Extraterrestrial Gynecology: Could Spaceflight Increase the Risk of Developing Cancer in Female Astronauts? An Updated Review
Abstract
1. Introduction
2. Space-Environmental Factors: Microgravity and Space Radiation
2.1. Microgravity
2.2. Space Radiation
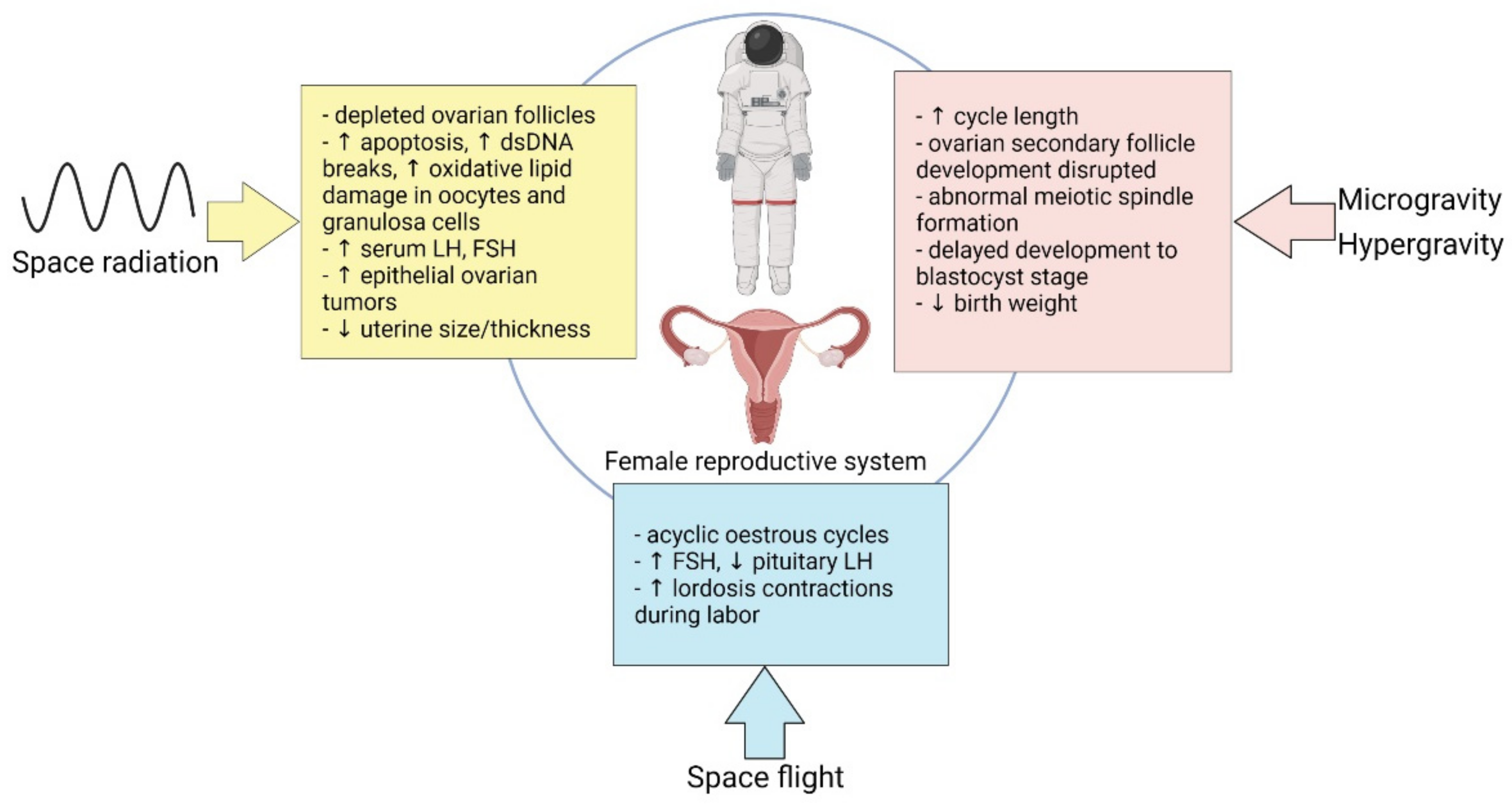
3. Effect of Spaceflight on Female Reproduction
3.1. Microgravity
3.2. Hormonal Modalities in Spaceflight
3.3. Space Radiation
4. Effect of Space Travel on Cancer
| Cancer Type | Microgravity Model | Model | Effect | Study |
|---|---|---|---|---|
| Breast cancer | 6 min of r-µg *; PF ** maneuvers | MCF-7 cell line | Rearrangement of F-actin and tubulin, appearance of filopodia- and lamellipodia-like structures; PF-induced differential regulation of KRT8, RDX, TIMP1, CXCL8 (up), VCL, and CDH1 (down) genes | Nassef et al., 2019 [56] |
| Breast cancer | Exposure to an RPM ## | MCF-7 cell line | Cells formed multicellular spheroids resembling epithelial ducts; microgravity-induced differential regulation of IL8, VEGFA, FLT1, ESR1 (up), ACTB, TUBB, FN1, CASP9, CASP3, and PGR1 (down) genes | Kopp et al., 2016 [57] |
| Breast cancer | PF ** maneuvers; incubator RPM ## | MDA-MB-231 cells | Differential regulation of ICAM1, CD44, ERK1, NFKB1, FAK1 (up), ANXA2, and BAX (down) genes | Nassef et al., 2019 [58] |
| Glioma | Exposure to an RPM ## | U251 cells | Induction of apoptosis; reduced FAK/RhoA/Rock and FAK/Nek2 signaling events | Deng et al., 2019 [59] |
| Lung cancer (non small cell) | Exposure to an RPM ## | NCI-H1703 (CRL-5889) cells | Formation of multicellular spheroids; spherical rearrangement of actin filaments in the outer region of cytoplasm; increased apoptosis, upregulation of TP53, CDKN2A, RB1, PTEN, and SOX2 in stimulated adherent cells | Dietz et al., 2019 [60] |
| Melanoma | Exposure to a 3-D Clinostat # | A375 cells | Decreased cell viability; increase in caspase 3/7 activity; reduced cell proliferation; change in cell morphology (presence of membrane blebbing lamellipodia, and stress fibers, absence of filopodia) | Przystupski et al., 2021 [61] |
| Thyroid cancer | Exposure to an RPM ## | FTC-133 cells | Cells formed multicellular spheroids; differential regulation of ERK1, EGF (up), CTGF, and CAV (down) genes in multicellular spheroids | Warnke et al., 2014 [62] |
| Thyroid cancer | 10 day of r-µg * | FTC-133 cells | Differential expression of IL6, IL7, IL8, VEGF, TIMP1, MMP3, CCL4, and B2M (up) proteins | Riwaldt et al., 2015 [63] |
| Cell Type | Radiation Model | Cell/Animal Model | Effect | Study |
|---|---|---|---|---|
| Lung cells | Iron ion (Fe) beam (180 MeV/nucleon; LET 300 keV/µm) for 0.1 Gy | SV40-immortalized human bronchial epithelial cells (NL20) | Progeny of Fe-irradiated cells showed elevated micronucleus formation, increased markers for DNA double-strand breaks (γ-H2AX foci), reduced cell proliferation, persistent oxidative stress, and increased colony formation. | Cao et al., 2018 [64] |
| Lung cells | 56Fe (600 MeV/u at 0, 0.1, 0.3, 1.0 Gy) and 28Si (300 MeV/u at 0, 0.3, 1.0 Gy) high LET irradiation | Immortalized human bronchial epithelial cell line (HBEC3-KT) | Global differential CpG island methylation in response to 56Fe and 28Si ion exposure suggests a lasting impact on the epigenome relevant to lung cancer | Kennedy et al., 2018 [65] |
| Hematopoietic stem cells | 100 cGy of 1000 MeV/n protons (LET 0.23 keV/micron); 28Si 300 MeV/n ions (LET 70 keV/micron) | Mlh1+/− mice (B6.129-Mlh1tm1Rak/NCI) representing loss of MLH1 that occurs in human hematopoietic stem cells with age | High LET 28Si ion irradiation affected hematopoietic stem cell differentiation; high LET irradiation caused early and higher incidence of tumorigenesis in Mlh1 heterozygous mice; frequent occurrence of T-cell rich B-cell (TRB) lymphomas with altered mismatch repair pathway | Patel et al., 2020 [66] |
| Spleen cells | 0.5 Gy Proton irradiation (1-GeV; LET 0.24-keV/µm) | Murine Lewis lung carcinoma (LLC) cells-bearing C57BL/6 mice | Upregulation of genes involved in DNA repair and cell cycle, including CDK2, MCM7, CD74, and RUVBL2 | Wage et al., 2015 [67] |
| Intestinal cells | 56Fe-irradiation (1.6 Gy; energy-1000 MeV/nucleon; LET-148 keV/µm) | Intestinal tissue from Female C57BL/6J mice | 56Fe-irradiation upregulated metabolites belonging to prostanoid biosynthesis and eicosanoid signaling pathways linked with cellular inflammation, which has been associated with intestinal inflammatory disease and colon cancer | Cheema et al., 2014 [68] |
| Liver cells | 56Fe ion irradiation (1 GeV/nucleon) | CBA/CaJ mice | Higher incidence of hepatocellular carcinoma than γ-irradiated mice | Weil et al., 2009 [69] |
| Kidney cells | 56Fe ions irradiation (1 GeV/amu, 151 keV/μm) | Aprt heterozygous (Aprt+/−) B6D2F1 mice | Increased mutant frequencies leading to DNA damage | Turker et al., 2017 [70] |
| Cervical cancer cells | Kept at the Russian Mir space station (40 days); American space shuttle (10 days) | HeLa cells | DNA damage | Ohnishi et al., 2002 [71] |
| Normal human foreskin fibroblast cells | Kept at the International Space Station (14 days) | AG1522 cells | Larger size γ-H2AX foci suggest DNA damage | Lu et al., 2017 [72] |
| Normal human foreskin fibroblast cells | Kept at the International Space Station (14 days) | AG1522 cells | Downregulation of miRNA Let-7a, which was found to be downregulated to γ ray and UV ray radiation in another study | Zhang et al., 2016 [73] |
5. Gynecological Cancers and Space
5.1. Brief Overview on Gynecological Cancers
5.2. Effects of Microgravity and Radiation on Gynecological Cancers: In Vitro and In Vivo Studies
5.2.1. Ovarian Normal and Cancer Cells Exposed to Microgravity and/or Radiation
Altered Gravity and Microgravity
Radiation
5.2.2. Cervical Normal and Cancer Cells Exposed to Microgravity and/or Radiation
Microgravity
Spaceflight Studies
Radiation
Viral Reactivation
5.2.3. Endometrial Normal and Cancer Cells Exposed to Microgravity and/or Radiation
Microgravity
Radiation
| Tissue Type | Microgravity/Space Flight | Cell/Animal Models | Effect | Study |
|---|---|---|---|---|
| Ovarian | simulated microgravity RWV | LN1 human ovarian tumor cells | LN1 cells grew as spheroids free in suspension | Becker et al., 1993; Goodwin et al., 1997 [134,135] |
| spaceflight (cells were cultured on the ISS) | LN1 human ovarian tumor cells | Cells showed reduced expression of VIM and EMA | Hammond et al., 2005 [136] | |
| simulated microgravity 3D-C | SKOV-3 human ovarian cancer cells | Cells showed reduced proliferation, migration, and higher sensitivity of cancer cells to the cisplatin | Przystupski et al., 2021 [137] | |
| microgravity | set of systems-biology tools and databases | identified several cancer related signatures induced by microgravity | Mukhopadhyay et al., 2016 [138] | |
| Cervical | simulated microgravity RWV | Co-culture of HUVEC and tumor primary cells | Co-culture presented tubular structures penetrating the tumor cell masses, | Chopra et al., 1997 [143] |
| simulated microgravity HFB and RCCS | HeLa human cervical cancer cells | HFB exposure increased CD133-positive cell growth | Kelly et al., 2010 [144] | |
| spaceflight (cells were flown on “Shen Zhou IV” space shuttle mission) | Human cervical carcinoma CaSki cells | Cells exhibited morphologic differences, characterized by rounder, smoother, decreased, smaller, and low adhesion cells. Furthermore, space-grown cells showed altered gene expression that generally corresponded to changes in genes regulating the cell cycle, cell morphology, apoptosis, and signal transduction | Zhang et al., 2011; Guo et al., 2012 [145,146] | |
| Endometrial | simulated microgravity 3D-C | human endometrial stromal cells (eSCs) | Cells showed reduced proliferation and migration. This was accompanied by a simultaneous decrease in the phosphorylation of Akt and the level of matrix metalloproteinase (MMP)-2 and FOXO3a. | Cho et al., 2019 [156] |
| simulated microgravity RCCS | Human tumor primary cells | 3D model endometrial cancer cell culture was established | Grun et al., 2009 [157] |
| Tissue Type | Radiation Type | Cell/Animal Models | Effect | Study |
|---|---|---|---|---|
| Ovarian | 0.439 Gy as a 290 MeV/u carbon-ion beam (LET 10 keV/micron) | B6C3F1 mice | Induction of ovarian tumors | Watanabe et al., 1998 [139] |
| 0.426 Gy heavy ion irradiation of 290 MeV/u carbon-ion beam (LET 60–210 KeV/micron) at the dose rate of 0.4 +/− 0.2 Gy/min; 0.5 Gy of X-ray irradiation at 0.1 Gy/min or 5 Gy of X-ray irradiation at 1 Gy/min. | B6C3F1 mice | Tumorigenicity was lower for heavy ion than for 0.5 Gy and 5 Gy X-ray irradiation | Watanabe et al., 1998 [140] | |
| high and low LET radiations. 1.0 Gy monoenergetic neutrons (0.317, 0.525 and 1.026 MeV), 252Cf fission neutron (2.13 MeV) or 137Cs γ-rays | C57BL/6N mice | Higher effectiveness of neutrons than γ-rays to induce oocyte and pregranulosa cell apoptosis correlates with the inhibition of granulosa cell tumor development | Nitta & Hoshi, 2003 [141] | |
| HZE particles. 50 cGy iron ions | C57BL/6J | Induction of ovarian tumors | Mishra et al., 2018 [142] | |
| Cervical | spaceflight (cells were flown on “Russian MIR” space station or on the Space Shuttle) | HeLa human cervical cancer cells | Increased DNA damage | Ohnishi, et al., 2002 [71] |
| Endometrial | Monoenergetic protons (1–10 Gy; LET 8.35 keV/μm and 4.86 MeV) and γ-rays (0.2–1.6 Gy) | Human endometrial carcinoma cell lines (HEC1B and AN3CA cells) | Decreased cell survival | Palumbo et al., 2001 [158] |
6. Current Challenges in Gynecological Cancer Risk Prediction for Spaceflight
6.1. Gynecologic Medical Standards for Career and Private Astronauts
6.2. Countermeasures
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACMG | American College of Medical Genetics and Genomics |
| ACOG | American College of Obstetricians and Gynecologists |
| ASCCP | American Society for Colposcopy and Cervical Pathology |
| CC | cervical cancer |
| CSA | Canadian Space Agency |
| 3D-C | 3D-clinostat |
| EC | endometrial cancer |
| ESA | European Space Agency |
| GCR | galactic cosmic radiation |
| HFB | hydrofocusing bioreactor |
| HPV | human papillomavirus |
| HZE | high Z and energy |
| ISS | International Space Station |
| JAXA | Japan Aerospace Exploration Agency |
| LEO | low Earth orbit |
| LET | Linear Energy Transfer |
| MSMBIP | Multilateral Space Medicine Board of the ISS International Partners |
| NAC | N-Acetyl-L-Cysteine |
| NASA | National Aeronautics and Space Administration |
| NSGC | National Society of Genetic Counselors |
| OC | ovarian cancer |
| RBE | relative biological effectiveness |
| RCCS | rotary cell culture system |
| RER | radiation effects ratio |
| RWV | rotating-wall vessel |
| SGO | Society of Gynecologic Oncology |
| SPE | solar particle events |
References
- Steller, J.G.; Alberts, J.R.; Ronca, A.E. Oxidative Stress as Cause, Consequence, or Biomarker of Altered Female Reproduction and Development in the Space Environment. Int. J. Mol. Sci. 2018, 19, 3729. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Sánchez, J.L.; Callant, J.; Krüger, M.; Sahana, J.; Kraus, A.; Baselet, B.; Infanger, M.; Baatout, S.; Grimm, D. Cancer Studies under Space Conditions: Finding Answers Abroad. Biomedicines 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Afshinnekoo, E.; Scott, R.T.; MacKay, M.J.; Pariset, E.; Cekanaviciute, E.; Barker, R.; Gilroy, S.; Hassane, D.; Smith, S.M.; Zwart, S.R.; et al. Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration. Cell 2020, 183, 1162–1184. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Chan, D.; Abegaz, M.F.; Schreurs, A.S.; Alwood, J.S.; Globus, R.K.; Appel, E.A. A human mission to Mars: Predicting the bone mineral density loss of astronauts. PLoS ONE 2020, 15, e0226434. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Brunstetter, T.J.; Tarver, W.J.; Whitmire, A.M.; Zwart, S.R.; Smith, S.M.; Huff, J.L. Red risks for a journey to the red planet: The highest priority human health risks for a mission to Mars. NPJ Microgravity 2020, 6, 33. [Google Scholar] [CrossRef]
- Crucian, B.; Simpson, R.J.; Mehta, S.; Stowe, R.; Chouker, A.; Hwang, S.A.; Actor, J.K.; Salam, A.P.; Pierson, D.; Sams, C. Terrestrial stress analogs for spaceflight associated immune system dysregulation. Brain Behav. Immun. 2014, 39, 23–32. [Google Scholar] [CrossRef]
- Leser, N.; Wagner, S. The effects of acute social isolation on long-term social recognition memory. Neurobiol. Learn. Mem. 2015, 124, 97–103. [Google Scholar] [CrossRef]
- Reschke, M.F.; Cohen, H.S.; Cerisano, J.M.; Clayton, J.A.; Cromwell, R.; Danielson, R.W.; Hwang, E.Y.; Tingen, C.; Allen, J.R.; Tomko, D.L. Effects of sex and gender on adaptation to space: Neurosensory systems. J. Women’s Health 2014, 23, 959–962. [Google Scholar] [CrossRef]
- Clark, B.C.; Fernhall, B.; Ploutz-Snyder, L.L. Adaptations in human neuromuscular function following prolonged unweighting: I. Skeletal muscle contractile properties and applied ischemia efficacy. J. Appl. Physiol. 2006, 101, 256–263. [Google Scholar] [CrossRef]
- Ronca, A.E.; Baker, E.S.; Bavendam, T.G.; Beck, K.D.; Miller, V.M.; Tash, J.S.; Jenkins, M. Effects of sex and gender on adaptations to space: Reproductive health. J. Women’s Health 2014, 23, 967–974. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef]
- RCR—Royal College of Radiologists. Radiotherapy Dose Fractionation Third Edition. 2019. Available online: https://www.rcr.ac.uk/publication/radiotherapy-dose-fractionation-third-edition (accessed on 3 March 2022).
- Freese, S.; Reddy, A.P.; Lehnhardt, K. Radiation impacts on human health during spaceflight beyond Low Earth Orbit. REACH 2016, 2–4, 1–7. [Google Scholar] [CrossRef]
- Jones, C.B.; Mange, A.; Granata, L.; Johnson, B.; Hienz, R.D.; Davis, C.M. Short and Long-Term Changes in Social Odor Recognition and Plasma Cytokine Levels Following Oxygen (16O) Ion Radiation Exposure. Int. J. Mol. Sci. 2019, 20, 339. [Google Scholar] [CrossRef]
- Barr, Y.R.; Bacal, K.; Jones, J.A.; Hamilton, D.R. Breast cancer and spaceflight: Risk and management. Aviat. Space Environ. Med. 2007, 78, A26–A37. [Google Scholar]
- Steller, J.G.; Blue, R.S.; Burns, R.; Bayuse, T.; Antonsen, E.L.; Jain, V.; Blackwell, M.M.; Jennings, R.T. Gynecologic Risk Mitigation Considerations for Long-Duration Spaceflight. Aerosp. Med. Hum. Perform. 2020, 91, 543–564. [Google Scholar] [CrossRef]
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Böttcher, S.; Böhm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef]
- Wilson, J.W. Environmental geophysics and SPS shielding. In Proceedings of the Workshop on the Radiation Environment of the Satellite Power System, Berkeley, CA, USA, 15 September 1978. [Google Scholar]
- Chancellor, J.C.; Scott, G.B.; Sutton, J.P. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef]
- Simpson, J.A. Elemental and isotopic composition of the galactic cosmic rays. Ann. Rev. Nucl. Part. Sci. 1983, 33, 323–381. [Google Scholar] [CrossRef]
- Ackermann, M.; Ajello, M.; Allafort, A.; Baldini, L.; Ballet, J.; Barbiellini, G.; Baring, M.G.; Bastieri, D.; Bechtol, K.; Bellazzini, R.; et al. Detection of the characteristic pion-decay signature in supernova remnants. Science 2013, 339, 807–811. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Durante, M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar] [CrossRef]
- Onorato, G.; Di Schiavi, E.; Di Cunto, F. Understanding the Effects of Deep Space Radiation on Nervous System: The Role of Genetically Tractable Experimental Models. Front. Phys. 2020, 8, 362. [Google Scholar] [CrossRef]
- Dymova, M.; Dmitrieva, M.; Kuligina, E.; Richter, V.; Savinov, S.; Shchudlo, I.; Sycheva, T.; Taskaeva, I.; Taskaev, S. Method of Measuring High-LET Particles Dose. Radiat. Res. 2021, 196, 192–196. [Google Scholar] [CrossRef]
- Cortese, F.; Klokov, D.; Osipov, A.; Stefaniak, J.; Moskalev, A.; Schastnaya, J.; Cantor, C.; Aliper, A.; Mamoshina, P.; Ushakov, I.; et al. Vive la radiorésistance!: Converging research in radiobiology and biogerontology to enhance human radioresistance for deep space exploration and colonization. Oncotarget 2018, 9, 14692–14722. [Google Scholar] [CrossRef]
- Tommasino, F.; Durante, M. Proton radiobiology. Cancers 2015, 7, 353–381. [Google Scholar] [CrossRef]
- Saha, J.; Wilson, P.; Thieberger, P.; Lowenstein, D.; Wang, M.; Cucinotta, F.A. Biological characterization of low-energy ions with high-energy deposition on human cells. Radiat. Res. 2014, 182, 282–291. [Google Scholar] [CrossRef]
- Benton, E.R.; Benton, E.V. Space radiation dosimetry in low-Earth orbit and beyond. Nucl. Instrum. Methods Phys. Res. B. 2001, 184, 255–294. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection. 1990 Recommendations of the International Commission on Radiological Protection. In Annals of the ICRP; Pergamon Press: Oxford, UK, 1991; Volume 21, pp. 1–201. [Google Scholar]
- Chancellor, J.C.; Blue, R.S.; Cengel, K.A.; Auñón-Chancellor, S.M.; Rubins, K.H.; Katzgraber, H.G.; Kennedy, A.R. Limitations in predicting the space radiation health risk for exploration astronauts. NPJ Microgravity 2018, 4, 8. [Google Scholar] [CrossRef]
- Schwadron, N.A.; Blake, J.B.; Case, A.W.; Joyce, C.J.; Kasper, J.; Mazur, J.; Petro, N.; Quinn, M.; Porter, J.A.; Smith, C.W.; et al. Does the worsening galactic cosmic radiation environment observed by CRaTER preclude future manned deep space exploration? Space Weather 2014, 12, 622–632. [Google Scholar] [CrossRef]
- Papaioannou, A.; Anastasiadis, A.; Sandberg, I.; Jiggens, P. Nowcasting of Solar Energetic Particle Events using near real-time Coronal Mass Ejection characteristics in the framework of the FORSPEF tool. J. Space Weather Space Clim. 2018, 8, A37. [Google Scholar] [CrossRef]
- Restier-Verlet, J.; El-Nachef, L.; Ferlazzo, M.L.; Al-Choboq, J.; Granzotto, A.; Bouchet, A.; Foray, N. Radiation on Earth or in Space: What Does It Change? Int. J. Mol. Sci. 2021, 22, 3739. [Google Scholar] [CrossRef]
- Zeitlin, C.; Hassler, D.M.; Ehresmann, B.; Rafkin, S.C.R.; Guo, J.; Wimmer-Schweingruber, R.F.; Berger, T.; Matthiä, D. Measurements of radiation quality factor on Mars with the Mars Science Laboratory Radiation Assessment Detector. Life Sci. Space Res. 2019, 22, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Luderer, U. Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 2019, 15, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Serova, L.V.; Denisova, L.A. The effect of weightlessness on the reproductive function of mammals. Physiologist 1982, 25, S9–S12. [Google Scholar] [PubMed]
- Hong, X.; Ratri, A.; Choi, S.Y.; Tash, J.S.; Ronca, A.E.; Alwood, J.S.; Christenson, L.K. Effects of spaceflight aboard the International Space Station on mouse estrous cycle and ovarian gene expression. NPJ Microgravity 2021, 7, 11. [Google Scholar] [CrossRef]
- Lei, X.; Cao, Y.; Ma, B.; Zhang, Y.; Ning, L.; Qian, J.; Zhang, L.; Qu, Y.; Zhang, T.; Li, D.; et al. Development of mouse preimplantation embryos in space. Natl. Sci. Rev. 2020, 7, 1437–1446. [Google Scholar] [CrossRef]
- Alberts, J.R.; Ronca, A.E. Development as adaptation: A paradigm for gravitational and space biology. Adv. Space Biol. Med. 2005, 10, 175–207. [Google Scholar]
- Ronca, A.E.; Alberts, J.R. Physiology of a microgravity environment selected contribution: Effects of spaceflight during pregnancy on labor and birth at 1 G. J. Appl. Physiol. 2000, 89, 849–854. [Google Scholar] [CrossRef]
- Burden, H.W.; Zary, J.; Alberts, J.R. Effects of space flight on the immunohistochemical demonstration of connexin 26 and connexin 43 in the postpartum uterus of rats. J. Reprod. Fertil. 1999, 116, 229–234. [Google Scholar] [CrossRef]
- Oyama, J.; Platt, W.T. Reproduction and Growth of Mice and Rats under Conditions of Simulated Increased Gravity. Am. J. Physiol. 1967, 212, 164–166. [Google Scholar] [CrossRef]
- Plaut, K.; Maple, R.L.; Wade, C.E.; Baer, L.A.; Ronca, A.E. Effects of hypergravity on mammary metabolic function: Gravity acts as a continuum. J. Appl. Physiol. 2003, 95, 2350–2354. [Google Scholar] [CrossRef]
- Ronca, A.E.; Baer, L.A.; Daunton, N.G.; Wade, C.E. Maternal reproductive experience enhances early postnatal outcome following gestation and birth of rats in hypergravity. Biol. Reprod. 2001, 65, 805–813. [Google Scholar] [CrossRef][Green Version]
- Wu, Q.J.; Li, Y.Y.; Tu, C.; Zhu, J.; Qian, K.Q.; Feng, T.B.; Li, C.; Wu, L.; Ma, X.X. Parity and endometrial cancer risk: A meta-analysis of epidemiological studies. Sci. Rep. 2015, 5, 14243. [Google Scholar] [CrossRef]
- Lambe, M.; Hsieh, C.C.; Chan, H.W.; Ekbom, A.; Trichopoulos, D.; Adami, H.O. Parity, age at first and last birth, and risk of breast cancer: A population-based study in Sweden. Breast Cancer Res. Treat. 1996, 38, 305–311. [Google Scholar] [CrossRef]
- Sung, H.K.; Ma, S.H.; Choi, J.Y.; Hwang, Y.; Ahn, C.; Kim, B.G.; Kim, Y.M.; Kim, J.W.; Kang, S.; Kim, J.; et al. The Effect of Breastfeeding Duration and Parity on the Risk of Epithelial Ovarian Cancer: A Systematic Review and Meta-analysis. J. Prev. Med. Public Health 2016, 49, 349–366. [Google Scholar] [CrossRef]
- Jain, V.; Wotring, V.E. Medically induced amenorrhea in female astronauts. NPJ Microgravity 2016, 2, 16008. [Google Scholar] [CrossRef]
- Prasad, K.N. (Ed.) Handbook of Radiobiology; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Ogilvy-Stuart, A.L.; Shalet, S.M. Effect of radiation on the human reproductive system. Environ. Health Perspect. 1993, 101, 109–116. [Google Scholar]
- Marci, R.; Mallozzi, M.; Di Benedetto, L.; Schimberni, M.; Mossa, S.; Soave, I.; Palomba, S.; Caserta, D. Radiations and female fertility. Reprod. Biol. Endocrinol. 2018, 16, 112. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Grimm, D.; Bauer, J.; Wise, P.; Krüger, M.; Simonsen, U.; Wehland, M.; Infanger, M.; Corydon, T.J. The role of SOX family members in solid tumours and metastasis. Semin. Cancer Biol. 2020, 67, 122–153. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Moreno-Villanueva, M.; Wu, H. Radiation and microgravity—Associated stress factors and carcinogensis. REACH 2019, 13, 100027. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Krüger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schütte, A.; et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3156. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.; Slumstrup, L.; Corydon, T.J.; Sahana, J.; Aleshcheva, G.; Islam, T.; Magnusson, N.E.; Wehland, M.; Bauer, J.; Infanger, M.; et al. Identifications of novel mechanisms in breast cancer cells involving duct-like multicellular spheroid formation after exposure to the Random Positioning Machine. Sci. Rep. 2016, 6, 26887. [Google Scholar] [CrossRef] [PubMed]
- Nassef, M.Z.; Kopp, S.; Melnik, D.; Corydon, T.J.; Sahana, J.; Krüger, M.; Wehland, M.; Bauer, T.J.; Liemersdorf, C.; Hemmersbach, R.; et al. Short-Term Microgravity Influences Cell Adhesion in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5730. [Google Scholar] [CrossRef]
- Deng, B.; Liu, R.; Tian, X.; Han, Z.; Chen, J. Simulated microgravity inhibits the viability and migration of glioma via FAK/RhoA/Rock and FAK/Nek2 signaling. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 260–271. [Google Scholar] [CrossRef]
- Dietz, C.; Infanger, M.; Romswinkel, A.; Strube, F.; Kraus, A. Apoptosis Induction and Alteration of Cell Adherence in Human Lung Cancer Cells under Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 3601. [Google Scholar] [CrossRef]
- Przystupski, D.; Górska, A.; Michel, O.; Podwin, A.; Śniadek, P.; Łapczyński, R.; Saczko, J.; Kulbacka, J. Testing Lab-on-a-Chip Technology for Culturing Human Melanoma Cells under Simulated Microgravity. Cancers 2021, 13, 402. [Google Scholar] [CrossRef]
- Warnke, E.; Pietsch, J.; Wehland, M.; Bauer, J.; Infanger, M.; Görög, M.; Hemmersbach, R.; Braun, M.; Ma, X.; Sahana, J.; et al. Spheroid formation of human thyroid cancer cells under simulated microgravity: A possible role of CTGF and CAV1. Cell Commun. Signal. 2014, 12, 32. [Google Scholar] [CrossRef]
- Riwaldt, S.; Bauer, J.; Pietsch, J.; Braun, M.; Segerer, J.; Schwarzwälder, A.; Corydon, T.J.; Infanger, M.; Grimm, D. The Importance of Caveolin-1 as Key-Regulator of Three-Dimensional Growth in Thyroid Cancer Cells Cultured under Real and Simulated Microgravity Conditions. Int. J. Mol. Sci. 2015, 16, 28296–28310. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, W.; Wang, J.; Cao, J.; Yang, H. A single low dose of Fe ions can cause long-term biological responses in NL20 human bronchial epithelial cells. Radiat. Environ. Biophys. 2018, 57, 31–40. [Google Scholar] [CrossRef]
- Kennedy, E.M.; Powell, D.R.; Li, Z.; Bell, J.S.K.; Barwick, B.G.; Feng, H.; McCrary, M.R.; Dwivedi, B.; Kowalski, J.; Dynan, W.S.; et al. Galactic Cosmic Radiation Induces Persistent Epigenome Alterations Relevant to Human Lung Cancer. Sci. Rep. 2018, 8, 6709. [Google Scholar] [CrossRef]
- Patel, R.; Zhang, L.; Desai, A.; Hoenerhoff, M.J.; Kennedy, L.H.; Radivoyevitch, T.; La Tessa, C.; Gerson, S.L.; Welford, S.M. Protons and High-Linear Energy Transfer Radiation Induce Genetically Similar Lymphomas with High Penetrance in a Mouse Model of the Aging Human Hematopoietic System. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1091–1102. [Google Scholar] [CrossRef]
- Wage, J.; Ma, L.; Peluso, M.; Lamont, C.; Evens, A.M.; Hahnfeldt, P.; Hlatky, L.; Beheshti, A. Proton irradiation impacts age-driven modulations of cancer progression influenced by immune system transcriptome modifications from splenic tissue. J. Radiat. Res. 2015, 56, 792–803. [Google Scholar] [CrossRef]
- Cheema, A.K.; Suman, S.; Kaur, P.; Singh, R.; Fornace, A.J., Jr.; Datta, K. Long-term differential changes in mouse intestinal metabolomics after γ and heavy ion radiation exposure. PLoS ONE 2014, 9, e87079. [Google Scholar] [CrossRef]
- Weil, M.M.; Bedford, J.S.; Bielefeldt-Ohmann, H.; Ray, F.A.; Genik, P.C.; Ehrhart, E.J.; Fallgren, C.M.; Hailu, F.; Battaglia, C.L.; Charles, B.; et al. Incidence of acute myeloid leukemia and hepatocellular carcinoma in mice irradiated with 1 GeV/nucleon (56)Fe ions. Radiat. Res. 2009, 172, 213–219. [Google Scholar] [CrossRef]
- Turker, M.S.; Grygoryev, D.; Lasarev, M.; Ohlrich, A.; Rwatambuga, F.A.; Johnson, S.; Dan, C.; Eckelmann, B.; Hryciw, G.; Mao, J.H.; et al. Simulated space radiation-induced mutants in the mouse kidney display widespread genomic change. PLoS ONE 2017, 12, e0180412. [Google Scholar] [CrossRef]
- Ohnishi, T.; Ohnishi, K.; Takahashi, A.; Taniguchi, Y.; Sato, M.; Nakano, T.; Nagaoka, S. Detection of DNA damage induced by space radiation in Mir and space shuttle. J. Radiat. Res. 2002, 43, S133–S136. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Y.; Wong, M.; Feiveson, A.; Gaza, R.; Stoffle, N.; Wang, H.; Wilson, B.; Rohde, L.; Stodieck, L.; et al. Detection of DNA damage by space radiation in human fibroblasts flown on the International Space Station. Life Sci. Space Res. 2017, 12, 24–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, T.; Wong, M.; Wang, X.; Stodieck, L.; Karouia, F.; Story, M.; Wu, H. Transient gene and microRNA expression profile changes of confluent human fibroblast cells in spaceflight. FASEB J. 2016, 30, 2211–2224. [Google Scholar] [CrossRef]
- Blakely, E.A.; Kronenberg, A. Heavy-ion radiobiology: New approaches to delineate mechanisms underlying enhanced biological effectiveness. Radiat. Res. 1998, 150, S126–S145. [Google Scholar] [CrossRef]
- Koontz, B.F.; Verhaegen, F.; De Ruysscher, D. Tumour and normal tissue radiobiology in mouse models: How close are mice to mini-humans? Br. J. Radiol. 2017, 90, 20160441. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Blakely, E.A.; Burma, S.; Fornace, A.J., Jr.; Gerson, S.; Hlatky, L.; Kirsch, D.G.; Luderer, U.; Shay, J.; Wang, Y.; et al. Concepts and challenges in cancer risk prediction for the space radiation environment. Life Sci. Space Res. 2015, 6, 92–103. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; To, K.; Cacao, E. Predictions of space radiation fatality risk for exploration missions. Life Sci. Space Res. 2017, 13, 1–11. [Google Scholar] [CrossRef]
- Shuryak, I.; Fornace, A.J., Jr.; Datta, K.; Suman, S.; Kumar, S.; Sachs, R.K.; Brenner, D.J. Scaling Human Cancer Risks from Low LET to High LET when Dose-Effect Relationships are Complex. Radiat. Res. 2017, 187, 476–482. [Google Scholar] [CrossRef]
- Mishra, K.P. Carcinogenic risk from low-dose radiation exposure is overestimated. J. Radiat. Cancer Res. 2017, 8, 1–3. [Google Scholar] [CrossRef]
- Prasad, N.R. Radiation carcinogenesis: Mechanisms and experimental models—A meeting report. J. Radiat. Cancer Res. 2017, 8, 114–117. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Wong, M.; Lu, T.; Zhang, Y.; Wu, H. Interplay of space radiation and microgravity in DNA damage and DNA damage response. NPJ Microgravity 2017, 3, 14. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Y.C.; Zhao, T.Z.; Zhang, S.; Du, T.Y.; Yang, C.B.; Li, Y.H.; Sun, X.Q. Effects of simulated microgravity on human umbilical vein endothelial cell angiogenesis and role of the PI3K-Akt-eNOS signal pathway. PLoS ONE 2012, 7, e40365. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, F.; Wu, F.; Xu, H.; Yang, C.; Li, J.; Liang, P.; Zhang, H.; Qu, L.; Tan, Y.; et al. Integrin αvβ3 mediates the synergetic regulation of core-binding factor α1 transcriptional activity by gravity and insulin-like growth factor-1 through phosphoinositide 3-kinase signaling. Bone 2014, 69, 126–132. [Google Scholar] [CrossRef]
- Najrana, T.; Sanchez-Esteban, J. Mechanotransduction as an Adaptation to Gravity. Front. Pediatr. 2016, 4, 140. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Arun, R.P.; Sivanesan, D.; Vidyasekar, P.; Verma, R.S. PTEN/FOXO3/AKT pathway regulates cell death and mediates morphogenetic differentiation of Colorectal Cancer Cells under Simulated Microgravity. Sci. Rep. 2017, 7, 5952. [Google Scholar] [CrossRef] [PubMed]
- Monti, N.; Masiello, M.G.; Proietti, S.; Catizone, A.; Ricci, G.; Harrath, A.H.; Alwasel, S.H.; Cucina, A.; Bizzarri, M. Survival Pathways Are Differently Affected by Microgravity in Normal and Cancerous Breast Cells. Int. J. Mol. Sci. 2021, 22, 862. [Google Scholar] [CrossRef] [PubMed]
- Hybel, T.E.; Dietrichs, D.; Sahana, J.; Corydon, T.J.; Nassef, M.Z.; Wehland, M.; Krüger, M.; Magnusson, N.E.; Bauer, J.; Utpatel, K.; et al. Simulated Microgravity Influences VEGF, MAPK, and PAM Signaling in Prostate Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1263. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Pampaloni, F. In Vitro Models of Bone Marrow Remodelling and Immune Dysfunction in Space: Present State and Future Directions. Biomedicines 2022, 10, 766. [Google Scholar] [CrossRef]
- Elgart, S.R.; Little, M.P.; Chappell, L.J.; Milder, C.M.; Shavers, M.R.; Huff, J.L.; Patel, Z.S. Radiation Exposure and Mortality from Cardiovascular Disease and Cancer in Early NASA Astronauts. Sci. Rep. 2018, 8, 8480. [Google Scholar] [CrossRef]
- Di Trolio, R.; Di Lorenzo, G.; Fumo, B.; Ascierto, P.A. Cosmic radiation and cancer: Is there a link? Future Oncol. 2015, 11, 1123–1135. [Google Scholar] [CrossRef]
- Rahimian, N.; Razavi, Z.S.; Aslanbeigi, F.; Mirkhabbaz, A.M.; Piroozmand, H.; Shahrzad, M.K.; Hamblin, M.R.; Mirzaei, H. Non-Coding RNAs Related to Angiogenesis in Gynecological Cancer. Gynecol. Oncol. 2021, 161, 896–912. [Google Scholar] [CrossRef]
- Di Fiore, R.; Suleiman, S.; Pentimalli, F.; O’Toole, S.A.; O’Leary, J.J.; Ward, M.P.; Conlon, N.T.; Sabol, M.; Ozretić, P.; Erson-Bensan, A.E.; et al. Could MicroRNAs Be Useful Tools to Improve the Diagnosis and Treatment of Rare Gynecological Cancers? A Brief Overview. Int. J. Mol. Sci. 2021, 22, 3822. [Google Scholar] [CrossRef]
- Mandilaras, V.; Karakasis, K.; Clarke, B.; Amit Oza, A.; Lheureux, S. Rare tumors in gynaecological cancers and the lack of therapeutic options and clinical trials. Expert Opin. Orphan Drugs 2017, 5, 71–83. [Google Scholar] [CrossRef]
- Xie, W.; Sun, H.; Li, X.; Lin, F.; Wang, Z.; Wang, X. Ovarian cancer: Epigenetics, drug resistance, and progression. Cancer Cell Int. 2021, 21, 434. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Meryet-Figuiere, M.; Sabzalipoor, H.; Kashani, H.H.; Nikzad, H.; Asemi, Z. Dysregulated expression of long noncoding RNAs in gynecologic cancers. Mol. Cancer 2017, 16, 107. [Google Scholar] [CrossRef]
- Mota, A.; Oltra, S.S.; Moreno-Bueno, G. Insight updating of the molecular hallmarks in ovarian carcinoma. EJC Suppl. 2020, 15, 16–26. [Google Scholar] [CrossRef]
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.J.; Bast, R.C., Jr.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Frazer, I.H. Prevention of cervical cancer through papillomavirus vaccination. Nat. Rev. Immunol. 2004, 4, 46–54. [Google Scholar] [CrossRef]
- Kokka, F.; Bryant, A.; Brockbank, E.; Jeyarajah, A. Surgical treatment of stage IA2 cervical cancer. Cochrane Database Syst. Rev. 2014, 5, CD010870. [Google Scholar] [CrossRef]
- Louie, K.S.; de Sanjosé, S.; Diaz, M.; Castellsagué, X.; Herrero, R.; Meijer, C.J.; Shah, K.; Franceschi, S.; Muñoz, N.; Bosch, F.X. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br. J. Cancer 2009, 100, 1191–1197. [Google Scholar] [CrossRef]
- Liu, Z.C.; Liu, W.D.; Liu, Y.H.; Ye, X.H.; Chen, S.D. Multiple Sexual Partners as a Potential Independent Risk Factor for Cervical Cancer: A Meta-analysis of Epidemiological Studies. Asian Pac. J. Cancer Prev. 2015, 16, 3893–3900. [Google Scholar] [CrossRef]
- Husain, R.S.; Ramakrishnan, V. Global Variation of Human Papillomavirus Genotypes and Selected Genes Involved in Cervical Malignancies. Ann. Glob. Health 2015, 81, 675–683. [Google Scholar] [CrossRef]
- Yi, Y.; Fang, Y.; Wu, K.; Liu, Y.; Zhang, W. Comprehensive gene and pathway analysis of cervical cancer progression. Oncol. Lett. 2020, 19, 3316–3332. [Google Scholar] [CrossRef]
- Roura, E.; Castellsagué, X.; Pawlita, M.; Travier, N.; Waterboer, T.; Margall, N.; Bosch, F.X.; de Sanjosé, S.; Dillner, J.; Gram, I.T.; et al. Smoking as a major risk factor for cervical cancer and pre-cancer: Results from the EPIC cohort. Int. J. Cancer 2014, 135, 453–466. [Google Scholar] [CrossRef]
- Peirson, L.; Fitzpatrick-Lewis, D.; Ciliska, D.; Warren, R. Screening for cervical cancer: A systematic review and meta-analysis. Syst. Rev. 2013, 2, 35. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Fan, L.; Ma, J.; Yu, L.; Yi, H.; Chen, X.; Wei, W.; Wu, P.; Liang, L.; et al. Preoperative SCC-Ag and thrombocytosis as predictive markers for pelvic lymphatic metastasis of squamous cervical cancer in early FIGO stage. J. Cancer 2018, 9, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Nanthamongkolkul, K.; Hanprasertpong, J. Predictive Factors of Pelvic Lymph Node Metastasis in Early-Stage Cervical Cancer. Oncol. Res. Treat. 2018, 41, 194–198. [Google Scholar] [CrossRef]
- Wright, J.D.; Huang, Y.; Ananth, C.V.; Tergas, A.I.; Duffy, C.; Deutsch, I.; Burke, W.M.; Hou, J.Y.; Neugut, A.I.; Hershman, D.L. Influence of treatment center and hospital volume on survival for locally advanced cervical cancer. Gynecol. Oncol. 2015, 139, 506–512. [Google Scholar] [CrossRef]
- Vaccarella, S.; Lortet-Tieulent, J.; Plummer, M.; Franceschi, S.; Bray, F. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. Eur. J. Cancer 2013, 49, 3262–3273. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, R.; Suleiman, S.; Drago-Ferrante, R.; Felix, A.; O’Toole, S.A.; O’Leary, J.J.; Ward, M.P.; Beirne, J.; Yordanov, A.; Vasileva-Slaveva, M.; et al. LncRNA MORT (ZNF667-AS1) in Cancer—Is There a Possible Role in Gynecological Malignancies? Int. J. Mol. Sci. 2021, 22, 7829. [Google Scholar] [CrossRef] [PubMed]
- Yasin, H.K.; Taylor, A.H.; Ayakannu, T. A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer. Cancers 2021, 13, 2149. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Evrard, C.; Alexandre, J. Predictive and Prognostic Value of Microsatellite Instability in Gynecologic Cancer (Endometrial and Ovarian). Cancers 2021, 13, 2434. [Google Scholar] [CrossRef]
- Lax, S.F. Molecular genetic pathways in various types of endometrial carcinoma: From a phenotypical to a molecular-based classification. Virchows Arch. 2004, 444, 213–223. [Google Scholar] [CrossRef]
- Lax, S.F.; Kendall, B.; Tashiro, H.; Slebos, R.J.; Hedrick, L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: Evidence of distinct molecular genetic pathways. Cancer 2000, 88, 814–824. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Di Tucci, C.; Capone, C.; Galati, G.; Iacobelli, V.; Schiavi, M.C.; Di Donato, V.; Muzii, L.; Panici, P.B. Immunotherapy in endometrial cancer: New scenarios on the horizon. J. Gynecol. Oncol. 2019, 30, e46. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Daling, J.R.; Madeleine, M.M.; Schwartz, S.M.; Shera, K.A.; Carter, J.J.; McKnight, B.; Porter, P.L.; Galloway, D.A.; McDougall, J.K.; Tamimi, H. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol. Oncol. 2002, 84, 263–270. [Google Scholar] [CrossRef]
- Saraiya, M.; Watson, M.; Wu, X.; King, J.B.; Chen, V.W.; Smith, J.S.; Giuliano, A.R. Incidence of in situ and invasive vulvar cancer in the US, 1998–2003. Cancer 2008, 113, 2865–2872. [Google Scholar] [CrossRef]
- Shah, C.A.; Goff, B.A.; Lowe, K.; Peters, W.A., 3rd; Li, C.I. Factors affecting risk of mortality in women with vaginal cancer. Obstet. Gynecol. 2009, 113, 1038–1045. [Google Scholar] [CrossRef]
- How, J.A.; Jazaeri, A.A.; Soliman, P.T.; Fleming, N.D.; Gong, J.; Piha-Paul, S.A.; Janku, F.; Stephen, B.; Naing, A. Pembrolizumab in vaginal and vulvar squamous cell carcinoma: A case series from a phase II basket trial. Sci. Rep. 2021, 11, 3667. [Google Scholar] [CrossRef]
- Krüger, M.; Bauer, J.; Grimm, D. Cancer Research in Space. In Biotechnology in Space; Ruyters, G., Betzel, C., Grimm, D., Eds.; Springer: Cham, Switzerland, 2017; pp. 87–106. [Google Scholar]
- Grimm, D.; Schulz, H.; Krüger, M.; Cortés-Sánchez, J.L.; Egli, M.; Kraus, A.; Sahana, J.; Corydon, T.J.; Hemmersbach, R.; Wise, P.M.; et al. The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model. Int. J. Mol. Sci. 2022, 23, 3073. [Google Scholar] [CrossRef]
- Becker, J.L.; Souza, G.R. Using space-based investigations to inform cancer research on Earth. Nat. Rev. Cancer 2013, 13, 315–327. [Google Scholar] [CrossRef]
- Mehner, C.; Krishnan, S.; Chou, J.; Freeman, M.L.; Freeman, W.D.; Patel, T.; Turnbull, M.T. Real versus simulated galactic cosmic radiation for investigating cancer risk in the hematopoietic system-are we comparing apples to apples? Life Sci. Space Res. 2021, 29, 8–14. [Google Scholar] [CrossRef]
- Becker, J.L.; Prewett, T.L.; Spaulding, G.F.; Goodwin, T.J. Three-dimensional growth and differentiation of ovarian tumor cell line in high aspect rotating-wall vessel: Morphologic and embryologic considerations. J. Cell Biochem. 1993, 51, 283–289. [Google Scholar] [CrossRef]
- Goodwin, T.J.; Prewett, T.L.; Spaulding, G.F.; Becker, J.L. Three-dimensional culture of a mixed mullerian tumor of the ovary: Expression of in vivo characteristics. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 366–374. [Google Scholar] [CrossRef]
- Hammond, D.K.; Becker, J.; Elliott, T.F.; Holubee, K.; Baker, T.L.; Love, J.E. Antigenic protein in microgravity-grown human mixed Mullerian ovarian tumor (LN1) cells preserved in RNA stabilizing agent. Gravit. Space Biol. Bull. 2005, 18, 99–100. [Google Scholar]
- Przystupski, D.; Górska, A.; Szewczyk, A.; Drag-Zalensinka, M.; Kulbacka, J. 3D Clinorotation Affects Drug Sensitivity of Human Ovarian Cancer Cells. Microgravity Sci. Technol. 2021, 33, 42. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Saha, R.; Palanisamy, A.; Ghosh, M.; Biswas, A.; Roy, S.; Pal, A.; Sarkar, K.; Bagh, S. A systems biology pipeline identifies new immune and disease related molecular signatures and networks in human cells during microgravity exposure. Sci. Rep. 2016, 6, 25975. [Google Scholar] [CrossRef]
- Watanabe, H.; Ogiu, T.; Nishizaki, M.; Fujimoto, N.; Kido, S.; Ishimura, Y.; Shiraki, K.; Kuramoto, K.; Hirata, S.; Shoji, S.; et al. Induction of ovarian tumors by heavy ion irradiation in B6C3F1 mice. Oncol. Rep. 1998, 5, 1377–1380. [Google Scholar] [CrossRef]
- Watanabe, H.; Ogiu, T.; Nishimura, M.; Masaoka, Y.; Kurosumi, M.; Takahashi, T.; Oguri, T.; Shoji, S.; Katoh, O. Comparison of tumorigenesis between accelerated heavy ion and X-ray in B6C3F1 mice. J. Radiat. Res. 1998, 39, 93–100. [Google Scholar] [CrossRef][Green Version]
- Nitta, Y.; Hoshi, M. Relationship between oocyte apoptosis and ovarian tumours induced by high and low LET radiations in mice. Int. J. Radiat. Biol. 2003, 79, 241–250. [Google Scholar] [CrossRef]
- Mishra, B.; Lawson, G.W.; Ripperdan, R.; Ortiz, L.; Luderer, U. Charged-Iron-Particles Found in Galactic Cosmic Rays are Potent Inducers of Epithelial Ovarian Tumors. Radiat. Res. 2018, 190, 142–150. [Google Scholar] [CrossRef]
- Chopra, V.; Dinh, T.V.; Hannigan, E.V. Three-dimensional endothelial-tumor epithelial cell interactions in human cervical cancers. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 432–442. [Google Scholar] [CrossRef]
- Kelly, S.E.; Di Benedetto, A.; Greco, A.; Howard, C.M.; Sollars, V.E.; Primerano, D.A.; Valluri, J.V.; Claudio, P.P. Rapid selection and proliferation of CD133+ cells from cancer cell lines: Chemotherapeutic implications. PLoS ONE 2010, 5, e10035. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Tong, Y.Q.; Wang, J.J.; Yang, C.; Zhou, G.H.; Li, Y.H.; Xie, P.L.; Hu, J.Y.; Li, G.C. Spaceflight alters the gene expression profile of cervical cancer cells. Chin. J. Cancer 2011, 30, 842–852. [Google Scholar] [CrossRef]
- Guo, F.; Li, Y.; Liu, Y.; Huang, J.; Zhang, Z.; Wang, J.; Li, Y.; Hu, J.; Li, G. Identification of genes associated with tumor development in CaSki cells in the cosmic space. Mol. Biol. Rep. 2012, 39, 6923–6931. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Maglennon, G.A.; Doorbar, J. The biology of papillomavirus latency. Open Virol. J. 2012, 6, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Guidry, J.T.; Scott, R.S. The interaction between human papillomavirus and other viruses. Virus Res. 2017, 231, 139–147. [Google Scholar] [CrossRef]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Feiveson, A.H.; Sams, C.F.; Pierson, D.L. Latent virus reactivation in astronauts on the international space station. NPJ Microgravity 2017, 3, 11. [Google Scholar] [CrossRef]
- Bigley, A.B.; Agha, N.H.; Baker, F.L.; Spielmann, G.; Kunz, H.E.; Mylabathula, P.L.; Rooney, B.V.; Laughlin, M.S.; Mehta, S.K.; Pierson, D.L.; et al. NK cell function is impaired during long-duration spaceflight. J. Appl. Physiol. 2019, 126, 842–853. [Google Scholar] [CrossRef]
- Martinez, E.M.; Yoshida, M.C.; Candelario, T.L.; Hughes-Fulford, M. Spaceflight and simulated microgravity cause a significant reduction of key gene expression in early T-cell activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R480–R488. [Google Scholar] [CrossRef]
- Rooney, B.V.; Crucian, B.E.; Pierson, D.L.; Laudenslager, M.L.; Mehta, S.K. Herpes Virus Reactivation in Astronauts during Spaceflight and Its Application on Earth. Front. Microbiol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Jennings, R.T.; Baker, E.S. Gynecological and reproductive issues for women in space: A review. Obstet. Gynecol. Surv. 2000, 55, 109–116. [Google Scholar] [CrossRef]
- Cho, H.J.; Baek, M.O.; Khaliq, S.A.; Chon, S.J.; Son, K.H.; Lee, S.H.; Yoon, M.S. Microgravity inhibits decidualization via decreasing Akt activity and FOXO3a expression in human endometrial stromal cells. Sci. Rep. 2019, 9, 12094. [Google Scholar] [CrossRef]
- Grun, B.; Benjamin, E.; Sinclair, J.; Timms, J.F.; Jacobs, I.J.; Gayther, S.A.; Dafou, D. Three-dimensional in vitro cell biology models of ovarian and endometrial cancer. Cell Prolif. 2009, 42, 219–228. [Google Scholar] [CrossRef]
- Palumbo, G.; Varriale, L.; Paba, V.; Sasso, A.; Crescenzi, E.; Gialanella, G.; Grossi, G.; Pugliese, M.G.; Scampoli, P. Effect of space radiation on expression of apoptosis-related genes in endometrial cells: A preliminary study. Phys. Med. 2001, 17 (Suppl. S1), 241–246. [Google Scholar]
- Jennings, R.; Baker, E. Gynecologic and Reproductive Concerns. In Principles of Clinical Medicine for Space Flight; Barratt, M.R., Pool, S.L., Eds.; Springer Science & Business Media: Berlin, Germany, 2008; Chapter 18; pp. 381–390. [Google Scholar]
- Werneth, C.M.; Slaba, T.C.; Huff, J.L.; Patel, Z.S.; Simonsen, L.C. Medical Countermeasure Requirements to Meet NASA’s Space Radiation Permissible Exposure Limits for a Mars Mission Scenario. Health Phys. 2022, 123, 116–127. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.J.; Kim, S.M. Endometrial evaluation with transvaginal ultrasonography for the screening of endometrial hyperplasia or cancer in premenopausal and perimenopausal women. Obstet. Gynecol. Sci. 2016, 59, 192–200. [Google Scholar] [CrossRef]
- Steller, J.G.; Blue, R.; Zahner, C.; Frisch, E.H.; Bayuse, T.; Auñon-Chancellor, S.; Jennings, R.T. Menstrual management considerations in the space environment. Reach 2021, 23–24, 100044. [Google Scholar] [CrossRef]
- Bogomolov, V.V.; Castrucci, F.; Comtois, J.M.; Damann, V.; Davis, J.R.; Duncan, J.M.; Johnston, S.L.; Gray, G.W.; Grigoriev, A.I.; Koike, Y.; et al. International Space Station medical standards and certification for space flight participants. Aviat. Space Environ. Med. 2007, 78, 1162–1169. [Google Scholar]
- Barthel, J.; Sarigul-Klijn, N. A review of radiation shielding needs and concepts for space voyages beyond Earth’s magnetic influence. Prog. Aerosp. Sci. 2019, 110, 100553. [Google Scholar] [CrossRef]
- Montesinos, C.A.; Khalid, R.; Cristea, O.; Greenberger, J.S.; Epperly, M.W.; Lemon, J.A.; Boreham, D.R.; Popov, D.; Gorthi, G.; Ramkumar, N.; et al. Space Radiation Protection Countermeasures in Microgravity and Planetary Exploration. Life 2021, 11, 829. [Google Scholar] [CrossRef]
- Reliene, R.; Pollard, J.M.; Sobol, Z.; Trouiller, B.; Gatti, R.A.; Schiestl, R.H. N-acetyl cysteine protects against ionizing radiation-induced DNA damage but not against cell killing in yeast and mammals. Mutat. Res. 2009, 665, 37–43. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Fan, L.H.; Jing, Y.; Li, J.; Ouyang, Y.C.; Wang, Z.B.; Hou, Y.; Sun, Q.Y. N-acetyl-L-cysteine (NAC) delays post-ovulatory oocyte aging in mouse. Aging 2019, 11, 2020–2030. [Google Scholar] [CrossRef]
- Mendelev, N.; Mehta, S.L.; Idris, H.; Kumari, S.; Li, P.A. Selenite stimulates mitochondrial biogenesis signaling and enhances mitochondrial functional performance in murine hippocampal neuronal cells. PLoS ONE 2012, 7, e47910. [Google Scholar] [CrossRef]
- McKenzie, R.C.; Beckett, G.J.; Arthur, J.R. Effects of selenium on immunity and aging. In Selenium: Its Molecular Biology and Role in Human Health, 2nd ed.; Hatfield, D.L., Berry, M.J., Gladyshev, V.N., Eds.; Springer: New York, NY, USA, 2006; pp. 311–323. [Google Scholar]
- Suzuki, T.; Suzuki, T.; Wada, T.; Saigo, K.; Watanabe, K. Taurine as a constituent of mitochondrial tRNAs: New insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002, 21, 6581–6589. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Hashimoto, T.; Horikawa, D.D.; Saito, Y.; Kuwahara, H.; Kozuka-Hata, H.; Shin-I, T.; Minakuchi, Y.; Ohishi, K.; Motoyama, A.; Aizu, T.; et al. Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 2016, 7, 12808. [Google Scholar] [CrossRef] [PubMed]
- Sanzari, J.K.; Krigsfeld, G.S.; Shuman, A.L.; Diener, A.K.; Lin, L.; Mai, W.; Kennedy, A.R. Effects of a granulocyte colony stimulating factor, Neulasta, in mini pigs exposed to total body proton irradiation. Life Sci. Space Res. 2015, 5, 13–20. [Google Scholar] [CrossRef]
- Bahamondes, L.; Valeria Bahamondes, M.; Shulman, L.P. Non-contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Hum. Reprod. Update 2015, 21, 640–651. [Google Scholar] [CrossRef]
- Asthana, S.; Busa, V.; Labani, S. Oral contraceptives use and risk of cervical cancer—A systematic review & meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 163–175. [Google Scholar]
- Iversen, L.; Fielding, S.; Lidegaard, Ø.; Hannaford, P.C. Contemporary hormonal contraception and cervical cancer in women of reproductive age. Int. J. Cancer 2021, 149, 769–777. [Google Scholar] [CrossRef]
- Cogliano, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005, 6, 204. [Google Scholar] [CrossRef]
- Cibula, D.; Gompel, A.; Mueck, A.O.; La Vecchia, C.; Hannaford, P.C.; Skouby, S.O.; Zikan, M.; Dusek, L. Hormonal contraception and risk of cancer. Hum. Reprod. Update 2010, 16, 631–650. [Google Scholar] [CrossRef]
- Mørch, L.S.; Hannaford, P.C.; Lidegaard, Ø. Contemporary Hormonal Contraception and the Risk of Breast Cancer. N. Engl J. Med. 2018, 378, 1265–1266. [Google Scholar] [CrossRef] [PubMed]
- Conz, L.; Mota, B.S.; Bahamondes, L.; Teixeira Dória, M.; Françoise Mauricette Derchain, S.; Rieira, R.; Sarian, L.O. Levonorgestrel-releasing intrauterine system and breast cancer risk: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2020, 99, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, J.S. Radioprotection. In Vivo 2009, 23, 323–336. [Google Scholar] [PubMed]
- Belli, M.; Tabocchini, M.A. Ionizing Radiation-Induced Epigenetic Modifications and Their Relevance to Radiation Protection. Int. J. Mol. Sci. 2020, 21, 5993. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J. Natl. Compr. Canc. Netw. 2020, 18, 380–391. [Google Scholar] [CrossRef]
- Nuñez, J.K.; Chen, J.; Pommier, G.C.; Cogan, J.Z.; Replogle, J.M.; Adriaens, C.; Ramadoss, G.N.; Shi, Q.; Hung, K.L.; Samelson, A.J.; et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell 2021, 184, 2503–2519.e17. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Q.; Jin, S.; Gao, C. The CRISPR-Cas toolbox and gene editing technologies. Mol. Cell. 2022, 82, 333–347. [Google Scholar] [CrossRef]
- Berrios, D.C.; Galazka, J.; Grigorev, K.; Gebre, S.; Costes, S.V. NASA GeneLab: Interfaces for the exploration of space omics data. Nucleic Acids Res. 2021, 49, D1515–D1522. [Google Scholar] [CrossRef]
- Datta, K.; Hyduke, D.R.; Suman, S.; Moon, B.H.; Johnson, M.D.; Fornace, A.J., Jr. Exposure to ionizing radiation induced persistent gene expression changes in mousemammary gland. Radiat. Oncol. 2012, 7, 205. [Google Scholar] [CrossRef]
| Type of Radiation | Radiation Weighting Factor (WR) |
|---|---|
| X-rays/Gamma rays | 1 |
| Electrons | 1 |
| Protons | 2–5 |
| Neutrons | 5–20 |
| Heavy ions | 20 |
| Age | Dose, mGy | Effect |
|---|---|---|
| All ages | 1700 | Temporary sterility lasting 1–3 years |
| 1250–1500 | Amenorrhea in 50% | |
| 3200–6250 | Permanent sterility | |
| Ages 15–40 | 1250–2500 | Temporary amenorrhea |
| 2500–5000 | Ovulary suppression in 40–100% | |
| 5000–8000 | Permanent ovulary suppression in 40–100% | |
| 8000–20,000 | Permanent ovulary suppression in 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drago-Ferrante, R.; Di Fiore, R.; Karouia, F.; Subbannayya, Y.; Das, S.; Aydogan Mathyk, B.; Arif, S.; Guevara-Cerdán, A.P.; Seylani, A.; Galsinh, A.S.; et al. Extraterrestrial Gynecology: Could Spaceflight Increase the Risk of Developing Cancer in Female Astronauts? An Updated Review. Int. J. Mol. Sci. 2022, 23, 7465. https://doi.org/10.3390/ijms23137465
Drago-Ferrante R, Di Fiore R, Karouia F, Subbannayya Y, Das S, Aydogan Mathyk B, Arif S, Guevara-Cerdán AP, Seylani A, Galsinh AS, et al. Extraterrestrial Gynecology: Could Spaceflight Increase the Risk of Developing Cancer in Female Astronauts? An Updated Review. International Journal of Molecular Sciences. 2022; 23(13):7465. https://doi.org/10.3390/ijms23137465
Chicago/Turabian StyleDrago-Ferrante, Rosa, Riccardo Di Fiore, Fathi Karouia, Yashwanth Subbannayya, Saswati Das, Begum Aydogan Mathyk, Shehbeel Arif, Ana Paula Guevara-Cerdán, Allen Seylani, Aman Singh Galsinh, and et al. 2022. "Extraterrestrial Gynecology: Could Spaceflight Increase the Risk of Developing Cancer in Female Astronauts? An Updated Review" International Journal of Molecular Sciences 23, no. 13: 7465. https://doi.org/10.3390/ijms23137465
APA StyleDrago-Ferrante, R., Di Fiore, R., Karouia, F., Subbannayya, Y., Das, S., Aydogan Mathyk, B., Arif, S., Guevara-Cerdán, A. P., Seylani, A., Galsinh, A. S., Kukulska, W., Borg, J., Suleiman, S., Porterfield, D. M., Camera, A., Christenson, L. K., Ronca, A. E., Steller, J. G., Beheshti, A., & Calleja-Agius, J. (2022). Extraterrestrial Gynecology: Could Spaceflight Increase the Risk of Developing Cancer in Female Astronauts? An Updated Review. International Journal of Molecular Sciences, 23(13), 7465. https://doi.org/10.3390/ijms23137465












