAT1 in atherosclerotic cardiovascular disease. J. Mol. Cell. Cardiol. 2021, 160, 111–120. [Google Scholar] [CrossRef]
- Fei, J.; Cui, X.-B.; Wang, J.-N.; Dong, K.; Chen, S.-Y. ADAR1-Mediated RNA Editing, A Novel Mechanism Controlling Phenotypic Modulation of Vascular Smooth Muscle Cells. Circ. Res. 2016, 119, 463–469. [Google Scholar] [CrossRef]
- van der Kwast, R.V.C.T.; van Ingen, E.; Parma, L.; Peters, H.A.B.; Quax, P.H.A.; Nossent, A.Y. Adenosine-to-Inosine editing of MicroRNA-487b alters target gene selection after ischemia and promotes neovascularization. Circ. Res. 2018, 122, 444–456. [Google Scholar] [CrossRef]
- Schirle, N.T.; Goodman, R.A.; Krishnamurthy, M.; Beal, P.A. Selective inhibition of ADAR2-catalyzed editing of the serotonin 2c receptor pre-mRNA by a helix-threading peptide. Org. Biomol. Chem. 2010, 8, 4898–4904. [Google Scholar] [CrossRef]
- Montiel-González, M.F.; Vallecillo-Viejo, I.C.; Rosenthal, J.J.C. An efficient system for selectively altering genetic information within mRNAs. Nucleic Acids Res. 2016, 44, e157. [Google Scholar] [CrossRef]
- Suzuki, A.; Tomita, H.; Okada, H. Form follows function: The endothelial glycocalyx. Transl. Res. 2022, in press. [Google Scholar] [CrossRef]
- Hu, Z.; Cano, I.; D’Amore, P.A. Update on the Role of the Endothelial Glycocalyx in Angiogenesis and Vascular Inflammation. Front. Cell Dev. Biol. 2021, 9, 734276. [Google Scholar] [CrossRef]
- Gaudette, S.; Hughes, D.; Boller, M. The endothelial glycocalyx: Structure and function in health and critical illness. J. Vet. Emerg. Crit. Care 2020, 30, 117–134. [Google Scholar] [CrossRef]
- Dogné, S.; Flamion, B. Endothelial Glycocalyx Impairment in Disease. Am. J. Pathol. 2020, 190, 768–780. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Koutlas, V.; Pappas, C.; Mitsis, M.; Dounousi, E. The Endothelial Glycocalyx as a Target of Ischemia and Reperfusion Injury in Kidney Transplantation—Where Have We Gone So Far? Int. J. Mol. Sci. 2021, 22, 2157. [Google Scholar] [CrossRef]
- Milusev, A.; Rieben, R.; Sorvillo, N. The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front. Cardiovasc. Med. 2022, 9, 897087. [Google Scholar] [CrossRef]
- Salmon, A.H.; Neal, C.R.; Sage, L.M.; Glass, C.A.; Harper, S.J.; Bates, D.O. Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc. Res. 2009, 83, 24–33. [Google Scholar] [CrossRef]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Bruegger, D.; Rehm, M.; Conzen, P.; Welsch, U.; Becker, B.F. Hydrocortisone Preserves the Vascular Barrier by Protecting the Endothelial Glycocalyx. Anesthesiology 2007, 107, 776–784. [Google Scholar] [CrossRef]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc. Res. 2009, 83, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gayosso, I.; Platts, S.H.; Duling, B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Circ. Physiol. 2006, 290, H2247–H2256. [Google Scholar] [CrossRef] [PubMed]
- Filho, I.P.T.; Torres, L.N.; Salgado, C.; Dubick, M.A. Novel Adjunct Drugs Reverse Endothelial Glycocalyx Damage after Hemorrhagic Shock in Rats. Shock 2017, 48, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Kozar, R.A.; Peng, Z.; Zhang, R.; Holcomb, J.B.; Pati, S.; Park, P.; Ko, T.C.; Paredes, A. Plasma Restoration of Endothelial Glycocalyx in a Rodent Model of Hemorrhagic Shock. Anesth. Analg. 2011, 112, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Lipowsky, H.H.; Lescanic, A. Inhibition of inflammation induced shedding of the endothelial glycocalyx with low molecular weight heparin. Microvasc. Res. 2017, 112, 72–78. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Liu, X.; Wang, T.; Xu, H.; Xia, B.; Kong, G.; Li, J.; Zhu, W.; Hu, H.; et al. Both UFH and NAH alleviate shedding of endothelial glycocalyx and coagulopathy in LPSinduced sepsis. Exp. Ther. Med. 2019, 19, 913–922. [Google Scholar] [CrossRef]
- Masola, V.; Onisto, M.; Zaza, G.; Lupo, A.; Gambaro, G. A new mechanism of action of sulodexide in diabetic nephropathy: Inhibits heparanase-1 and prevents FGF-2-induced renal epithelial-mesenchymal transition. J. Transl. Med. 2012, 10, 213. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, Y.; Zhang, J.; Wu, Y.; Xiang, X.; Zhang, Z.; Li, T.; Liu, L. The Beneficial Effect of HES on Vascular Permeability and Its Relationship with Endothelial Glycocalyx and Intercellular Junction after Hemorrhagic Shock. Front. Pharmacol. 2020, 11, 597. [Google Scholar] [CrossRef]
- Gumanova, N.G.; Gorshkov, A.U.; Klimushina, M.V.; Kots, A.Y. Associations of endothelial biomarkers, nitric oxide metabolites and endothelin, with blood pressure and coronary lesions depend on cardiovascular risk and sex to mark endothelial dysfunction on the SCORE scale. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20200024. [Google Scholar] [CrossRef]
- Beckman, J.A.; Hu, J.-R.; Huang, S.; Farber-Eger, E.; Wells, Q.S.; Wang, T.J.; Gerszten, R.E.; Ferguson, J.F. Metabolomics reveals the impact of Type 2 diabetes on local muscle and vascular responses to ischemic stress. Clin. Sci. 2020, 134, 2369–2379. [Google Scholar] [CrossRef]
- Wu, K.K.; Kuo, C.C.; Yet, S.F.; Lee, C.M.; Liou, J.Y. 5-methoxytryptophan: An arsenal against vascular injury and inflammation. J. Biomed. Sci. 2020, 27, 79. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Morga, M.; Nattich-Rak, M.; Sadowska, M. Nanoparticle and bioparticle deposition kinetics. Adv. Colloid Interface Sci. 2022, 302, 102630. [Google Scholar] [CrossRef]
- Chacko, A.M.; Hood, E.D.; Zern, B.J.; Muzykantov, V.R. Targeted nano-carriers for imaging and therapy of vascular inflammation. Curr. Opin. Colloid Interface Sci. 2011, 16, 215–227. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Yoo, J.W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bio-engineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef]
- Robbins, G.P.; Saunders, R.L.; Haun, J.B.; Rawson, J.; Therien, M.J.; Hammer, D.A. Tunable leuko-polymersomes that adhere specifically to in-flammatory markers. Langmuir 2010, 26, 14089–14096. [Google Scholar] [CrossRef]
- Parodi, A.; Quattrocchi, N.; van de Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V.; et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013, 8, 61–68. [Google Scholar] [CrossRef]
- McMahon, K.M.; Mutharasan, R.K.; Tripathy, S.; Veliceasa, D.; Bobeica, M.; Shumaker, D.K.; Luthi, A.J.; Helfand, B.T.; Ardehali, H.; Mirkin, C.A.; et al. Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett. 2011, 11, 1208–1214. [Google Scholar] [CrossRef]
- Taneja, G.; Sud, A.; Pendse, N.; Panigrahi, B.; Kumar, A.; Sharma, A.K. Nano-Medicine and Vascular Endothelial Dysfunction: Options and Delivery Strategies. Cardiovasc. Toxicol. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 3149223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, R.; Liu, C.; Pan, L.; Qi, Y.; Cheng, J.; Guo, J.; Jia, Y.; Ding, J.; Zhang, J.; et al. A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases. Biomaterials 2019, 230, 119605. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, S.; Bru, R.; Clément, C.; Jacquard, C.; Nabavi, S.F.; Khayatkashani, M.; Batiha, G.E.-S.; Khan, H.; et al. Resveratrol and cyclodextrins, an easy alliance: Applications in nanomedicine, green chemistry and biotechnology. Biotechnol. Adv. 2021, 53, 107844. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.H.; Park, J.R.; Nakamura, M.T.; Odintsov, B.M.; Wallig, M.A.; Chung, B.H. Synthetic dimyristoylphosphatidylcholine liposomes assimilating into high-density lipoprotein promote regression of atherosclerotic lesions in cholesterol-fed rab-bits. Exp. Biol. Med. 2010, 235, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.C.; Borah, A.; Jacob, E.M.; Kumar, D.S. Nanotechnological approach to delivering nutraceuticals as promising drug candidates for the treatment of atherosclerosis. Drug Deliv. 2021, 28, 550–568. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Cox, D.B.T.; Platt, R.J.; Zhang, F. Therapeutic genome editing: Prospects and challenges. Nat. Med. 2015, 21, 121–131. [Google Scholar] [CrossRef]
- Varshney, G.K.; Pei, W.; LaFave, M.C.; Idol, J.; Xu, L.; Gallardo, V.; Carrington, B.; Bishop, K.; Jones, M.; Li, M.; et al. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 2015, 25, 1030–1042. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, H.; Huang, X.; Chaurasiya, B.; Dong, D.; Shanley, T.P.; Zhao, Y.-Y. Robust genome editing in adult vascular endothelium by nanoparticle delivery of CRISPR-Cas9 plasmid DNA. Cell Rep. 2022, 38, 110196. [Google Scholar] [CrossRef]
- Botto, C.; Dalkara, D.; El-Amraoui, A. Progress in Gene Editing Tools and Their Potential for Correcting Mutations Underlying Hearing and Vision Loss. Front. Genome Ed. 2021, 3, 737632. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balistreri, C.R. Promising Strategies for Preserving Adult Endothelium Health and Reversing Its Dysfunction: From Liquid Biopsy to New Omics Technologies and Noninvasive Circulating Biomarkers. Int. J. Mol. Sci. 2022, 23, 7548. https://doi.org/10.3390/ijms23147548
Balistreri CR. Promising Strategies for Preserving Adult Endothelium Health and Reversing Its Dysfunction: From Liquid Biopsy to New Omics Technologies and Noninvasive Circulating Biomarkers. International Journal of Molecular Sciences. 2022; 23(14):7548. https://doi.org/10.3390/ijms23147548
Chicago/Turabian StyleBalistreri, Carmela Rita. 2022. "Promising Strategies for Preserving Adult Endothelium Health and Reversing Its Dysfunction: From Liquid Biopsy to New Omics Technologies and Noninvasive Circulating Biomarkers" International Journal of Molecular Sciences 23, no. 14: 7548. https://doi.org/10.3390/ijms23147548
APA StyleBalistreri, C. R. (2022). Promising Strategies for Preserving Adult Endothelium Health and Reversing Its Dysfunction: From Liquid Biopsy to New Omics Technologies and Noninvasive Circulating Biomarkers. International Journal of Molecular Sciences, 23(14), 7548. https://doi.org/10.3390/ijms23147548





