Increase in Anticancer Drug-Induced Toxicity by Fisetin in Lung Adenocarcinoma A549 Spheroid Cells Mediated by the Reduction of Claudin-2 Expression
Abstract
:1. Introduction
2. Results
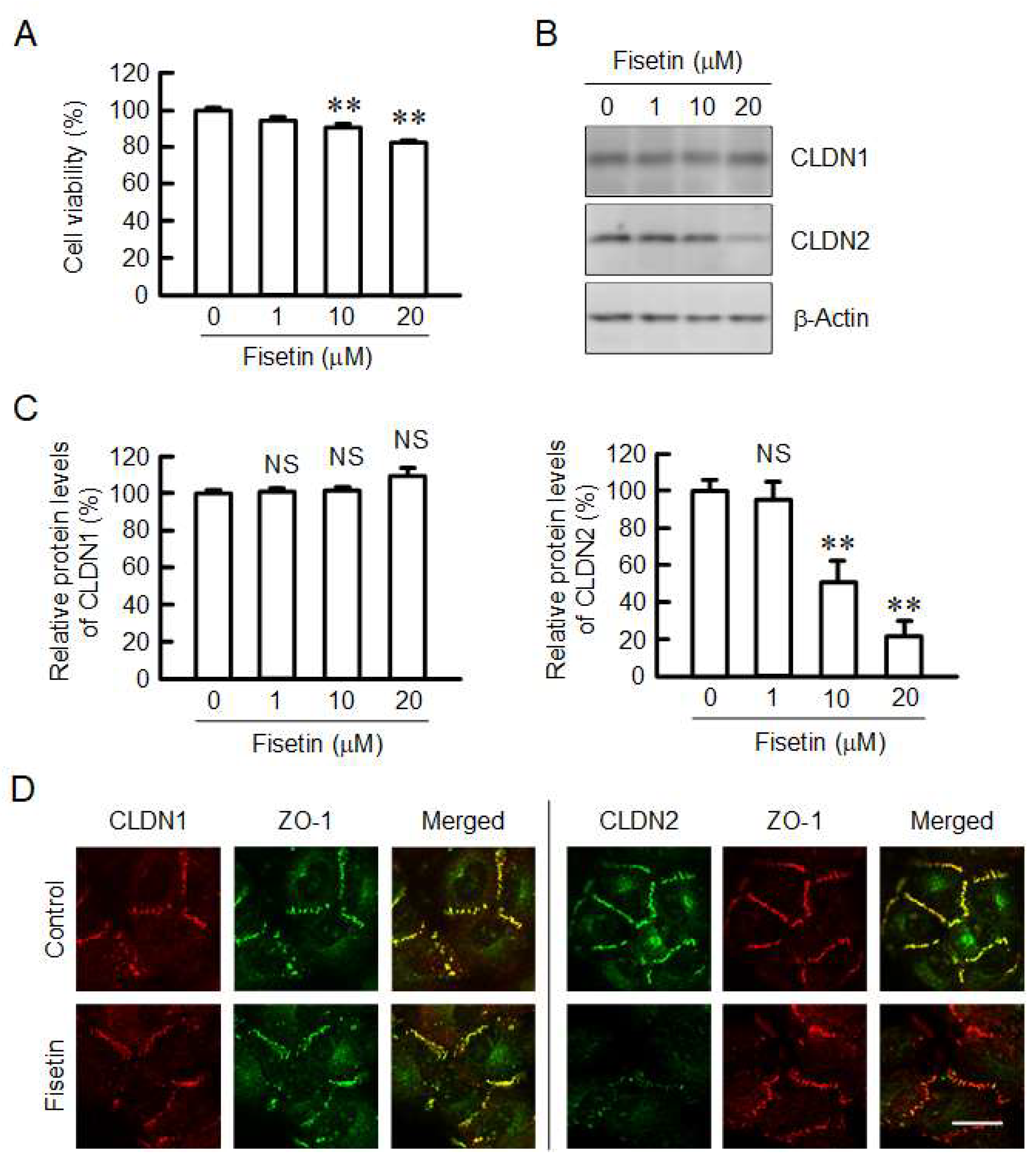
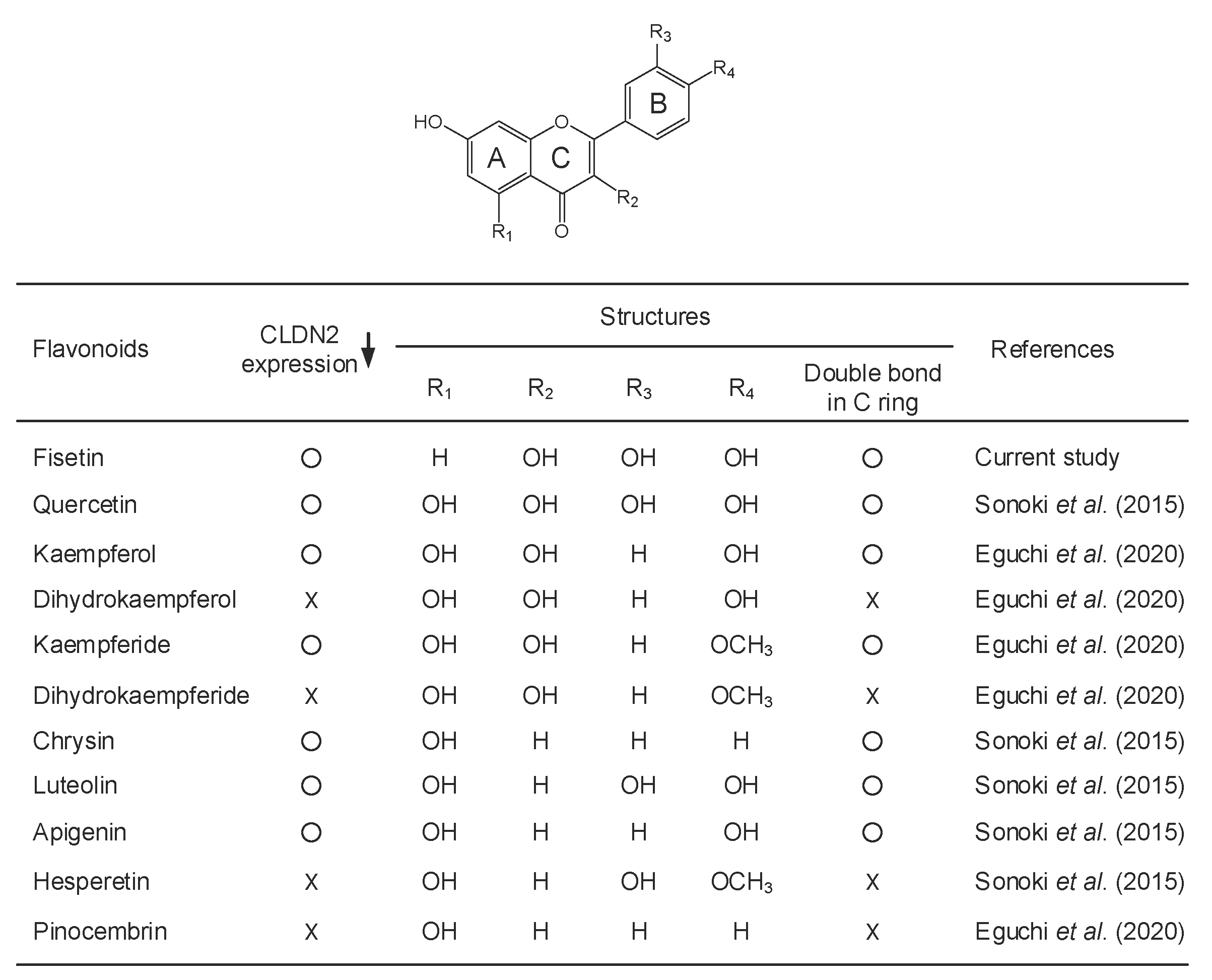
2.1. Decrease in Protein Level of CLDN2 in A549 Cells by Fisetin
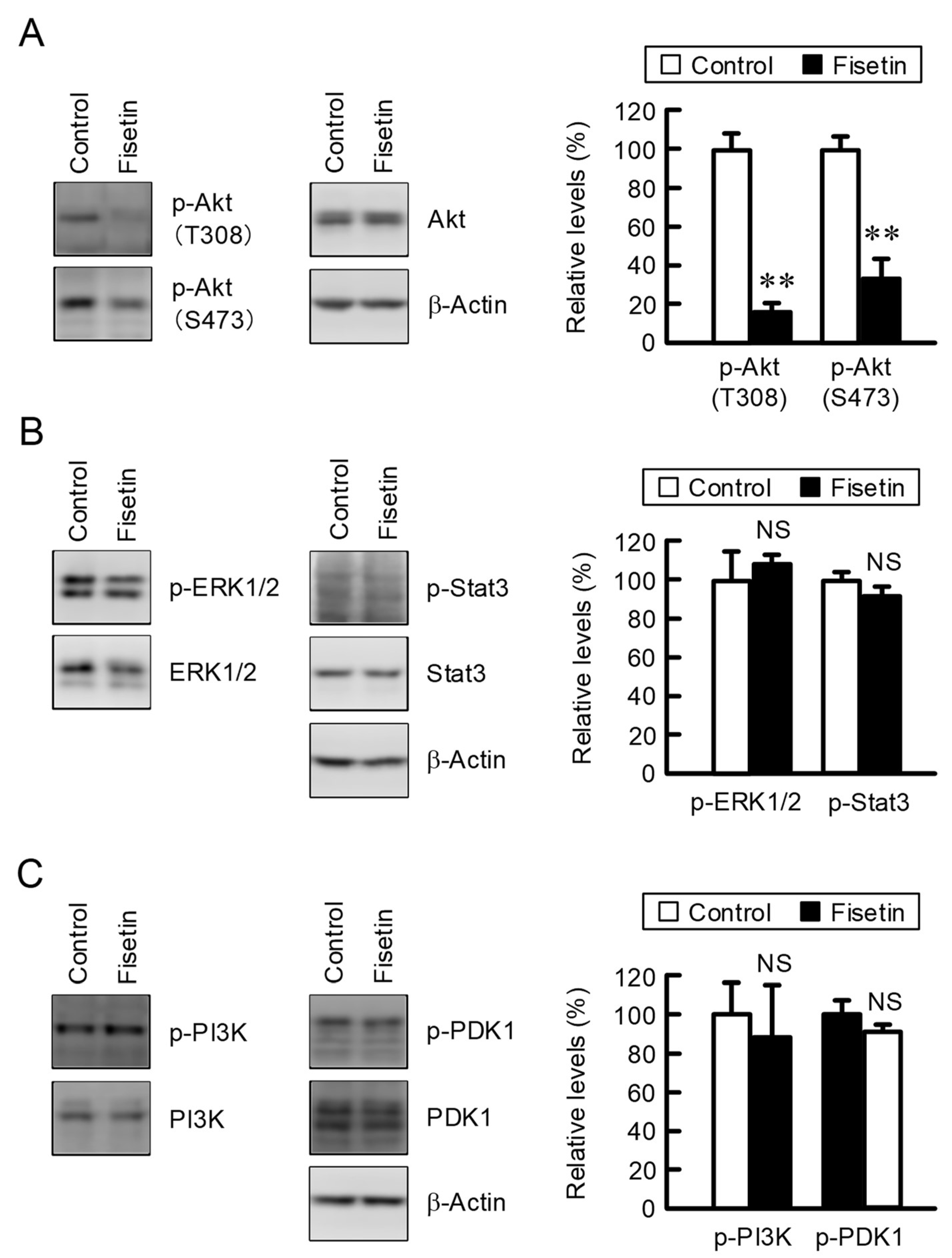
2.2. Effect of Fisetin on Intracellular Signaling Factors
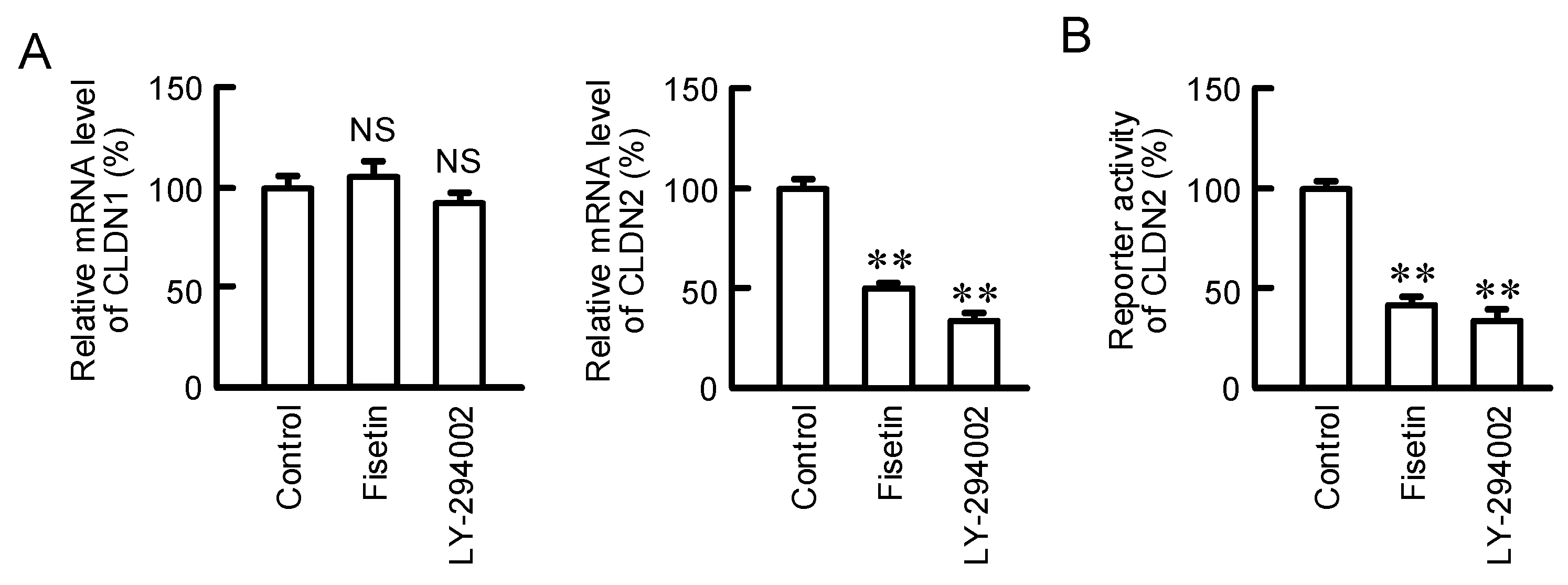
2.3. Effects of Fisetin and PI3K Inhibitor on the Expression of CLDN2 mRNA
2.4. Effects of Fisetin on Property of Spheroids
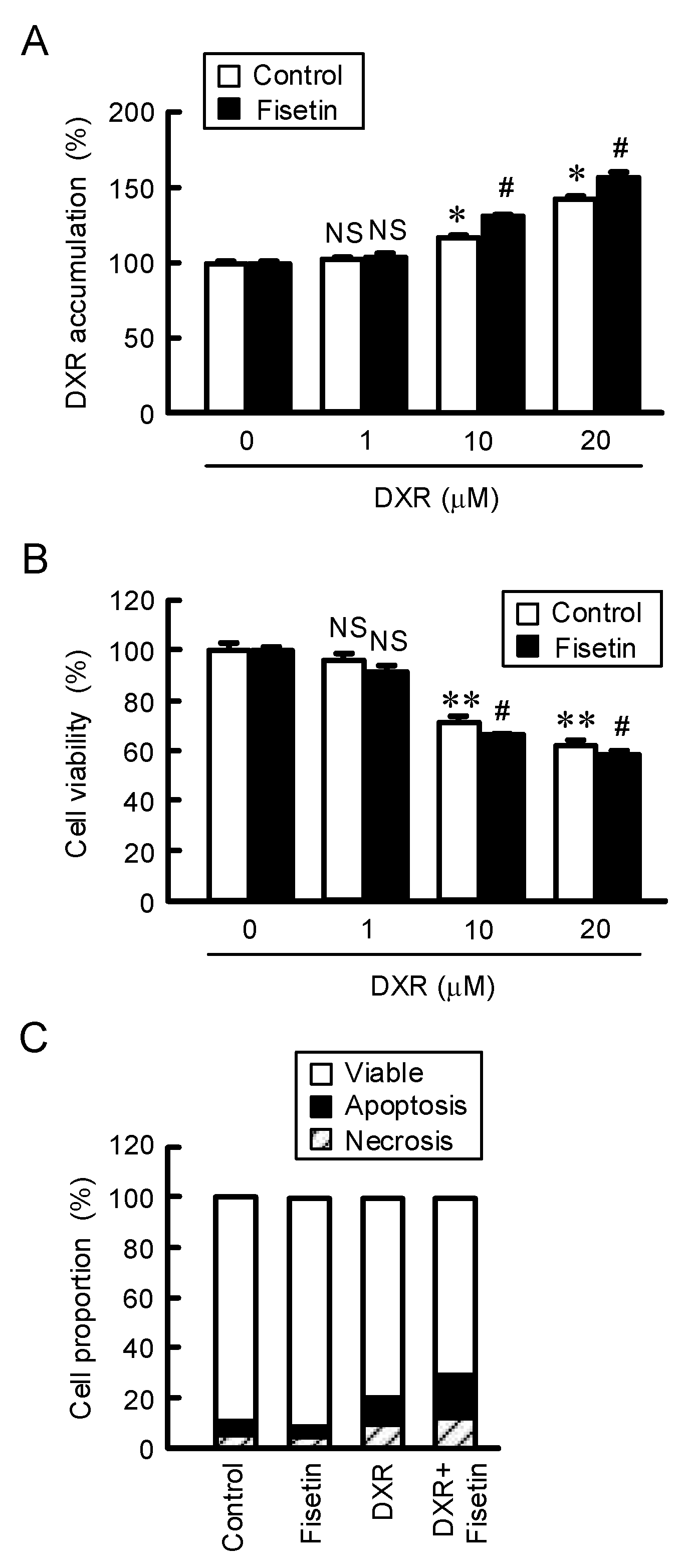
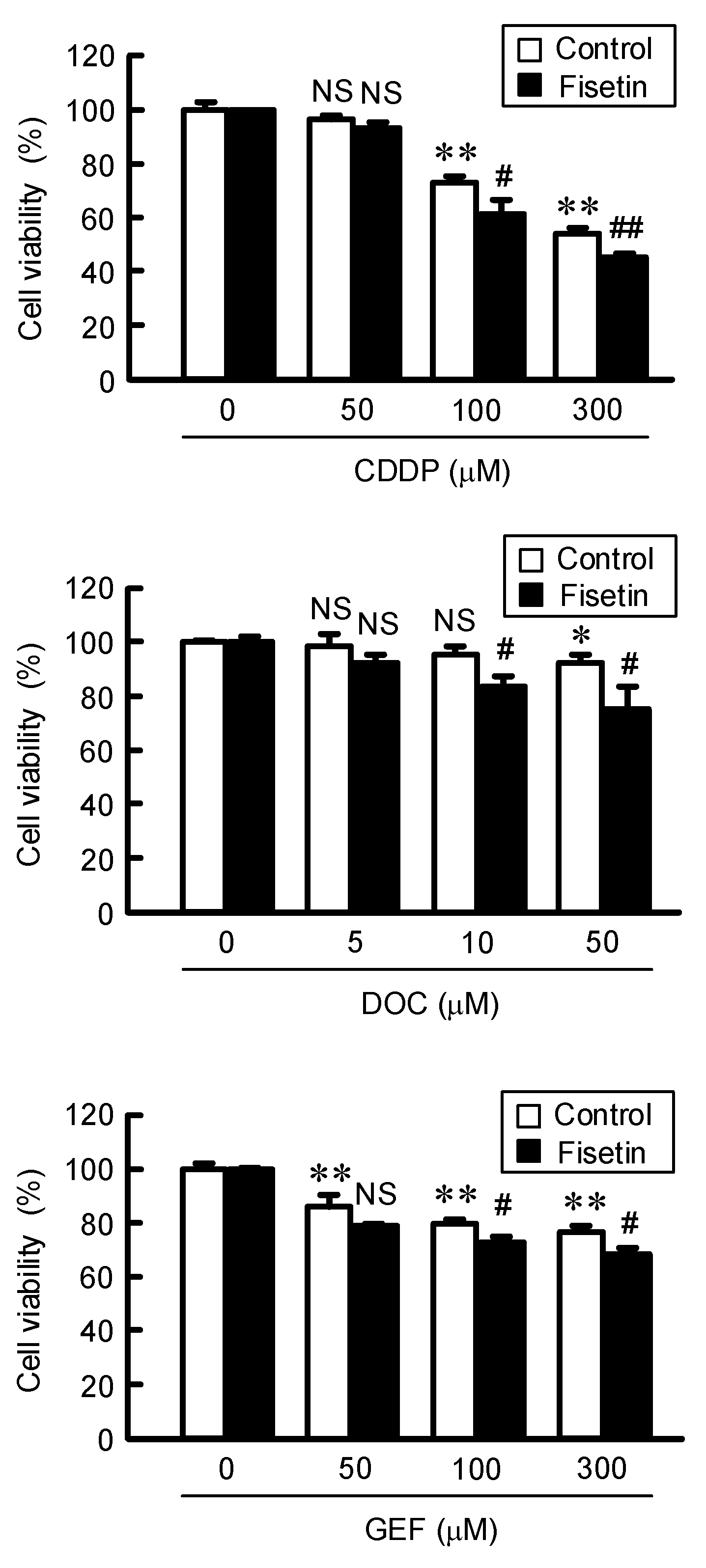
2.5. Enhancement of Anticancer Drug-Induced Toxicity by Fisetin in Spheroids
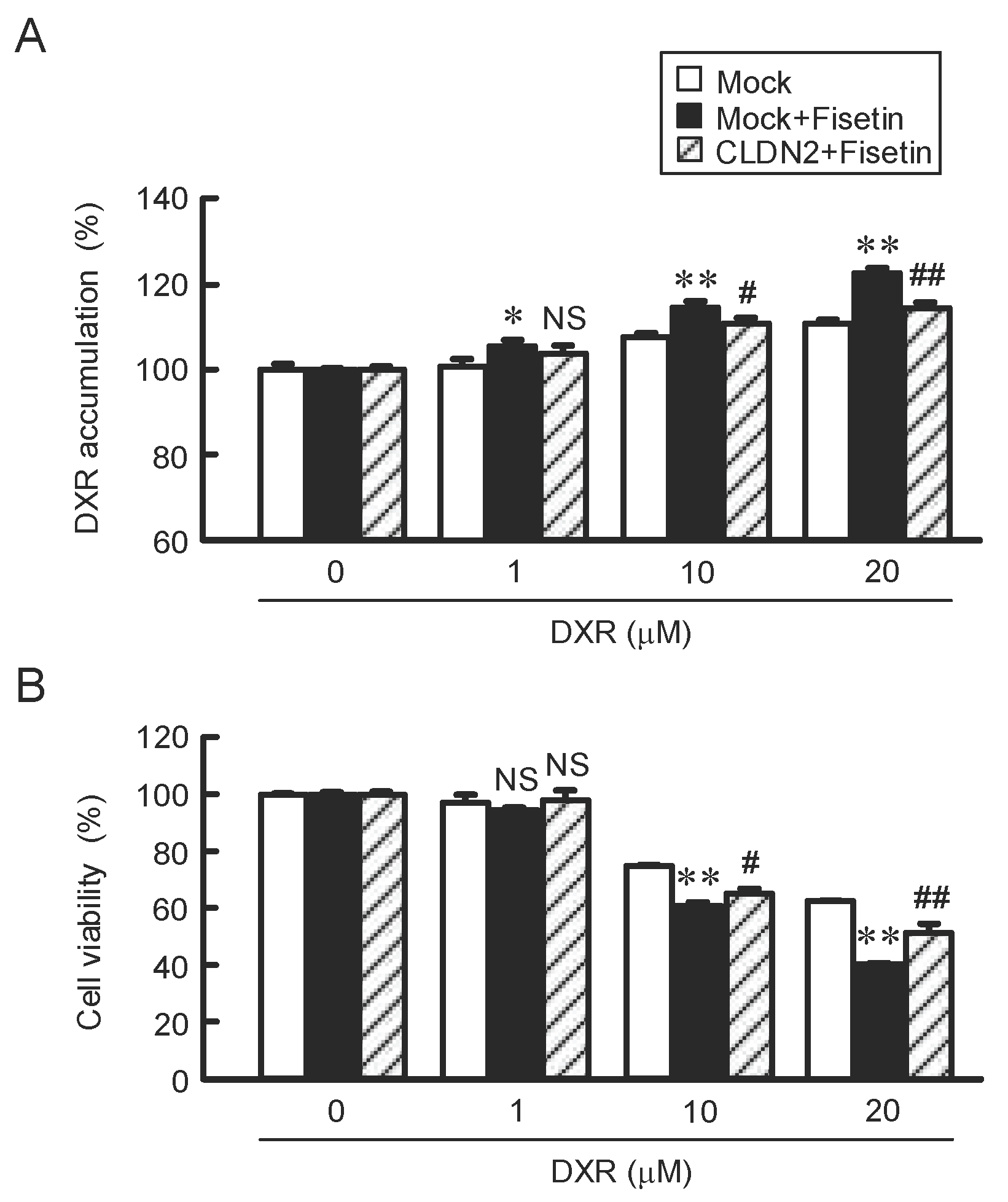
2.6. Attenuation of Fisetin-Induced Accumulation and Toxicity of DXR by CLDN2 Overexpression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Transfection
4.3. Cell Viability Assay
4.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blotting
4.5. Cell Distribution of CLDN
4.6. Reverse Transcription and Quantitative Real-Time PCR
4.7. Spheroid Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwan, T.Y.; Chowdhury, E.H. Clinical Outcomes of Chemotherapeutic Molecules as Single and Multiple Agents in Advanced Non-Small-Cell Lung Carcinoma (NSCLC) Patients. Medicina 2021, 57, 1252. [Google Scholar] [CrossRef] [PubMed]
- Passiglia, F.; Reale, M.L.; Cetoretta, V.; Novello, S. Immune-Checkpoint Inhibitors Combinations in Metastatic NSCLC: New Options on the Horizon? Immunotargets Ther. 2021, 10, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Kryczka, J.; Kryczka, J.; Czarnecka-Chrebelska, K.H.; Brzezianska-Lasota, E. Molecular Mechanisms of Chemoresistance Induced by Cisplatin in NSCLC Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 8885. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipp, A.P.; Deubel, S.; Arner, E.S.J.; Johansson, K. Time- and cell-resolved dynamics of redox-sensitive Nrf2, HIF and NF-kappaB activities in 3D spheroids enriched for cancer stem cells. Redox Biol. 2017, 12, 403–409. [Google Scholar] [CrossRef]
- Piontek, J.; Krug, S.M.; Protze, J.; Krause, G.; Fromm, M. Molecular architecture and assembly of the tight junction backbone. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183279. [Google Scholar] [CrossRef]
- Anderson, J.M.; van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. Gastrointest. Liver Physiol. 1995, 269, G467–G475. [Google Scholar] [CrossRef]
- Morita, K.; Furuse, M.; Fujimoto, K.; Tsukita, S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA 1999, 96, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schoneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, R.; Milatz, S.; Krug, S.M.; Oelrich, B.; Schulzke, J.D.; Amasheh, S.; Gunzel, D.; Fromm, M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 2010, 123, 1913–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabaries, S.; Dong, Z.; Annis, M.G.; Omeroglu, A.; Pepin, F.; Ouellet, V.; Russo, C.; Hassanain, M.; Metrakos, P.; Diaz, Z.; et al. Claudin-2 is selectively enriched in and promotes the formation of breast cancer liver metastases through engagement of integrin complexes. Oncogene 2011, 30, 1318–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabaries, S.; Dupuy, F.; Dong, Z.; Monast, A.; Annis, M.G.; Spicer, J.; Ferri, L.E.; Omeroglu, A.; Basik, M.; Amir, E.; et al. Claudin-2 promotes breast cancer liver metastasis by facilitating tumor cell interactions with hepatocytes. Mol. Cell. Biol. 2012, 32, 2979–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, I.; Kiss, A.; Schaff, Z.; Sobel, G. Claudins as diagnostic and prognostic markers in gynecological cancer. Histol. Histopathol. 2009, 24, 1607–1615. [Google Scholar] [PubMed]
- Aung, P.P.; Mitani, Y.; Sanada, Y.; Nakayama, H.; Matsusaki, K.; Yasui, W. Differential expression of claudin-2 in normal human tissues and gastrointestinal carcinomas. Virchows Arch. 2006, 448, 428–434. [Google Scholar] [CrossRef]
- Dhawan, P.; Ahmad, R.; Chaturvedi, R.; Smith, J.J.; Midha, R.; Mittal, M.K.; Krishnan, M.; Chen, X.; Eschrich, S.; Yeatman, T.J.; et al. Claudin-2 expression increases tumorigenicity of colon cancer cells: Role of epidermal growth factor receptor activation. Oncogene 2011, 30, 3234–3247. [Google Scholar] [CrossRef] [Green Version]
- Ikari, A.; Sato, T.; Watanabe, R.; Yamazaki, Y.; Sugatani, J. Increase in claudin-2 expression by an EGFR/MEK/ERK/c-Fos pathway in lung adenocarcinoma A549 cells. Biochim. Biophys. Acta 2012, 1823, 1110–1118. [Google Scholar] [CrossRef] [Green Version]
- Hichino, A.; Okamoto, M.; Taga, S.; Akizuki, R.; Endo, S.; Matsunaga, T.; Ikari, A. Down-regulation of Claudin-2 Expression and Proliferation by Epigenetic Inhibitors in Human Lung Adenocarcinoma A549 Cells. J. Biol. Chem. 2017, 292, 2411–2421. [Google Scholar] [CrossRef] [Green Version]
- Ikari, A.; Sato, T.; Takiguchi, A.; Atomi, K.; Yamazaki, Y.; Sugatani, J. Claudin-2 knockdown decreases matrix metalloproteinase-9 activity and cell migration via suppression of nuclear Sp1 in A549 cells. Life Sci. 2011, 88, 628–633. [Google Scholar] [CrossRef]
- Maruhashi, R.; Akizuki, R.; Sato, T.; Matsunaga, T.; Endo, S.; Yamaguchi, M.; Yamazaki, Y.; Sakai, H.; Ikari, A. Elevation of sensitivity to anticancer agents of human lung adenocarcinoma A549 cells by knockdown of claudin-2 expression in monolayer and spheroid culture models. Biochim. Biophys. Acta 2018, 1865, 470–479. [Google Scholar] [CrossRef]
- Ye, R.J.; Yang, J.M.; Hai, D.M.; Liu, N.; Ma, L.; Lan, X.B.; Niu, J.G.; Zheng, P.; Yu, J.Q. Interplay between male reproductive system dysfunction and the therapeutic effect of flavonoids. Fitoterapia 2020, 147, 104756. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients 2017, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, H.; Sato, T.; Endo, S.; Matsunaga, T.; Yamaguchi, M.; Yamazaki, Y.; Sugatani, J.; Ikari, A. Quercetin Decreases Claudin-2 Expression Mediated by Up-Regulation of microRNA miR-16 in Lung Adenocarcinoma A549 Cells. Nutrients 2015, 7, 4578–4592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, H.; Matsunaga, T.; Endo, S.; Ichihara, K.; Ikari, A. Kaempferide Enhances Chemosensitivity of Human Lung Adenocarcinoma A549 Cells Mediated by the Decrease in Phosphorylation of Akt and Claudin-2 Expression. Nutrients 2020, 12, 1190. [Google Scholar] [CrossRef]
- Ikari, A.; Watanabe, R.; Sato, T.; Taga, S.; Shimobaba, S.; Yamaguchi, M.; Yamazaki, Y.; Endo, S.; Matsunaga, T.; Sugatani, J. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim. Biophys. Acta 2014, 1843, 2079–2088. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huang, S. Fisetin inhibits the growth and migration in the A549 human lung cancer cell line via the ERK1/2 pathway. Exp. Ther. Med. 2018, 15, 2667–2673. [Google Scholar] [CrossRef] [Green Version]
- Sundarraj, K.; Raghunath, A.; Perumal, E. A review on the chemotherapeutic potential of fisetin: In vitro evidences. Biomed. Pharmacother. 2018, 97, 928–940. [Google Scholar] [CrossRef]
- Markovic, Z.S.; Mentus, S.V.; Dimitric Markovic, J.M. Electrochemical and density functional theory study on the reactivity of fisetin and its radicals: Implications on in vitro antioxidant activity. J. Phys. Chem. A 2009, 113, 14170–14179. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Madduma Hewage, S.R.; Ryu, Y.S.; Oh, M.C.; Kwon, T.K.; Chae, S.; Hyun, J.W. Fisetin induces apoptosis and endoplasmic reticulum stress in human non-small cell lung cancer through inhibition of the MAPK signaling pathway. Tumour Biol. 2016, 37, 9615–9624. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Gao, Z.; Li, X.; Weng, M.; Shi, C.; Wang, C.; Sun, L. Fisetin inhibits the proliferation, migration and invasion of pancreatic cancer by targeting PI3K/AKT/mTOR signaling. Aging 2021, 13, 24753–24767. [Google Scholar] [CrossRef]
- Li, J.; Gong, X.; Jiang, R.; Lin, D.; Zhou, T.; Zhang, A.; Li, H.; Zhang, X.; Wan, J.; Kuang, G.; et al. Fisetin Inhibited Growth and Metastasis of Triple-Negative Breast Cancer by Reversing Epithelial-to-Mesenchymal Transition via PTEN/Akt/GSK3beta Signal Pathway. Front. Pharmacol. 2018, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Syed, D.N.; Mukhtar, H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis 2008, 29, 1049–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Afaq, F.; Khusro, F.H.; Mustafa Adhami, V.; Suh, Y.; Mukhtar, H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int. J. Cancer 2012, 130, 1695–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xun, T.; Lin, Z.; Zhan, X.; Song, S.; Mo, L.; Feng, H.; Yang, Q.; Guo, D.; Yang, X. Advanced oxidation protein products upregulate efflux transporter expression and activity through activation of the Nrf-2-mediated signaling pathway in vitro and in vivo. Eur. J. Pharm. Sci. 2020, 149, 105342. [Google Scholar] [CrossRef]
- Sanchez-Ortega, M.; Carrera, A.C.; Garrido, A. Role of NRF2 in Lung Cancer. Cells 2021, 10, 1879. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, F.; Zhou, H.; Chen, Y.; Du, J.; Zhang, X.; Song, D.; Zhao, H. MDIG promotes cisplatin resistance of lung adenocarcinoma by regulating ABC transporter expression via activation of the WNT/beta-catenin signaling pathway. Oncol. Lett. 2019, 18, 4294–4307. [Google Scholar]
- Zhang, J.; Jiao, K.; Liu, J.; Xia, Y. Metformin reverses the resistance mechanism of lung adenocarcinoma cells that knocks down the Nrf2 gene. Oncol. Lett. 2018, 16, 6071–6080. [Google Scholar] [CrossRef] [Green Version]
- Hortobagyi, G.N. Anthracyclines in the treatment of cancer. An overview. Drugs 1997, 54 (Suppl. S4), 1–7. [Google Scholar]
- Fic, M.; Podhorska-Okolow, M.; Dziegiel, P.; Gebarowska, E.; Wysocka, T.; Drag-Zalesinska, M.; Zabel, M. Effect of melatonin on cytotoxicity of doxorubicin toward selected cell lines (human keratinocytes, lung cancer cell line A-549, laryngeal cancer cell line Hep-2). In Vivo 2007, 21, 513–518. [Google Scholar]
- Ito, A.; Nasako, H.; Akizuki, R.; Takashina, Y.; Eguchi, H.; Matsunaga, T.; Yoshino, Y.; Endo, S.; Ikari, A. Elevation of Chemosensitivity of Lung Adenocarcinoma A549 Spheroid Cells by Claudin-2 Knockdown through Activation of Glucose Transport and Inhibition of Nrf2 Signal. Int. J. Mol. Sci. 2021, 22, 6582. [Google Scholar] [CrossRef]
- Eguchi, H.; Matsunaga, H.; Onuma, S.; Yoshino, Y.; Matsunaga, T.; Ikari, A. Down-Regulation of Claudin-2 Expression by Cyanidin-3-Glucoside Enhances Sensitivity to Anticancer Drugs in the Spheroid of Human Lung Adenocarcinoma A549 Cells. Int. J. Mol. Sci. 2021, 22, 499. [Google Scholar] [CrossRef] [PubMed]

| Genes | Direction | Sequence (5′→3′) |
|---|---|---|
| CLDN1 | Sense | ATGAGGATGGCTGTCATTGG |
| Antisense | ATTGACTGGGGTCATAGGGT | |
| CLDN2 | Sense | ATTGTGACAGCAGTTGGCTT |
| Antisense | CTATAGATGTCACACTGGGTGATG | |
| HIF-1a | Sense | AACGTCGAAAAGAAAAGTCTCG |
| Antisense | AAATCACCAGCATCCAGAAGTT | |
| Nrf2 | Sense | TCCAGTCAGAAACCAGTGGAT |
| Antisense | GAATGTCTGCGCCAAAAGCTG | |
| ABCB1 | Sense | CCCATCATTGCAATAGCAGG |
| Antisense | TGTTCAAACTTCTGCTCCTGA | |
| ABCC1 | Sense | ATGTCACGTGGAATACCAGC |
| Antisense | GAAGACTGAACTCCCTTCCT | |
| ABCC2 | Sense | ACAGAGGCTGGTGGCAACC |
| Antisense | ACCATTACCTTGTCACTGTCCATGA | |
| ABCG2 | Sense | AGATGGGTTTCCAAGCGTTCAT |
| Antisense | CCAGTCCCAGTACGACTGTGACA | |
| β-Actin | Sense | CCTGAGGCACTCTTCCAGCCTT |
| Antisense | TGCGGATGTCCACGTCACACTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eguchi, H.; Kimura, R.; Matsunaga, H.; Matsunaga, T.; Yoshino, Y.; Endo, S.; Ikari, A. Increase in Anticancer Drug-Induced Toxicity by Fisetin in Lung Adenocarcinoma A549 Spheroid Cells Mediated by the Reduction of Claudin-2 Expression. Int. J. Mol. Sci. 2022, 23, 7536. https://doi.org/10.3390/ijms23147536
Eguchi H, Kimura R, Matsunaga H, Matsunaga T, Yoshino Y, Endo S, Ikari A. Increase in Anticancer Drug-Induced Toxicity by Fisetin in Lung Adenocarcinoma A549 Spheroid Cells Mediated by the Reduction of Claudin-2 Expression. International Journal of Molecular Sciences. 2022; 23(14):7536. https://doi.org/10.3390/ijms23147536
Chicago/Turabian StyleEguchi, Hiroaki, Riho Kimura, Haruka Matsunaga, Toshiyuki Matsunaga, Yuta Yoshino, Satoshi Endo, and Akira Ikari. 2022. "Increase in Anticancer Drug-Induced Toxicity by Fisetin in Lung Adenocarcinoma A549 Spheroid Cells Mediated by the Reduction of Claudin-2 Expression" International Journal of Molecular Sciences 23, no. 14: 7536. https://doi.org/10.3390/ijms23147536
APA StyleEguchi, H., Kimura, R., Matsunaga, H., Matsunaga, T., Yoshino, Y., Endo, S., & Ikari, A. (2022). Increase in Anticancer Drug-Induced Toxicity by Fisetin in Lung Adenocarcinoma A549 Spheroid Cells Mediated by the Reduction of Claudin-2 Expression. International Journal of Molecular Sciences, 23(14), 7536. https://doi.org/10.3390/ijms23147536







