Silencing GmBIR1 in Soybean Results in Activated Defense Responses
Abstract
1. Introduction
2. Results
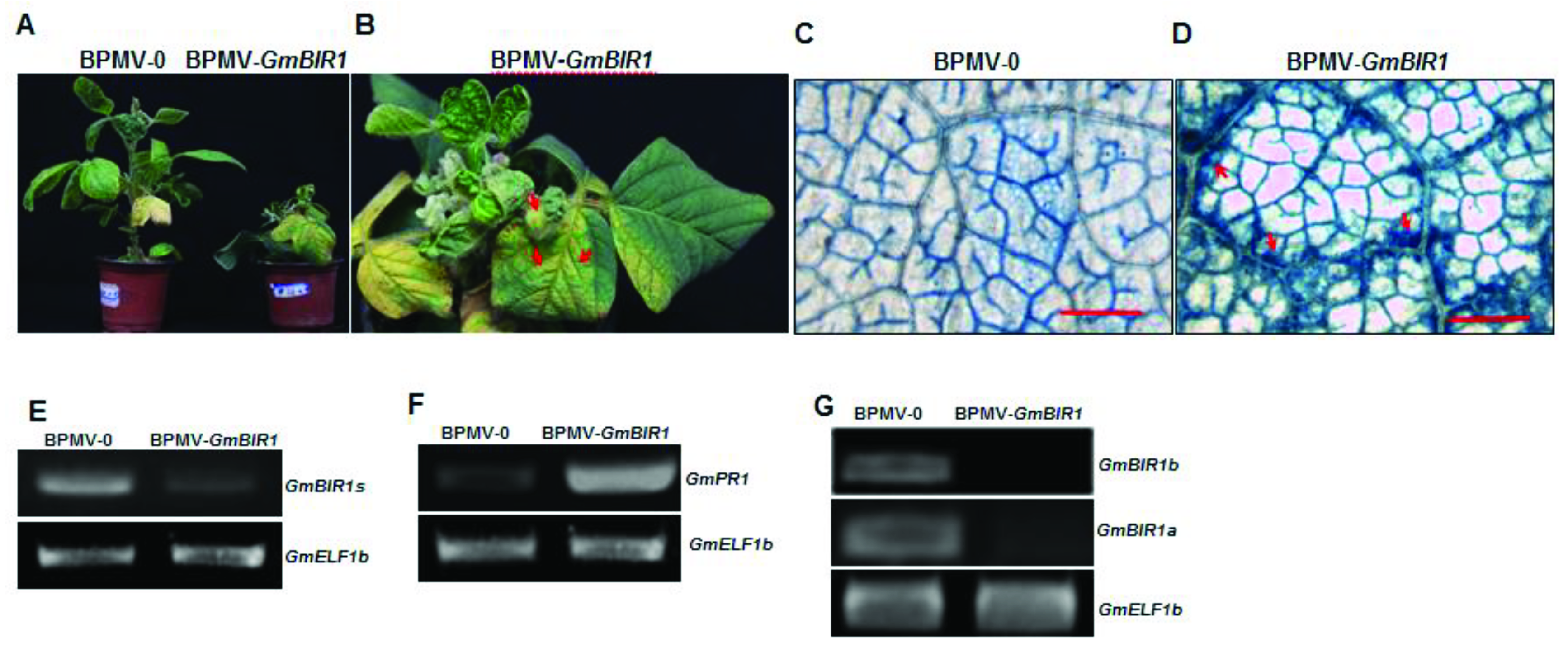
2.1. Silencing Glyma.18g246400 Results in Stunted Stature and Spontaneous Cell Death in Soybean
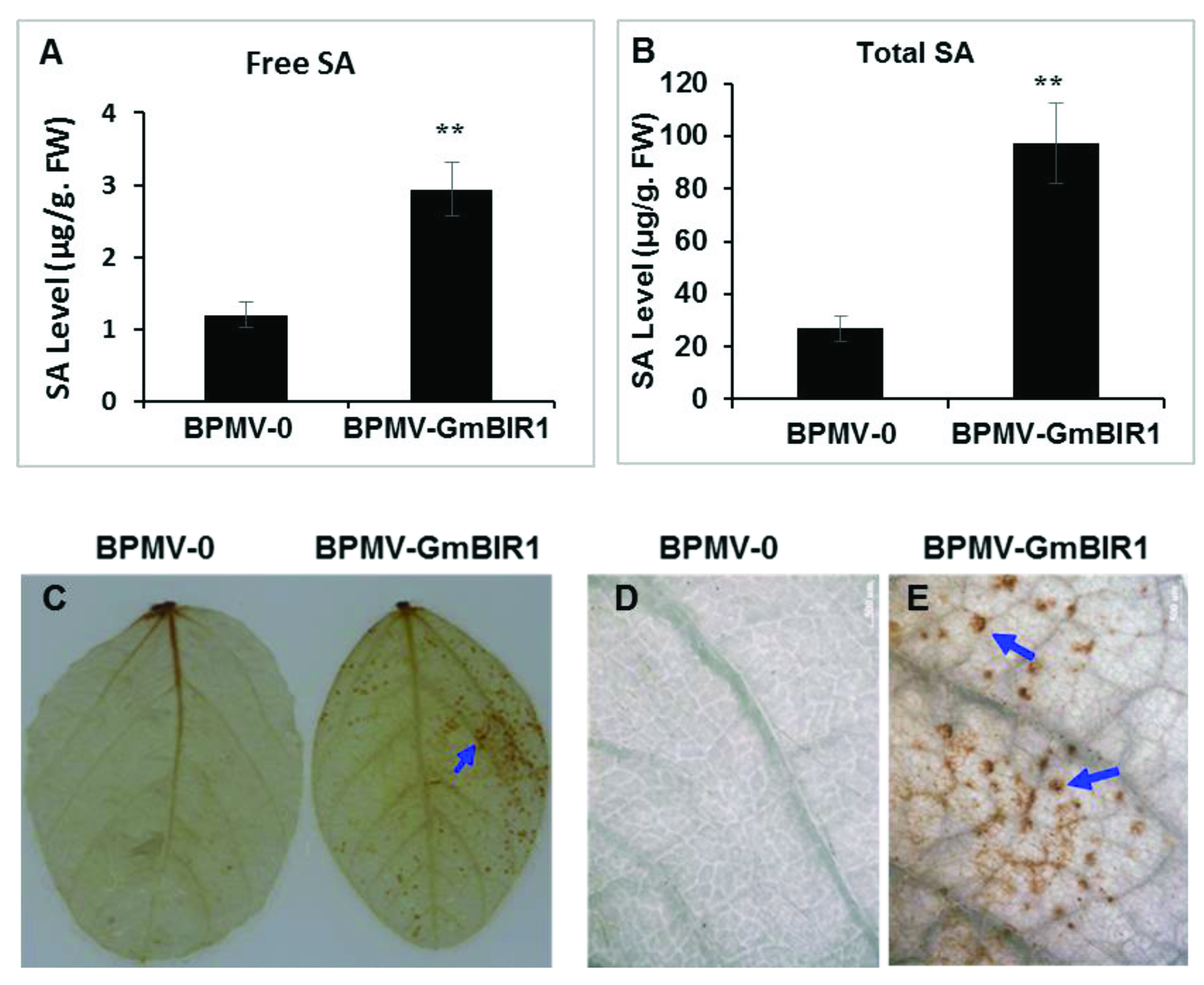
2.2. Both SA and H2O2 Are Over-Accumulated in the GmBIR1-Silenced Plants
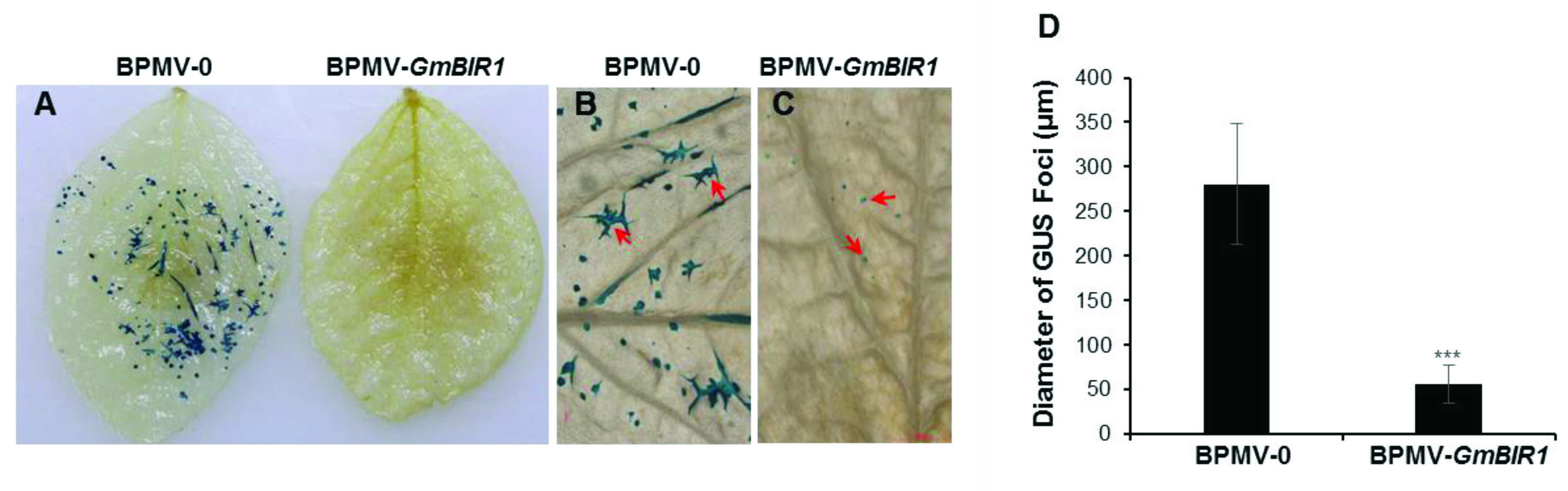
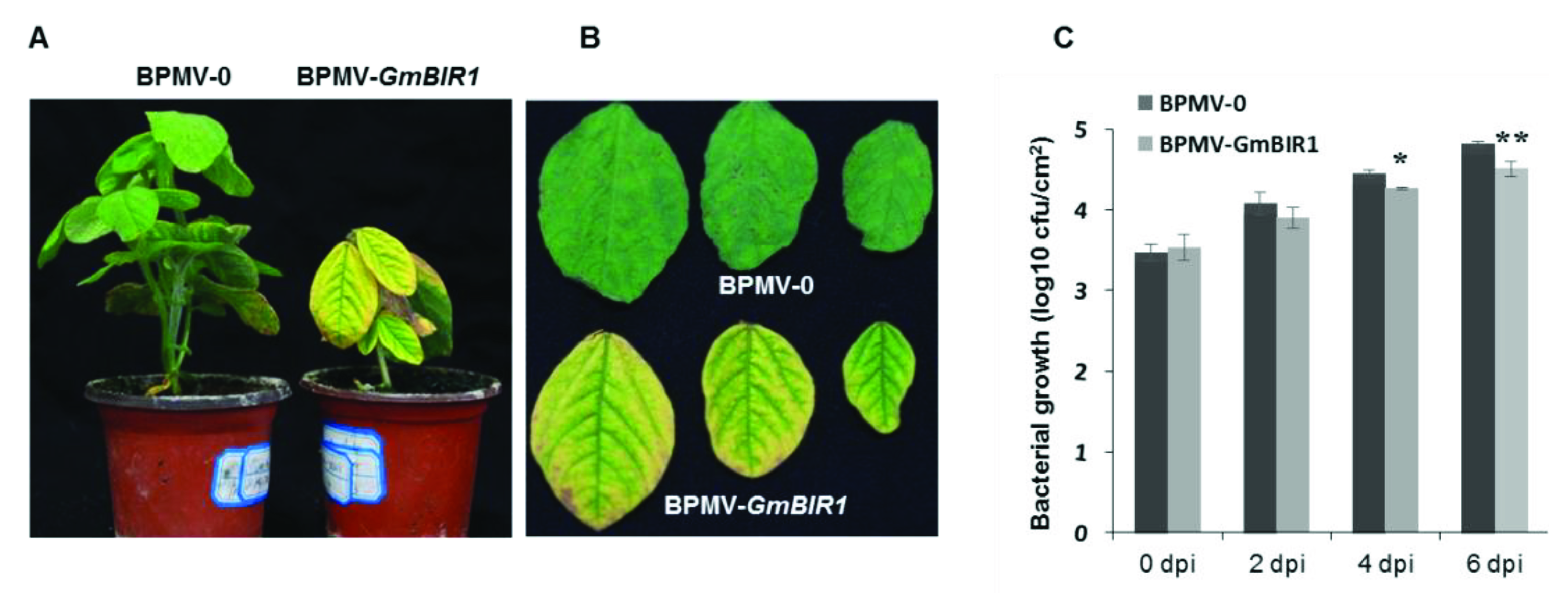
2.3. Silencing GmBIR1 Enhances Resistance against SMV and Pseudomonas syringae pv. glycinea (Psg), R4 Strain
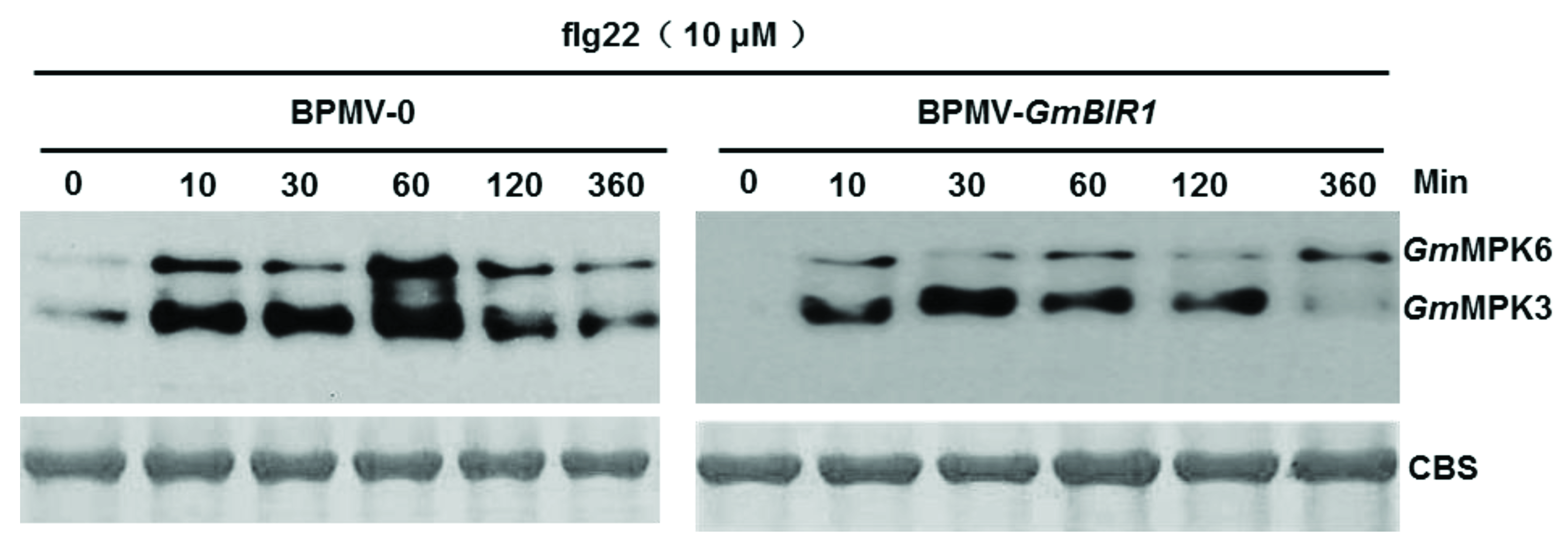
2.4. The Activated Defense Responses in GmBIR1-Silenced Plants Are Independent of GmMPK3/6 Activation
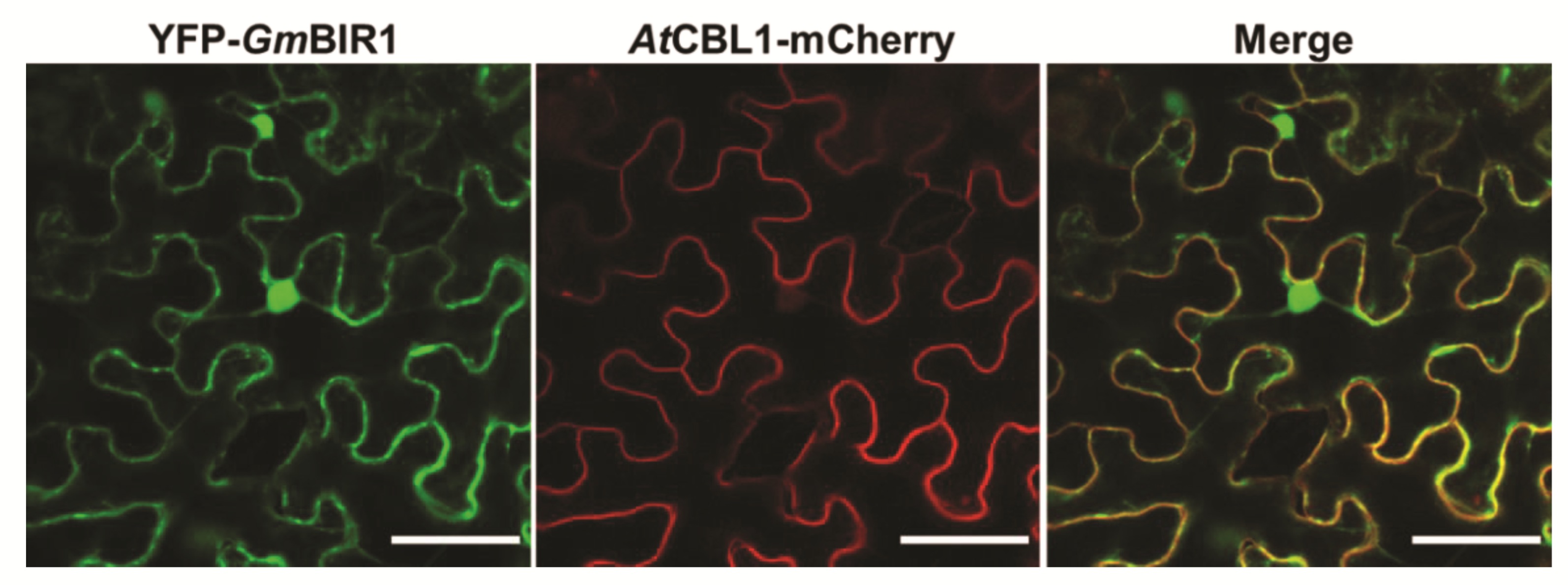
2.5. GmBIR1 Is Localized at the Plasma Membrane (PM) and in the Nucleus
3. Discussion
3.1. The Function of BIR1 Homologs Is Conserved from Different Plant Species
3.2. The Constitutively Activated Cell Death and Defense Responses in GmBIR1-Silenced Plants Is A Result of Co-Silencing of Both GmBIR1a and GmBIR1b
3.3. Does the Autoimmunity Observed in GmBIR1-Silenced Plants Result from Activated PTI or Activated ETI?
3.4. Is the Enhanced Resistance of GmBIR1-Silenced plants to SMV SA-Dependent?
4. Materials and Methods
4.1. Plant Materials
4.2. Flg22 Peptides
4.3. BPMV-Mediated VIGS
- GmBIR1-F: 5′-aagGGATCCCTCACTGGTCAAATTCCTGCTA-3′
- GmBIR1-R: 5′-ttgGGTACCCAGGGTCCTCTTCCTTCTTCCTA-3′
4.4. RNA Isolation and RT-PCR
- GmELF1b-F: 5′- ACCGAAGAGGGCATCAAATCCC -3′
- GmELF1b-R: 5′- CTCAACTGTCAAGCGTTCCTC -3′
- GmBIR1-full-F: 5′- ATAGGAAGAAGGAAGAGGACC -3′
- GmBIR1-full-R: 5′- TTTAGGAGTAGCCACCAAAGT -3′
- GmBIR1a-F: 5′- GACTTGCTCAATATCCCAGGATAAGT-3′
- GmBIR1a-R: 5′- TGTTGAGATTCCTGTAATCTCTTAACCAT -3′
- GmBIR1b-F: 5′- TACTTCCTCAATATCCCAGGTGG -3′
- GmBIR1b-R: 5′- TATTGAGATTCCTGCAATCTCTTGAC -3′.
4.5. Trypan Blue Staining
4.6. SA Quantification
4.7. H2O2 Detection by DAB Staining
4.8. Inoculation of Pseudomonas Syringae pv. Glycinea (Psg) onto the Leaves of Soybean Plants
4.9. SMV-N-GUS Inoculation, GUS Staining, and GUS Foci Measurements
4.10. Western Blot Analysis for Detecting Phosphorylated MPKs
4.11. Subcellular Localization of GmBIR1
- GmBIR1-PGDG-F-HinDIII: 5′-AAGCTTATGAAGATGTTTATGGGTGGC-3′
- GmBIR1-PGDG-R-BamHI: 5′-GGATCCTCAATCATGTCCCTCTCGAG-3′
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.H.; Bleecker, A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 2001, 98, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, P.A.; Shan, L.; He, P. Plant cell surface molecular cypher: Receptor-like proteins and their roles in immunity and development. Plant Sci. 2018, 274, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, L.; Boller, T. FLS2: An LRR Receptor–like Kinase Involved in the Perception of the Bacterial Elicitor Flagellin in Arabidopsis. Mol. Cell 2009, 5, 1003–1011. [Google Scholar] [CrossRef]
- Zipfel, C.; Kunze, G.; Chinchilla, D.; Caniard, A.; Jones, J.D.; Boller, T.; Felix, G. Perception of the Bacterial PAMP EF-Tu by the Receptor EFR Restricts Agrobacterium-Mediated Transformation. Cell 2006, 125, 749–760. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Lease, K.A.; Doke, J.T.; Tax, F.E.; Walker, J.C. BAK1, an Arabidopsis LRR Receptor-like Protein Kinase, Interacts with BRI1 and Modulates Brassinosteroid Signaling. Cell 2002, 110, 213–222. [Google Scholar] [CrossRef]
- Nam, K.H.; Li, J. BRI1/BAK1, a Receptor Kinase Pair Mediating Brassinosteroid Signaling. Cell 2002, 110, 203–212. [Google Scholar] [CrossRef]
- Chinchilla, D.; Zipfel, C.; Robatzek, S.; Kemmerling, B.; Nürnberger, T.; Jones, J.D.G.; Felix, G.; Boller, T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007, 448, 497–500. [Google Scholar] [CrossRef]
- Heese, A.; Hann, D.R.; Gimenez-Ibanez, S.; Jones, A.M.; He, K.; Li, J.; Schroeder, J.I.; Peck, S.C.; Rathjen, J.P. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA 2007, 104, 12217–12222. [Google Scholar] [CrossRef]
- Roux, M.; Schwessinger, B.; Albrecht, C.; Chinchilla, D.; Jones, A.; Holton, N.; Malinovsky, F.G.; Tör, M.; de Vries, S.; Zipfel, C. The Arabidopsis leucine-rich repeat receptor-like kinase BAK1/SERK3 and BKK1/SERK4 are required for the innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 2011, 23, 2440–2455. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.P.; Schwessinger, B.; Ntoukakis, V.; Brutus, A.; Segonzac, C.; Roy, S.; Kadota, Y.; Oh, M.H.; Sklenar, J.; Derbyshire, P.; et al. A bacterial tyrosine phosphatase inhibits plant pattern recognition receptor activation. Science 2014, 343, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, X.; Wang, D.; Xu, F.; Ding, X.; Zhang, Z.; Bi, D.; Cheng, Y.T.; Chen, S.; Li, X.; et al. Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 2009, 6, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, X.; Li, M.; He, P.; Zhang, Y. Loss-of-function of Arabidopsis receptor-like kinase BIR1 activates cell death and defense responses mediated by BAK1 and SOBIR1. New Phytol. 2016, 212, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Liebrand, T.W.; van den Berg, G.C.; Zhang, Z.; Smit, P.; Cordewener, J.H.; America, A.H.; Sklenar, J.; Jones, A.M.; Tameling, W.I.; Robatzek, S.; et al. Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc. Natl. Acad. Sci. USA 2013, 110, 10010–10015. [Google Scholar] [CrossRef]
- Liebrand, T.W.; van den Burg, H.A.; Joosten, M.H. Two for all: Receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci. 2014, 19, 123–132. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, P.; Zhang, X.; Ren, D.; Yang, S. BON1 interacts with the protein kinases BIR1 and BAK1 in modulation of temperature-dependent plant growth and cell death in Arabidopsis. Plant J. 2011, 67, 1081–1093. [Google Scholar] [CrossRef]
- Halter, T.; Imkampe, J.; Mazzotta, S.; Wierzba, M.; Postel, S.; Bücherl, C.; Kiefer, C.; Stahl, M.; Chinchilla, D.; Wang, X.; et al. The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr. Biol. 2014, 24, 134–143. [Google Scholar] [CrossRef]
- Liu, J.Z.; Horstman, H.D.; Braun, E.; Graham, M.A.; Zhang, C.; Navarre, D.; Qiu, W.L.; Lee, Y.; Nettleton, D.; Hill, J.H.; et al. Soybean homologs of MPK4 negatively regulate defense responses and positively regulate growth and development. Plant Physiol. 2011, 157, 1363–1378. [Google Scholar] [CrossRef]
- Liu, J.Z.; Whitham, S.A. Overexpresson of a soybean nuclear-localized type III DnaJ domain-containing HSP40 reveals it roles in cell death and disease resistance. Plant J. 2013, 74, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Braun, E.; Qiu, W.L.; Shi, Y.F.; Marcelino-Guimarães, F.C.; Navarre, D.; Hill, J.H.; Whitham, S.A. Positive and negative roles for soybean MPK6 in regulating defense responses. Mol. Plant Microbe Interact. 2014, 27, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, C.; Li, Z.C.; Wan, Z.R.; Jiang, X.X.; Tian, S.N.; Braun, E.; Mei, Y.; Qiu, W.L.; Li, S.; et al. The MAPK kinase kinase GmMEKK1 regulates cell death and defense responses. Plant Physiol. 2018, 178, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.N.; Liu, D.D.; Zhong, C.L.; Xu, H.Y.; Yang, S.; Fang, Y.; Ran, J.; Liu, J.Z. Silencing GmFLS2 enhances the susceptibility of soybean to bacterial pathogen through attenuating the activation of GmMAPK signaling pathway. Plant Sci. 2019, 292, 110386. [Google Scholar] [CrossRef]
- Hashimi, S.M.; Wu, N.N.; Ran, J.; Liu, J.Z. Silencing Autophagy-Related Gene 2 (ATG2) Results in Accelerated Senescence and Enhanced Immunity in Soybean. Int. J. Mol. Sci. 2021, 22, 11749. [Google Scholar] [CrossRef]
- Liu, J.Z.; Graham, M.A.; Pedley, K.F.; Whitham, S.A. Gaining insight into soybean defense responses using functional genomics approaches. Brief. Funct. Genom. 2015, 14, 283–290. [Google Scholar] [CrossRef]
- Liu, J.Z.; Fang, Y.; Pang, H. The current status of the soybean-soybean mosaic virus (SMV) pathosystem. Front. Microbiol. 2016, 7, 1906. [Google Scholar] [CrossRef]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E.; et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Delledonne, M.; Xia, Y.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.G.; Wei, Y.D.; Collinge, D.B. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Ren, D.; Yang, H.; Zhang, S. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J. Biol. Chem. 2002, 277, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Eggenberger, A.; Hill, J.; Bogdanove, A.J. Pseudomonas syringae effector avrB confers soybean cultivar-specific avirulence on Soybean mosaic virus adapted for transgene expression but effector avrPto does not. Mol. Plant Microbe Interact. 2006, 19, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Zhao, C.; Nie, H.; Shen, Q.; Zhang, S.; Lukowitz, W.; Tang, D. EDR1 physically interacts with MKK4/MKK5 and negatively regulates a MAP kinase cascade to modulate plant innate immunity. PLoS Genet. 2014, 10, e1004389. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Y.; Bai, B.; Han, Z.; Tang, J.; Zhang, H.; Yaghmaiean, H.; Zhang, Y.; Chai, J. Structural basis for BIR1-mediated negative regulation of plant immunity. Cell Res. 2017, 27, 1521–1524. [Google Scholar] [CrossRef]
- Wierzba, M.P.; Tax, F.E. An allelic series of bak1 mutations differentially alter bir1 cell death, immune response, growth, and root development phenotypes in Arabidopsis thaliana. Genetics 2016, 202, 689–702. [Google Scholar] [CrossRef][Green Version]
- Guzmán-Benito, I.; Donaire, L.; Amorim-Silva, V.; Vallarino, J.G.; Esteban, A.; Wierzbicki, A.T.; Ruiz-Ferrer, V.; Llave, C. The immune repressor BIR1 contributes to antiviral defense and undergoes transcriptional and post-transcriptional regulation during viral infections. New Phytol. 2019, 224, 421–438. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Gao, M.; Zhang, J.; Kong, Q.; Liu, Y.; Ba, H.; Zhou, J.; Zhang, Y. Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe 2012, 11, 253–263. [Google Scholar] [CrossRef]
- Kong, Q.; Qu, N.; Gao, M.; Zhang, Z.; Ding, X.; Yang, F.; Li, Y.; Dong, O.X.; Chen, S.; Li, X.; et al. The MEKK1-MKK1/MKK2-MPK4 kinase cascade negatively regulates immunity mediated by a mitogen-activated protein kinase kinase kinase in Arabidopsis. Plant Cell 2012, 24, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Hamano, K.; Takagi, H.; Morimoto, T.; Akimitsu, K.; Terauchi, R.; Shirasu, K.; Ichimura, K. Disruption of the MAMP-Induced MEKK1-MKK1/MKK2-MPK4 Pathway Activates the TNL Immune Receptor SMN1/RPS6. Plant Cell Physiol. 2019, 60, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Whitham, S.; Dinesh-Kumar, S.P.; Choi, D.; Hehl, R.; Corr, C.; Baker, B. The product of the tobacco mosaic virus resistance gene N: Similarity to toll and the interleukin-1 receptor. Cell 1994, 78, 1101–1115. [Google Scholar] [CrossRef]
- Yang, S.; Hua, J. A haplotype-specific Resistance gene regulated by BONZAI1 mediates temperature-dependent growth control in Arabidopsis. Plant Cell 2004, 16, 1060–1071. [Google Scholar] [CrossRef]
- Wiermer, M.; Feys, B.J.; Parker, J.E. Plant immunity: EDS1 regulatory node. Curr. Opin. Plant Biol. 2005, 8, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ahn, H.K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, C.; Whitham, S.A.; Hill, J.H. Development and use of an efficient DNA-based viral gene silencing vector for soybean. Mol. Plant Microbe Interact. 2009, 22, 123–131. [Google Scholar] [CrossRef]
- Zhang, C.; Bradshaw, J.D.; Whitham, S.A.; Hill, J.H. The development of an efficient multipurpose bean pod mottle virus viral vector set for foreign gene expression and RNA silencing. Plant Physiol. 2010, 153, 52–65. [Google Scholar] [CrossRef]
- Liu, J.Z.; Blancaflor, E.B.; Nelson, R.S. The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol. 2005, 138, 1853–1865. [Google Scholar] [CrossRef]
- Zhang, K.; Halitschke, R.; Yin, C.; Liu, C.J.; Gan, S.S. Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc. Natl. Acad. Sci. USA 2013, 110, 14807–14812. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Goodin, M.; Dietzgen, R.G.; Schichnes, D.; Ruzin, S.; Jackson, A.O. pGD vectors: Versatile tools for the expression of green and red fluorescent protein fusions in Agroinfiltrated plant leaves. Plant J. 2002, 31, 375–383. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.-D.; Lan, H.-J.; Masoud, H.S.; Ye, M.-Y.; Dai, X.-Y.; Zhong, C.-L.; Tian, S.-N.; Liu, J.-Z. Silencing GmBIR1 in Soybean Results in Activated Defense Responses. Int. J. Mol. Sci. 2022, 23, 7450. https://doi.org/10.3390/ijms23137450
Liu D-D, Lan H-J, Masoud HS, Ye M-Y, Dai X-Y, Zhong C-L, Tian S-N, Liu J-Z. Silencing GmBIR1 in Soybean Results in Activated Defense Responses. International Journal of Molecular Sciences. 2022; 23(13):7450. https://doi.org/10.3390/ijms23137450
Chicago/Turabian StyleLiu, Dan-Dan, Hu-Jiao Lan, Hashimi Said Masoud, Mei-Yan Ye, Xian-Yong Dai, Chen-Li Zhong, Sheng-Nan Tian, and Jian-Zhong Liu. 2022. "Silencing GmBIR1 in Soybean Results in Activated Defense Responses" International Journal of Molecular Sciences 23, no. 13: 7450. https://doi.org/10.3390/ijms23137450
APA StyleLiu, D.-D., Lan, H.-J., Masoud, H. S., Ye, M.-Y., Dai, X.-Y., Zhong, C.-L., Tian, S.-N., & Liu, J.-Z. (2022). Silencing GmBIR1 in Soybean Results in Activated Defense Responses. International Journal of Molecular Sciences, 23(13), 7450. https://doi.org/10.3390/ijms23137450






